Abstract
The renal-specific NKCC2 (Na+–K+–2Cl− co-transporter 2) is regulated by changes in phosphorylation state, however, the phosphorylation sites and kinases responsible have not been fully elucidated. In the present study, we demonstrate that the metabolic sensing kinase AMPK (AMP-activated protein kinase) phosphorylates NKCC2 on Ser126 in vitro. Co-precipitation experiments indicated that there is a physical association between AMPK and the N-terminal cytoplasmic domain of NKCC2. Activation of AMPK in the MMDD1 (mouse macula densa-derived 1) cell line resulted in an increase in Ser126 phosphorylation in situ, suggesting that AMPK may phosphorylate NKCC2 in vivo. The functional significance of Ser126 phosphorylation was examined by mutating the serine residue to an alanine residue resulting in a marked reduction in co-transporter activity when exogenously expressed in Xenopus laevis oocytes under isotonic conditions. Under hypertonic conditions no significant change of activity was observed. Therefore the present study identifies a novel phosphorylation site that maintains NKCC2-mediated transport under isotonic or basal conditions. Moreover, the metabolic-sensing kinase, AMPK, is able to phosphorylate this site, potentially linking the cellular energy state with changes in co-transporter activity.
Keywords: AMP-activated protein kinase (AMPK), cation co-transporter, co-transporter activity, Na+–K+–2Cl− co-transporter 2 (NKCC2), phosphorylation, Xenopus laevis oocytes
Abbreviations: ADR-1, alcohol dehydrogenase regulator 1; AMPK, AMP-activated protein kinase; DN-AMPK, dominant-negative AMPK; DTT, dithiothreitol; ECL, enhanced chemiluminescence; GFP, green fluorescent protein; GST, glutathione S-transferase; HEK, human embryonic kidney; MALDI-TOF, matrix-assisted laser-desorption ionization–time-of-flight; MMDD1, mouse macula densa-derived 1 cells; NCC, thiazide-sensitive sodium chloride co-transporter; NKCC2, Na+–K+–2Cl− co-transporter 2; NKCC1, Na+–K+–2Cl− co-transporter 1; SPAK, STE20/SPS1-related proline/alanine-rich kinase; TFA, trifluoroacetic acid; WNK, with no K (lysine) kinase
INTRODUCTION
Regulation of net renal NaCl reabsorption is a major determinant of extracellular fluid volume and, consequently, blood pressure. The kidney-specific Na+–K+–2Cl− co-transporter, NKCC2, mediates transport of Na+, K+ and Cl− across the luminal membrane of the thick ascending limb of the loop of Henle and the macula densa [1]. NKCC2 is important for overall salt reabsorption, as evidenced by loss-of-function mutations that lead to a severe form of salt-wasting in neonates, termed Bartter's syndrome [2]. Disruption of the NKCC2 gene by homologous recombination in mice causes a similar syndrome [3]. NKCC2 is also the target of the commonly used loop diuretics bumetanide and furosemide. A second function of NKCC2 is its involvement in tubuloglomerular feedback, one of the key determinants of renal blood flow. NKCC2 is required to detect the level of Cl− in the tubular fluid at the macula densa in the kidney. The macula densa then regulates blood flow through the glomerulus of the kidney, by mechanisms that have only partly been elucidated.
Studies of post-translational regulation of the Na+–K+–2Cl− co-transporter family have generally been performed in the more widely-distributed secretory form, NKCC1. NKCC1 is phosphorylated in response to a number of stimuli including changes in cell volume and intracellular Cl− concentration [4]. Five phosphoacceptor threonine residues have been identified in the N-terminus of NKCC1 (Thr174, Thr179, Thr184, Thr189 and Thr202 in the shark NKCC1 sequence) with phosphorylation at Thr189 being essential for activity [5]. The function of phosphorylation of the remaining phosphoacceptor residues is not as evident but they could modulate phosphorylation of Thr189. The N-terminus contains a high affinity binding site for SPAK (STE20/SPS1-related proline/alanine-rich kinase), although this site is not required for activation of NKCC1 [6]. Phosphorylation of Thr184 and Thr189 phosphoacceptor sites, however, is inhibited by expression of a dominant-negative SPAK. In addition SPAK has been demonstrated to directly phosphorylate NKCC1 on Thr174, Thr179 and Thr184 in response to sorbitol stimulation, thus providing the first evidence that the SPAK pathway is directly implicated in the phosphorylation of NKCC1 [7].
There have been few studies directly examining phosphorylation of NKCC2, however, the NKCC1 phosphoacceptor residues and surrounding residues are highly conserved in NKCC2 (Thr89, Thr94, Thr99, Thr104 and Thr117 in the rabbit NKCC2 sequence). In response to administration of vasopressin in mice, there was increased phosphorylation of the Thr99 and Thr104 residues [8], although site-directed mutagenesis of each of the Thr99, Thr104 and Thr117 demonstrated that, in contrast to NKCC1, no single residue was critical, rather, all three were required for maximal activity [9]. A second distinction between regulation of NKCC1 and NKCC2 by phosphorylation is the failure of the triple alanine mutation (Thr99, Thr104 and Thr117) to abolish co-transporter activity in NKCC2. There is residual co-transporter activity in NKCC2 when the three phosphorylation sites have been mutated to an alanine residue, suggesting the existence of another regulatory site [9].
We have recently described the distribution of the activated form of AMPK (AMP-activated protein kinase) in the kidney [10]. AMPK is a serine/threonine kinase with a pivotal role in regulation of energy expenditure (reviewed in [11]). Moreover, AMPK has been implicated in the regulation of electrolyte transport by directly modulating the CFTR (cystic fibrosis transmembrane conductance regulator) [12], whereas activation of AMPK has been implicated in the regulation of voltage-gated sodium channels [13], the ENaC (amiloride-sensitive sodium channel) [14,15] and K(ATP) (ATP-sensitive K+ channel) [16]. Furthermore, administration of rosiglitazone, a drug commonly used in the treatment of Type 2 diabetes and associated with an increase in AMPK activity in vitro [17] and in vivo [18,19], leads to sodium retention in rats and is associated with oedema and fluid retention [20,21]. Since the distribution of the activated form of AMPK was similar to that of NKCC2 in the kidney, in the present study we attempted to determine whether AMPK may interact and regulate NKCC2 directly.
EXPERIMENTAL
Materials
[γ-32P]ATP and materials for protein purification were obtained from Amersham Biosciences. Other chemicals were of the highest purity available and obtained from Sigma. ECL (enhanced chemiluminescence) reagents were obtained from Pierce. Cell culture medium was obtained from Invitrogen.
Antibodies
The anti-GFP (green fluorescent protein) monoclonal antibody was obtained from Molecular Probes. The T4 monoclonal antibody directed against NKCC1/2 was obtained from the Developmental Studies Hybridoma Bank. AMPK antibodies against the α1 (373–390) and phosphorylated-Thr172 (165–179) were raised and purified as described previously [22]. Polyclonal antibodies were raised against the C-terminal cytoplasmic domain (810–1096) of NKCC2 and phosphorylated-Ser126 peptide (P121KNRPSpLLEI130). All antibodies were antigen affinity purified and tested for reactivity by ELISA with the immunizing antigen and for specificity by Western blotting. Detection antibodies used in Western blot analysis were obtained from Dako, immunohistochemical secondary antibodies were obtained from Molecular Probes.
Plasmids
Rabbit NKCC2A in the oocyte expression vector Pol1 was obtained from Professor Biff Forbush (Department of Cellular and Molecular Physiology, Yale University, New Haven, Conneticut, U.S.A.). Rat AMPK constructs, as previously described [23], were cloned into the Pol1 oocyte expression vector. Mouse NKCC2N−term (amino acids 1–181) and NKCC2C−term (amino acids 813–1099) were PCR amplified from mouse kidney cDNA and cloned into the pEGFP-C2 vector (Clontech) to generate N-terminally tagged GFP–NKCC2N−term and GFP–NKCC2C−term and confirmed by sequencing.
Site-directed mutagenesis
Substitutions of single amino acids were achieved using PCR-based mutagenesis reactions (QuikChange®, Stratagene) and were confirmed by DNA sequencing.
Immunohistochemistry
Tissue was immersion-fixed in 4% paraformaldehyde (BDH), processed, and embedded in paraffin, as described previously [10]. Briefly, 4-μm-thick paraffin sections were incubated overnight with the T4 mouse monoclonal ascites (diluted 1:1000) and rabbit polyclonal anti-phospho-Thr172 antibody (5 μg/ml) at 4 °C. Rabbit IgG was detected using an anti-rabbit antibody conjugated to Alexa-Fluor® 594 (Molecular Probes), while mouse IgG was detected using an anti-mouse antibody conjugated to Alexa Fluor® 488 (Molecular Probes). Sections were visualized by confocal laser-scanning microscopy (Leica Microsystems, Heidelberg, Germany).
Culture, treatment and lysis of cells
MMDD1 (mouse macula densa-derived 1) cells (supplied by Professor Jurgen Schnermann, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health), HEK (human embryonic kidney)-293 and COS-7 cells were cultivated in Dulbecco's modified Eagle medium containing 10% FCS (foetal calf serum), 100 units/ml of penicillin and 0.1 mg/ml streptomycin. Transfection of HEK-293 and COS-7 cells was routinely performed using Effectene transfection reagent (Qiagen) according to the manufacturer's protocol. Cells were lysed in 25 mM Hepes, 300 mM NaCl, 1.5 mM MgCl2, 200 mM EDTA, 0.5% Triton X-100 for 5 min on ice and then centrifuged at 18000 g for 5 min at 4 °C and the resulting pellets discarded. The protein concentration was determined by the Bradford assay using a commercial protein assay solution (Bio-Rad), and the lysates were used for immunoprecipitation.
Immunoprecipitation and immunoblotting
Immunoprecipitations were performed by incubating 1 mg of cell lysate for 1 h with 2 μg of antibody directed against a specific antigen or unrelated antigen. Protein G-Sepharose was used to immunoprecipitate immune complexes. Samples were separated using SDS/PAGE (10% gels), transferred to PVDF membranes (Immobolin-P, Millipore), and immunoblotted with specific antibodies. Immunoreactive proteins were detected using ECL with Protein A-horseradish peroxidase and the SuperSignal chemiluminescent system (Pierce). If the membrane was to be reprobed with another primary antibody, bound antibody was stripped with Re-blot stripping solution (Chemicon) for 15 min. GST (glutathione S-transferase)-coupled AMPKα1 was affinity purified using glutathione coupled to agarose (Sigma–Aldrich).
AMPK in vitro phosphorylation assay
NKCC2 was immunoprecipitated from MMDD1 cell lysates using the T4 antibody coupled to Protein G-Sepharose. NKCC2N−term (amino acids 1–181) was immunoprecipitated from transfected HEK-293 cell lysates respectively using an anti-GFP antibody coupled to Protein G-Sepharose. The protein immuno-complexes were λ-phosphatase treated and then prepared in kinase assay buffer {50 mM Hepes (pH 7.5), 10 mM MgCl2, 5% glycerol, 1 mM DTT (dithiothrietol), 0.05% Triton X-100, 250 μM [γ-32P]ATP (5000 c.p.m./pmole) and 100 μM 5′-AMP}. The reactions were initiated through the addition of 5 μl of purified rat liver AMPK diluted in 50 mM Tris/HCl (pH 7.5) buffer to the assay mix and incubated at 30 °C for 1 h. The proteins were separated on SDS/PAGE (10% gel). The protein was visualized with Coomassie Blue, dried and phosphorylation was detected using autoradiography.
Identification of phosphorylation sites
In vitro AMPK phoshorylation of GFP–NKCC2N−term was performed as above in the presence of [γ-32P]ATP. Proteins were separated on SDS/PAGE (10% gel) and GFP–NKCC2N−term visualized with Coomassie Blue and autoradiography. The protein band corresponding to GFP–NKCC2N−term was excized and completely destained using 50 mM ammonium bicarbonate containing 50% (v/v) acetonitrile. The destained gel pieces were then dried in a centrifugal freeze-dryer (Savant). Gel slices were then rehydrated with 20 μl of digestion buffer [50 mM ammonium bicarbonate, 10% (v/v) acetonitrile] containing 1 μg of trypsin (Promega) followed by the addition of a further 150 μl of digestion buffer and incubated at 37 °C for 16 h. Peptides were recovered by three sequential 30 min washes of 200 μl of 2% (v/v) TFA (trifluoroacetic acid), 30% acetonitrile; 0.1% TFA and 60% acetonitrile; and 0.1% TFA, using a sonicating waterbath. The combined extracts were partially dried in a centrifugal freeze-dryer. Samples were chromatographed on a reverse-phase C-18 HPLC column equilibrated in 0.1% TFA and eluted using a linear gradient of 0–60% acetonitrile in 0.1% TFA. Fractions were collected and 32P was estimated using the Cherenkov counting method.
Two-dimensional peptide mapping
Phosphopeptides were separated in two-dimensions on thin layer cellulose plates by HVE (high voltage electrophoresis; 1000 V for 30 min at pH 1.9) in the first dimension and ascending chromatography (in butan-1-ol/pyridine/acetic acid/water, 37.5:25:7.5:30) in the second dimension. Phosphopeptides were visualized using phosphorimage analysis.
MS
Fractions corresponding to the major 32P-containing peaks were spotted on to the sample stage with α-cyano-4-hydroxy-cinnamic acid. Masses were analysed using a Bruker MALDI-TOF (matrix assisted laser-desorption ionization–time-of-flight) mass spectrometer.
Phosphate release sequencing
Purified 32P-labelled peptides were covalently linked to Sequelon AA filters (PerkinElmer) according to the manufacturer's protocol followed by solid-phase Edman degradation amino acid sequencing. The release of radioactivity was determined at each cycle of the sequencing by counting using the Cherenkov method.
AMPK activity assay
AMPK activity was measured by the extent to which the sample phosphorylated the AMPK consensus sequence of the ADR-1 (alcohol dehydrogenase regulator 1) peptide (LKKLTLRPSFSAQ-amide), as previously described [24,25]. Briefly, AMPK was immunoprecipitated and incubated at 30 °C with 100 μM ADR-1 peptide in reaction buffer [50 mM Hepes (pH 7.5), 10 mM MgCl2, 5% glycerol, 1 mM DTT, 0.05% Triton X-100] with 250 μM [γ-32P]ATP (500 c.p.m./pmol) for 8 min. After the reaction, 25 μl of reaction mixture was spotted on to the phosphocellulose P81 paper and extensively washed with phosphoric acid. The radioactivity on the filter paper was measured by scintillation counting.
Oocyte expression system
Defolliculated stage IV–VI Xenopus laevis oocytes were obtained as described previously [26]. cRNA was synthesized using T7 RNA polymerase (mMessage-mMachine kit, Ambion) from linearized cDNA templates. About 70–90 ng of RNA/oocyte was injected. Injected oocytes were kept for 3 days in ND-96 [96 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2 and 10 mM Hepes (pH 7.4)], supplemented with penicillin/streptomycin at 17 °C.
Rubidium uptake in Xenopus laevis oocytes
Rubidium uptake assays were used to measure the activity of NKCC2 expressed in Xenopus laevis oocytes in iso-osmotic (basal NKCC2 activity) or hyperosmotic (maximally stimulated NKCC2 activity) conditions. All fluxes were performed in a basic flux solution containing 78 mM Na, 83 mM Cl, 2 mM K, 1 mM Ca, 1 mM Mg, 1 mM SO4, 1 mM HPO4 and 5 mM Hepes (pH 7.4)]. To increase the osmolarity of the flux solution while maintaining ionic strength and ion concentrations constant, sucrose was added. Oocytes were allowed to equilibrate to room temperature (20 °C), transferred to uptake medium containing rubidium and then incubated in either iso-osmotic flux solution or hyperosmotic solution for 1 h. Flux was stopped by washing three times in ice cold 72 mM MgCl2, permeabilized by the addition of 100 μl of methanol, and finally 900 μl of 5 mM CsCl was added. Rubidium was measured by detection at 780 nm using a flame emission spectrophotometer compared with rubidium standard solutions.
Statistics
Statistics were performed using Instat Version 3.05 (GraphPad Software, San Diego, CA, U.S.A.). Data are presented as means±S.D. Unless stated, data were analysed by ANOVA using Bonferroni's test of multiple comparisons. P values <0.05 were considered significant.
RESULTS
Association of AMPK and NKCC2
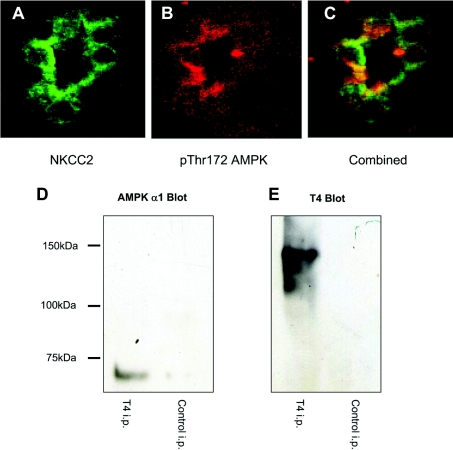
As a prerequisite for establishing a physical interaction between AMPK and NKCC2, both proteins should be co-expressed in the same cells and share overlapping subcellular distributions. We have previously demonstrated activated AMPK on the apical surface of the thick ascending limb [10] placing it in the proximity of the reported distribution of NKCC2 [27]. Two-colour confocal microscopy of immunohistochemical staining for activated AMPK (Figure 1A) and NKCC2 (Figure 1B) demonstrated an overlapping, apical distribution of phospho-Thr172 AMPK and NKCC2 in rat tubules (Figure 1C). To determine whether there was a physical interaction between AMPK and NKCC2, co-precipitation experiments were performed in the presence of 1% Triton X-100. When NKCC2 was immunoprecipitated from mouse kidney or the MMDD1 cell line, AMPK was detected (Figure 1D) suggesting a direct interaction. To determine whether the N- or C-terminal cytoplasmic domains mediated the interaction between AMPK and NKCC2, co-immunoprecipitations were performed on lysates of HEK-293 cells transfected with N- or C-terminal intracellular fragments of NKCC2. The N-terminal (amino acids 1–181) and C-terminal domains (amino acids 813–1099) of NKCC2 were PCR amplified from total mouse kidney cDNA and cloned into the pEGFP vector system (Clonetech). HEK-293 cells were transfected with GFP–NKCC2N−term or GFP–NKCC2C−term fusion protein constructs, with or without the AMPK heterotrimer constructs (α1, β1 and γ1). Lysates were prepared and co-immunoprecipitations performed using antibodies against the GFP-fusion proteins or glutathione agarose to purify the GST-tagged AMPKα1. Western blot analysis was then used to detect co-precipitated proteins. When GFP–NKCC2N−term was co-transfected with AMPK and immunoprecipitated with an anti-GFP antibody, a band corresponding to the α1 subunit of AMPK was detected with an anti-α1 antibody (Figure 2A). In contrast, when GFP–NKCC2C−term was co-transfected with AMPK and immunoprecipitated with an anti-GFP antibody, no AMPK band was detected (Figure 2A). Expression of the N-terminal fragment was greater than the C-terminal fragment in these transfections (Figure 2C). However, immunoprecipitation of these co-transfections with glutathione-Sepharose, which binds to the GST tag on AMPKα1, also demonstrated co-association with the N-terminus but not the C-terminus of NKCC2 (Figure 2D). Expression of AMPK in the cells co-transfected with the N- or C-terminus of NKCC2 was approximately equal (Figure 2B).
Figure 1. AMPK associates with NKCC2.
Laser scanning confocal microscopy was used to co-localize NKCC2 and AMPK in paraformaldehyde-fixed rat kidney. NKCC2 was detected using a T4 monoclonal antibody (A; green fluorescence), with active AMPK detected using an anti-AMPK phospho-Thr172 (B; red fluorescence). Areas of co-localization of AMPK and NKCC2 result in a cumulation of the emission, which appears yellow (C). The original magnification of (A), (B) and (C) is ×630. AMPK co-precipitates with NKCC2. Whole mouse kidney lysates were immunoprecipitated (i.p.) with the T4 mouse monoclonal antibody or an iso-typed matched irrelevant antibody and subjected to SDS/PAGE. The membrane was blotted with the anti-AMPKα1 antibody (D), stripped and re-probed with the T4 antibody (E).
Figure 2. Co-precipitation of cytoplasmic fragments and AMPK.
COS-7 cells transfected with AMPK (GST–α1β1γ1) and GFP–NKCC2N−term or GFP–NKCC2C−Term showed reciprocal association with each other. Transfected cell lysates were either immunoprecipitated using an anti-GFP antibody (A and C) or affinity-purified using glutathione-Sepharose (B and D). The anti-GFP immunoprecipitation was probed for AMPK (A) and the glutathione-Sepharose pulldown blotted for GFP (D). The blots were stripped and re-probed to measure the relative expression levels of (B) GST-AMPK and (C) GFP–NKCC. i.p., immunoprecipitation.
AMPK phosphorylates NKCC2 in vitro
To determine whether NKCC2 could serve as a substrate for AMPK, NKCC2 was immunoprecipitated from MMDD1 cell lysates using a polyclonal antibody raised against the C-terminus of NKCC2. The immunoprecipitates were then used to perform in vitro phosphorylation assays, using purified AMPK and [γ-32P]ATP, as described above. A band of approximately 120 kDa was specifically phosphorylated by purified AMPK (Figure 3A, lane 4). No specific bands were evident in the control lanes, NKCC2 only (lane 1), AMPK only (lane 2) or irrelevant protein immunoprecipitation (lane 3).
Figure 3. In vitro phosphorylation of NKCC2 by AMPK.
NKCC2 was immunoprecipitated from MMDD1 cell lysates before phosphorylation, SDS/PAGE, and autoradiography (A). A band was evident at approximately 120 kDa corresponding to NKCC2 in MMDD1 cells (lane 4, numbering from the left), no band was evident in the absence of exogenous AMPK (lane 1), in the absence of NKCC2 (lane 2) or when an irrelevant isotype-matched antibody was used in the immunoprecipitation (lane 3). GFP–NKCC2N−term was immunoprecipitated from transfected HEK-293 cells before phosphorylation, SDS/PAGE and autoradiography (B). Lane 1, GFP was immunoprecipitated and incubated in the presence of purified AMPK. Lane 2, GFP–NKCC2N−term was immunoprecipitated and incubated in the absence of AMPK. Lane 3 and lane 4, GFP–NKCC2N−term was immunoprecipitated and incubated in the presence of AMPK. Lane 5, recombinant HMG-CoA reductase, an established AMPK substrate, was incubated in the presence of AMPK.
Identification of an AMPK phosphorylation site in NKCC2
To identify the residues that are phosphorylated by AMPK in vitro, GFP-tagged NKCC2 cytoplasmic domains were immunoprecipitated from HEK-293 cells transfected with GFP–NKCC2N−term or GFP–NKCC2C−term, and then used to perform in vitro phosphorylation assays with purified AMPK. GFP–NKCC2N−term was readily phosphorylated in the presence of AMPK (Figure 3B), whereas GFP–NKCC2C−term was not phosphorylated (results not shown). Following tryptic digestion of the phosphorylated GFP–NKCC2N−term two-dimensional phosphopeptide mapping revealed three predominant phosphopeptides (Figure 4A). For the identification of the site(s) phosphorylated on NKCC2N−term by AMPK, the tryptic digests were separated by reverse-phase HPLC and a single predominant peak of radioactivity was detected (Figure 4B). This HPLC fraction was then subjected to solid-phase [32P]Pi Edman degradation, which demonstrated release of radioactivity during cycle five (Figure 4C). Assuming no missed cleavages, trypsin would be expected to generate three peptides from NKCC2N−term with a serine or threonine residue five amino acids distal to the cleavage site. Phosphorylated peptides were conclusively identified with MALDI-TOF spectroscopy by an appropriate mass-shift in reflectron mode (Figure 4D). The molecular mass of three phosphorylated peptides from fraction 26 with measurable levels of 32P incorporation was then matched with regions of the GFP–NKCC2N−term protein. One major peptide was identified in fraction 26 with a molecular mass of 1746 Da corresponding to the calculated mass of the peptide V122NRPSLLEIHEQLAK136 and containing a potential phosphoacceptor serine at position five, Ser126. Moreover, there was a smaller intensity peak of 1826 Da corresponding to the 122–136 peptide with the addition of a single phosphate group. Since Ser126 is the only phosphoacceptor residue in this peptide it suggests that this is the site of phosphorylation by AMPK. Multiple alignment of this site demonstrated a high degree of conservation across species, and the site was also conserved in NKCC1 and the thiazide-sensitive co-transporter (termed NCC) (Figure 4G). It was not present in the other members of the cation chloride co-transporter family, namely the KCC subfamily (Figure 4G).
Figure 4. Identification of sites within NKCC2 that are phosphorylated by AMPK.
(A) GFP–NKCC2N−term was incubated with purified AMPK in the presence of [γ-32P]ATP and subjected to digestion by trypsin, the resulting phosphopeptides were analysed by two-dimensional peptide mapping and autoradiography. Major radiolabelled phosphopeptide spots are indicated by arrows and labelled a, b and c. The direction of electrophoresis and chromatography are indicated on the axes of the autoradiograph. (B) GFP–NKCC2N−term was incubated with purified AMPK in the presence of [γ-32P]ATP and subjected to digestion by trypsin, and the resulting peptides were analysed by reverse-phase HPLC. The dotted line indicates the percentage (v/v) of CH3CN. (C) The radioactive fractions corresponding to peak 1 were subjected to solid-phase Edman sequencing, and the release of radioactivity was determined at each cycle. (D) MALDI-TOF spectrometry was used to analyse peptides contained in peak 1. Wild-type GFP–NKCC2N−term (E) or GFP–NKCC2N−term in which Ser126 had been mutated to alanine (F) were incubated with purified AMPK in the presence of [γ-32P]ATP and subjected to digestion by trypsin, the resulting phosphopeptides were analysed by two-dimensional peptide mapping and autoradiography. Major radiolabelled phosphopeptides obtained from the GFP–NKCC2N−term are indicated by arrows and labelled. This was superimposed on the corresponding GFP–NKCC2N−term S126A mutant phosphopeptide map. The direction of electrophoresis and chromatography are indicated on the axes of the autoradiograph. (G) ClustalW alignment of NKCC2 sequences obtained from different species and other members of the cation chloride co-transporter family. Ser126 (rabbit NKCC2 numbering) is identified and corresponding residues are highlighted.
To confirm Ser126 as a phosphoacceptor residue it was mutated to an alanine residue and incubated with AMPK. Following tryptic digestion, two-dimensional phosphopeptide mapping revealed the presence of a single principal phosphopeptide in the GFP–NKCC2N−term S126A mutant (Figure 4F, labelled c) compared with three major spots in the wild type GFP–NKCC2N−term (Figure 4E) thus confirming the mutation of a phosphoacceptor residue. As there was still one obvious phosphopeptide and other minor spots this suggests a secondary phosphorylation site, however, the radiolabel incorporation was low and did not permit identification of other sites.
Validation of the anti-NKCC2 phospho-Ser126 antibody
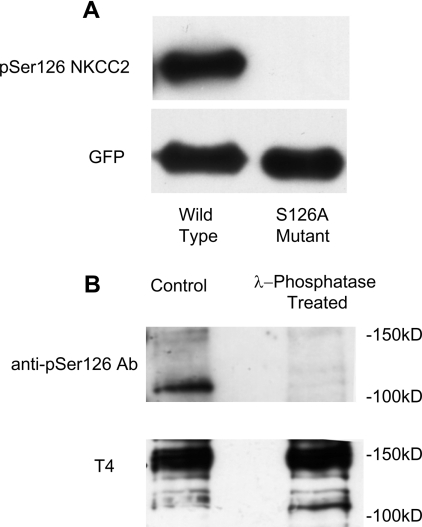
To examine the functional significance of phosphorylation of this site in vivo, a polyclonal rabbit antibody was raised against a phosphorylated peptide corresponding to this region in the mouse NKCC2 sequence (Pro121–Ile130). The specificity of the antibody was demonstrated by transfection of the NKCC2N−term or the mutant NKCC2N−term (S126A) in COS-7 cells. Western blot analysis demonstrated a band where the wild-type but not the mutated site was present (Figure 5A). Moreover, when T4 immunoprecipitations were performed from MMDD1 cell lysates a band was observed corresponding to NKCC2 but not the closely related NKCC1. This was also dependent on phosphorylation as treatment with λ-phosphatase resulted in no immunodetection (Figure 5B).
Figure 5. Validation of anti-phospho-Ser126 antibody.
Sequential immunoblotting with the phospho-Ser126 (pSer126) antibody and the T4 antibody demonstrates specificity of the phospho-antibody for the NKCC2 isoform in its phosphorylated state. (A) GFP–NKCC2N−term or GFP–NKCC2N−termS126A were immunoprecipitated from transfected HEK-293 lysates. λ-Phosphatase treatment was used to de-phosphorylate the protein before in vitro phosphorylation with AMPK. (B) T4 immunoprecipitations from MMDD1 cell lysates were performed.
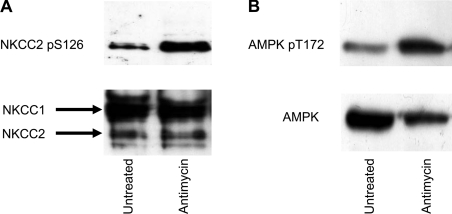
AMPK activation leads to NKCC2 Ser126 phosphorylation
To determine whether activation of AMPK leads to phosphorylation of NKCC2 Ser126 in vivo, MMDD1 cells were treated with the AMPK activator antimycin A. Cell lysates were immunoprecipitated for NKCC2 using the T4 monoclonal antibody and detected by Western blot analysis using the phospho-Ser126 specific antibody (Figure 6A). In cells where AMPK was activated, as demonstrated by AMPK phospho-Thr172 abundance (Figure 6B), there was an increase in phosphorylation of NKCC2.
Figure 6. The effect of AMPK activation on phosphorylation of NKCC2-Ser126.
MMDD1 cells were treated with antimycin A, lysed and immunoprecipitated with either T4 antibody (A) or AMPKα1 antibody (B) and subjected to SDS/PAGE. Membranes were then probed for the level of phosphorylation on NKCC2-Ser126 and AMPK-Thr172 (A and B respectively). Blots were then stripped and probed for total protein.
Functional significance of phosphorylation of NKCC2 Ser126 by AMPK
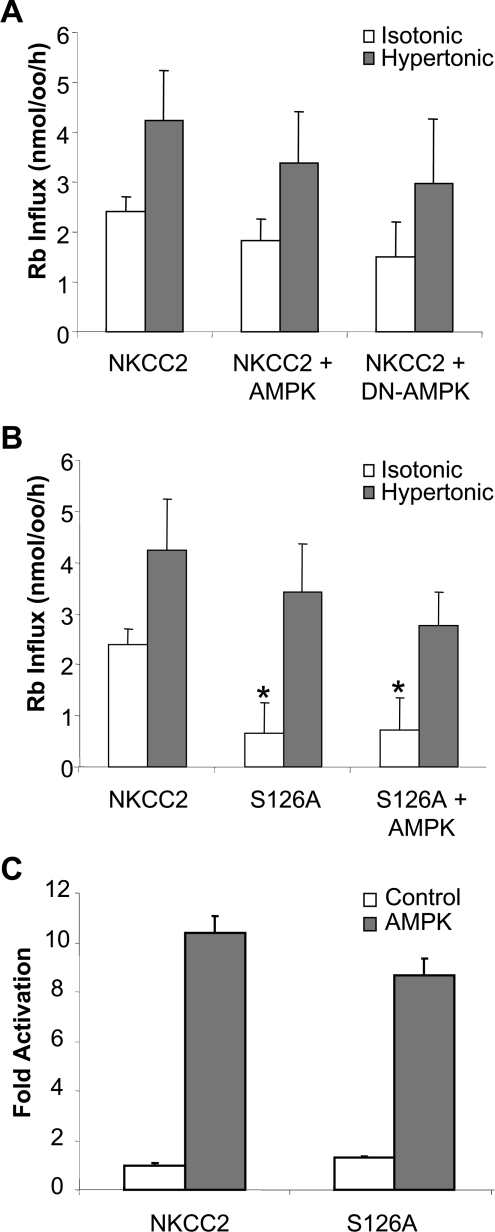
To test the functional effect of AMPK activity upon NKCC2 activation, Xenopus laevis oocytes were injected with NKCC2 cRNA with or without AMPKα1 or a kinase inactive, DN-AMPKα1 (dominant-negative-AMPKα1; K45R). Rubidium influx was measured under both isotonic, to measure the effect on basal NKCC2 activity, and hypertonic conditions, where NKCC2 activity is maximally stimulated (Figure 7A). In the presence of AMPK or the DN-AMPK there was no significant reduction in the level of NKCC2 activity in either isotonic or hypertonic conditions. Due to these unexpected data the level of AMPK activity was determined in these Xenopus laevis oocytes. Coinjection of AMPKα1 with NKCC2 or NKCC2S126A resulted in a 10-fold increase in AMPK activity over endogenous AMPK activity in NKCC2-injected oocytes (Figure 7C).
Figure 7. The effect of AMPK phosphorylation on NKCC2 activity.
(A) Rubidium influx in oocytes injected with wild-type NKCC2 and AMPKα1 or DN-AMPKα1 (n=24). (B) Rubidium influx in oocytes injected with wild-type NKCC2 or NKCC2 in which Ser126 has been mutated to alanine (n=24). (C) AMPK activity is increased in oocytes injected with AMPK (n=8). Protein lysates were made from oocytes co-injected with NKCC2, or NKCC2 in which Ser126 has been mutated to alanine, with or without AMPK. AMPK was immunoprecipitated and an AMPK activity assay was performed. Activity is expressed as the fold increase over un-injected oocytes. Bars show means±S.E.M. *Significantly greater than the NKCC2 alone isotonic stimulation, P<0.01.
To test the functional significance of phosphorylation at Ser126 a point mutation of the site was expressed in the Xenopus laevis oocytes (Figure 7B). Mutation of the Ser126 site resulted in a highly significant 73% reduction (P=0.00012) in rubidium influx under isotonic conditions. This point mutation had no significant effect in hypertonic-stimulated NKCC2 transport. Co-injection of the NKCC2S126A mutant with AMPKα1 or the DN-AMPK resulted in no significant change in co-transporter activity.
DISCUSSION
In the present study we have identified a novel activating phosphorylation site in the N-terminus of the NKCC2 on residue Ser126. This site was phosphorylated under non-stimulated conditions and could account for the high basal level of co-transporter activity seen in NKCC2 compared with NKCC1. The metabolic-sensing kinase, AMPK, was demonstrated to phosphorylate this site in vitro potentially linking cell co-transporter activity with cellular metabolic state, although co-expression of AMPK with NKCC2 in Xenopus laevis oocytes had no significant effect on activity.
Short-term regulation of the cation chloride co-transporter family is a function of their phosphorylation state. This has been well-documented for the secreted NKCC1 where 5–8 phosphorylation sites in the N-terminus are believed to be involved in its regulation [4]. Recent studies have shown that SPAK in conjunction with the WNK [with no K (lysine) protein kinase) 1, 3 and 4 are important for phosphorylation of the five sites so far reported in NKCC1 [5,28–30].
There are no data concerning the possible role of the stress-sensing kinase AMPK in phosphorylation of NKCC1 or NKCC2. We have previously reported [10], however, that AMPK activity in the kidney is increased in rats by variations in salt intake, suggesting a role for AMPK in salt handling. Additionally, activated AMPK detected by immunohistochemical staining was found to localize to the apical surface of the thick ascending limb of the loop of Henle in the kidney, placing it in proximity to the NKCC2 co-transporter that is also located at this site.
In the present study, co-immunoprecipitation experiments demonstrated a direct physical interaction between NKCC2 and AMPK in rat kidney and the cell line MMDD1. Transfection of the intracellular N- and C-terminal fragment demonstrated that the Nterminus of NKCC2 mediated this interaction; the N-termini of both NKCC1 and NKCC2 have previously been shown to be important targets for regulatory phosphorylation [5]. AMPK was found to phosphorylate a new site in vitro, Ser126, which demonstrates some, although not complete, conservation with the described consensus AMPK-recognition sequence [25]. Phosphorylation of Ser126 is in close proximity to the previously identified NKCC2 phosphoacceptor sites, Thr99, Thr104 and Thr117. Mutation of the three known sites has demonstrated that they are important in activating the co-transporter activity of NKCC2 in response to hypertonic conditions, however, they had no effect on reducing the basal activity attributed to NKCC2 [9]. Mutation of Ser126, however, did not affect co-transporter activity in hypertonic conditions. Its effect was seen in isotonic conditions, suggesting that its function is to maintain co-transporter activity at low solute levels. In previous studies three identified phosphorylation sites (Thr99, Thr104 and Thr117) were mutated, NKCC2 retained 50% of its activity [9]. It is interesting that this effect was not seen in NKCC1, which exhibits lower basal co-transport rates, where mutation of the corresponding phosphoacceptor threonines (Thr184, Thr189 and Thr202) abolished co-transporter activity. The site analogous to Ser126 is conserved in NKCC1 as well as the NCC, but it is not clear whether it will be functional in these molecules.
An antibody directed against the phosphorylated Ser126 site demonstrated that the site is phosphorylated in vivo, and can be increased by activation of AMPK. In the studies performed in Xenopus laevis oocytes, however, co-injection of AMPK resulted in a small but insignificant decrease in co-transporter activity. This was not due to kinase activity as co-expression of the kinase-inactive AMPK resulted in a comparable decrease. This discrepancy between the results of site-directed mutagenesis and co-expression with AMPK have several potential explanations. First Xenopus laevis oocytes possess endogenous AMPK [12], which might be responsible for constitutive phosphorylation or, secondly, Ser126 might be the target of another kinase expressed in these cells. Finally, phosphorylation of NKCC2 by AMPK may require another protein to produce a modification of co-transporter activity, possibly involving another phosphorylation event. Recent studies of NKCC1 have identified phosphorylation pathways involving at least two families of kinases, the WNK and the SPAK/OSR-1 (oxidative stress responsive kinase-1). In vivo, regulation of NKCC2 by AMPK may require these or other molecules.
In summary, the results of the present study demonstrate a novel phosphorylation site of the NKCC2 N-terminus that maintains co-transporter activity under isotonic or basal conditions. Moreover, the metabolic-sensing kinase, AMPK, is able to phosphorylate this site in vitro and, possibly, in vivo as activation of AMPK is associated with increased phosphorylation. We have previously identified the presence of activated AMPK in the apical membrane of the thick ascending limb of the loop of Henle and macula densa, where it is co-expressed with NKCC2. Phosphorylation of Ser126 by the AMPK-signalling cascade might contribute to the high basal level of salt re-absorption occurring in these regions under isotonic conditions. Further studies will be required to determine the conditions required for regulation of NKCC2 co-transporter activity in Xenopus laevis as well as the role of this site in other members of the cation co-transporter family, such as NKCC1 and NCC.
Acknowledgments
We wish to acknowledge NHMRC (National Health and Medical Research Council) of Australia and National Heart Foundation grant support to D. A. P. and B. E. K. and Spanish MEC (Ministerio de Educaión y Ciencia) grant (BFU2004–04469) support to I. G. B. E. K. is an ARC Foundation Fellow. S. F. and N. C. were the recipients of a Dora Lush NHMRC Postgraduate Scholarship. V. L. is a NHMRC Health Professional Fellow. I. G. is a ‘Ramón y Cajal’ Researcher.
References
- 1.Yang T., Huang Y. G., Singh I., Schnermann J., Briggs J. P. Localization of bumetanide- and thiazide-sensitive Na-K-Cl cotransporters along the rat nephron. Am. J. Physiol. 1996;271:F931–F939. doi: 10.1152/ajprenal.1996.271.4.F931. [DOI] [PubMed] [Google Scholar]
- 2.Starremans P. G., Kersten F. F., Knoers N. V., van den Heuvel L. P., Bindels R. J. Mutations in the human Na-K-2Cl cotransporter (NKCC2) identified in Bartter syndrome type I consistently result in nonfunctional transporters. J. Am. Soc. Nephrol. 2003;14:1419–1426. doi: 10.1097/01.asn.0000064948.39199.a0. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi N., Chernavvsky D. R., Gomez R. A., Igarashi P., Gitelman H. J., Smithies O. Uncompensated polyuria in a mouse model of Bartter's syndrome. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5434–5439. doi: 10.1073/pnas.090091297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lytle C., Forbush B., 3rd The Na-K-Cl cotransport protein of shark rectal gland. II. Regulation by direct phosphorylation. J. Biol. Chem. 1992;267:25438–25443. [PubMed] [Google Scholar]
- 5.Darman R. B., Forbush B. A regulatory locus of phosphorylation in the N-terminus of the Na-K-Cl cotransporter, NKCC1. J. Biol. Chem. 2002;277:37542–37550. doi: 10.1074/jbc.M206293200. [DOI] [PubMed] [Google Scholar]
- 6.Piechotta K., Lu J., Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J. Biol. Chem. 2002;277:50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 7.Dowd B. F., Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1) J. Biol. Chem. 2003;278:27347–27353. doi: 10.1074/jbc.M301899200. [DOI] [PubMed] [Google Scholar]
- 8.Gimenez I., Forbush B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J. Biol. Chem. 2003;278:26946–26951. doi: 10.1074/jbc.M303435200. [DOI] [PubMed] [Google Scholar]
- 9.Gimenez I., Forbush B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2) Am. J. Physiol. Renal Physiol. 2005;289:F1341–F1345. doi: 10.1152/ajprenal.00214.2005. [DOI] [PubMed] [Google Scholar]
- 10.Fraser S., Mount P., Hill R., Levidiotis V., Katsis F., Stapleton D., Kemp B. E., Power D. A. Regulation of the energy sensor AMP-activated protein kinase in the kidney by dietary salt intake and osmolality. Am. J. Physiol. Renal Physiol. 2005;288:F578–F586. doi: 10.1152/ajprenal.00190.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kemp B. E., Stapleton D., Campbell D. J., Chen Z. P., Murthy S., Walter M., Gupta A., Adams J. J., Katsis F., van Denderen B., et al. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 12.Hallows K. R., Raghuram V., Kemp B. E., Witters L. A., Foskett J. K. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J. Clin. Invest. 2000;105:1711–1721. doi: 10.1172/JCI9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Light P. E., Wallace C. H., Dyck J. R. Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation. 2003;107:1962–1965. doi: 10.1161/01.CIR.0000069269.60167.02. [DOI] [PubMed] [Google Scholar]
- 14.Carattino M. D., Edinger R. S., Grieser H. J., Wise R., Neumann D., Schlattner U., Johnson J. P., Kleyman T. R., Hallows K. R. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J. Biol. Chem. 2005;280:17608–17616. doi: 10.1074/jbc.M501770200. [DOI] [PubMed] [Google Scholar]
- 15.Woollhead A. M., Scott J. W., Hardie D. G., Baines D. L. Phenformin and 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) activation of AMP-activated protein kinase inhibits transepithelial Na+ transport across H441 lung cells. J. Physiol. 2005;566:781–792. doi: 10.1113/jphysiol.2005.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C. Z., Wang Y., Di A., Magnuson M. A., Ye H., Roe M. W., Nelson D. J., Bell G. I., Philipson L. H. 5-amino-imidazole carboxamide riboside acutely potentiates glucose-stimulated insulin secretion from mouse pancreatic islets by KATP channel-dependent and -independent pathways. Biochem. Biophys. Res. Commun. 2005;330:1073–1079. doi: 10.1016/j.bbrc.2005.03.093. [DOI] [PubMed] [Google Scholar]
- 17.Fryer L. G., Parbu-Patel A., Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 18.Ye J. M., Dzamko N., Hoy A. J., Iglesias M. A., Kemp B., Kraegen E. Rosiglitazone treatment enhances acute AMP-activated protein kinase-mediated muscle and adipose tissue glucose uptake in high-fat-fed rats. Diabetes. 2006;55:2797–2804. doi: 10.2337/db05-1315. [DOI] [PubMed] [Google Scholar]
- 19.Bandyopadhyay G. K., Yu J. G., Ofrecio J., Olefsky J. M. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006;55:2277–2285. doi: 10.2337/db06-0062. [DOI] [PubMed] [Google Scholar]
- 20.Song J., Knepper M. A., Hu X., Verbalis J. G., Ecelbarger C. A. Rosiglitazone activates renal sodium- and water-reabsorptive pathways and lowers blood pressure in normal rats. J. Pharmacol. Exp. Ther. 2004;308:426–433. doi: 10.1124/jpet.103.058008. [DOI] [PubMed] [Google Scholar]
- 21.Riazi S., Khan O., Tiwari S., Hu X., Ecelbarger C. A. Rosiglitazone regulates ENaC and Na-K-2Cl cotransporter (NKCC2) abundance in the obese Zucker rat. Am. J. Nephrol. 2006;26:245–257. doi: 10.1159/000093783. [DOI] [PubMed] [Google Scholar]
- 22.Stapleton D., Mitchelhill K. I., Gao G., Widmer J., Michell B. J., Teh T., House C. M., Fernandez C. S., Cox T., Witters L. A., Kemp B. E. Mammalian AMP-activated protein kinase subfamily. J. Biol. Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 23.Dyck J. R., Gao G., Widmer J., Stapleton D., Fernandez C. S., Kemp B. E., Witters L. A. Regulation of 5′-AMP-activated protein kinase activity by the noncatalytic β and γ subunits. J. Biol. Chem. 1996;271:17798–17803. doi: 10.1074/jbc.271.30.17798. [DOI] [PubMed] [Google Scholar]
- 24.Davies S. P., Helps N. R., Cohen P. T., Hardie D. G. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 25.Michell B. J., Stapleton D., Mitchelhill K. I., House C. M., Katsis F., Witters L. A., Kemp B. E. Isoform-specific purification and substrate specificity of the 5′-AMP-activated protein kinase. J. Biol. Chem. 1996;271:28445–28450. doi: 10.1074/jbc.271.45.28445. [DOI] [PubMed] [Google Scholar]
- 26.Gimenez I., Isenring P., Forbush B. Spatially distributed alternative splice variants of the renal Na-K-Cl cotransporter exhibit dramatically different affinities for the transported ions. J. Biol. Chem. 2002;277:8767–8770. doi: 10.1074/jbc.C200021200. [DOI] [PubMed] [Google Scholar]
- 27.Ecelbarger C. A., Terris J., Hoyer J. R., Nielsen S., Wade J. B., Knepper M. A. Localization and regulation of the rat renal Na(+)-K(+)-2Cl cotransporter, BSC-1. Am. J. Physiol. 1996;271:F619–F628. doi: 10.1152/ajprenal.1996.271.3.F619. [DOI] [PubMed] [Google Scholar]
- 28.Gagnon K. B., England R., Delpire E. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol. Cell. Biol. 2006;26:689–698. doi: 10.1128/MCB.26.2.689-698.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahle K. T., Rinehart J., de Los Heros P., Louvi A., Meade P., Vazquez N., Hebert S. C., Gamba G., Gimenez I., Lifton R. P. WNK3 modulates transport of Cl- in and out of cells: implications for control of cell volume and neuronal excitability. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16783–16788. doi: 10.1073/pnas.0508307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriguchi T., Urushiyama S., Hisamoto N., Iemura S., Uchida S., Natsume T., Matsumoto K., Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J. Biol. Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]