Abstract
Derivatives of the rotavirus SA11‐H96 strain, isolated in 1958 from an overtly healthy vervet monkey, have been used extensively to probe the viral life cycle. To gain insight into the phenotypic and genotypic differences among SA11 isolates, we sequenced the segmented double‐stranded RNA genomes of SA11‐H96 (P5B[2]:G3), two SA11‐4F‐like viruses (P6[1]:G3), two SA11‐4F‐like viruses with gene 5 rearrangements, and relevant segments of SA11 temperature‐sensitive mutants and the “O” (Offal) agent (P6[1]:G8), a rotavirus isolated in 1965 from abattoir waste. This analysis indicates that the only complete genomic sequence previously reported for SA11 (Both) is instead that of a reassortant, originating like the SA11‐4F‐like viruses, from the introduction of an “O”‐ agent gene into the SA11 genetic background. These results, combined with identification of mutations that correlate with altered growth properties and ts phenotype, emphasize the importance of considering segment origin and sequence variation in interpreting experimental outcomes with SA11 strains.
Keywords: rotavirus, Reoviridae, double‐strand RNA virus, reassortment, genome sequence
Introduction
Rotaviruses, members of the family Reoviridae, are causative agents of diarrheal diseases in a wide variety of animals. In humans, the group A rotaviruses are associated with endemic diarrhea in children under the age of 5, leading to approximately 600,000 deaths each year (Parashar et al., 2006). The mature rotavirion is an icosahedron consisting of three concentric shells of protein that surround a genome of eleven segments of double‐stranded RNA (Estes, 2001). This genome codes for six structural proteins (VP1‐VP4, VP6, VP7) and, usually, six nonstructural proteins (NSP1‐NSP6). The division of rotavirus into groups (e.g., A to G) is based on the antigenic properties of VP6, the protein forming the intermediate shell of the virion (Kapikian, 2001). The potential for the genetic exchange of genome segments between different rotavirus strains (i.e., reassortment) is restricted to those strains classified within the same group (Ramig, 1997).
Despite evidence suggesting that hundreds of unique strains of group A rotaviruses may exist in the world, the number of strains with fully sequenced genomes is limited, and includes only three human rotaviruses [B4106 (Matthijnssens et al., 2006), Tb‐Chen, KU] and five non‐human rotaviruses [pigeon PO‐13 (Ito et al., 2001), bovine RF and UK, simian SA11‐Both (Mitchell and Both, 1990), lapine‐30/96 (Matthijnssens et al., 2006)]. The bulk of sequences deposited in GenBank for rotaviruses have targeted those genes important for diagnostics (VP6), strain typing, virus neutralization (VP4, VP7), or virulence (NSP4). Unfortunately, with the lack of complete sequence information on rotavirus genomes, we are limited in our capacity to draw conclusions concerning the genetic fitness and selection of genome constellations, the reassortment frequency between co‐circulating strains, and the extent of sequence variation among both strains and groups of rotaviruses.
The rotavirus SA11‐H96 (P5B[2]:G3) strain was isolated in South Africa from the rectum of an overtly healthy vervet monkey in 1958 by Malherbe et al (1963). Early success in the high‐titer amplification of SA11 in cell culture systems supplemented with trypsin has led to the distribution of this virus throughout the world and its widespread application as the prototypic strain for studies on rotavirus growth, virulence, pathogenesis, virion architecture, genome replication, and viral protein structure and function (Estes, 2001). Electrophoretic comparison of the genome segments of the various SA11 strains used by different laboratories has shown that heterogeneity exists in several genome segments, including those encoding VP4 (gene 4), NSP1 (gene 5), and NSP2 (gene 8) or NSP3 (gene 7) (Pereira et al., 1984, 1986). Sequencing has indicated that the fast (F) migrating gene 4 RNA detected in some SA11 strains is similar to the gene 4 RNA of bovine strains NCDV (P6[1]:G6) (Mattion and Estes, 1991) and C486 (P6[1]:G6) (Mitchell and Both, 1989). This has raised the possibility that SA11 was recovered from a monkey co‐infected with a second bovine‐like rotavirus or that at some point following its isolation, the SA11‐H96 strain was contaminated with a bovine—like rotavirus, yielding a collection of reassortants (Kantharidis et al., 1988). For purposes of this communication, those SA11 strains with a bovine‐like gene 4 will be referred to as SA11‐4F‐like viruses, and include SA11‐4F (Pereira et al., 1984; Burns et al., 1989; Mattion and Estes, 1991), SA11‐5N (Patton et al., 2001), and SA11‐30/19 (Patton et al., 2001), SA11‐FEM (Nishikawa et al., 1988), and SA11‐4fM (Lopez and Arias, 1987). In 1965, the Malherbe laboratory recovered the “O” (Offal) agent, a rotavirus of undefined G and P type, from the sewage of a slaughterhouse processing cattle and sheep (Malherbe and Strickland‐Cholmley, 1967). Earlier studies indicated that the “O” agent grew well in cell culture (Kapikian et al., 1976a; 1976b).
To better define the segment origins and the extent of sequence variation among SA11 isolates, we sequenced the genomes of SA11‐H96, two SA11‐4F‐like viruses, and two SA11‐4F‐like viruses with gene 5 rearrangements (SA11‐5S and SA11‐30/1A) (Patton et al., 2001). We also sequenced relevant segments of the “O” agent and of mutant SA11 strains bearing temperature‐sensitive (ts) lesions. Our analysis indicates that the SA11‐4F‐like viruses represent reassortants derived from the introduction of the gene 4 (VP4) RNA of the bovine‐like “O” agent into the genetic background of SA11‐H96. Our analysis reveals that the only previously‐reported complete genome sequence for SA11 [SA11‐Both strain (Mitchell and Both, 1990)] is also that of a reassortant virus, in this case derived from the introduction of the gene 8 (NSP2) RNA of the “O” agent into the SA11‐H96 background. These findings suggest that the heterogeneity noted for the SA11 genome was due to the contamination of the SA11‐H96 strain with the “O” agent, and the subsequent distribution of the contaminated stock to different research laboratories. These results, combined with identification of variable residues in SA11 genome sequences that affect virus growth properties, stress the importance of considering the origin of all eleven genome segments and their sequence variation in interpreting and contrasting experimental outcomes with presumably homologous rotavirus strains used in different laboratories.
Results and Discussion
SA11 reassortment with the O agent
The SA11‐H96 virus was provided by Dr. H. Malherbe to the Laboratory of Infectious Diseases (LID) in 1975. This agent was subsequently passaged 6‐times in bovine kidney cells and 4‐times in African Green monkey kidney cells. In this study, we have sequenced the viral RNA recovered from an aliquot of this material, stored since 1975 at −70C. In addition, we obtained the genome sequence of two SA11‐4F‐like viruses, one (SA11‐30/19) derived from an SA11‐FEM sample received from Dr. G.W. Gary (Centers for Disease Control and Prevention, Atlanta, GA) (Patton et al., 2001), and the other (identified in this study as SA11g4“O”) derived from an SA11‐4F sample received from Dr. R.F. Ramig (Baylor College of Medicine, Houston, TX) (Burns et al., 1989). Complete sequences were obtained for the ORFs of all eleven genome segments of each strain. The extreme termini of some untranslated regions were not determined (UTRs) due to sequence masking brought on by the use of viral‐specific primers to produce and amplify cDNAs from viral RNAs.
The mVISTA (Frazer et al., 2004) visualization module was used to compare the genomes of SA11‐H96, SA11‐30/19, SA11g4“O”, and SA11‐Both (Fig. 1). The results showed that the gene 4 RNA of SA11‐H96 differed substantially from the gene 4 RNA of SA11g4“O” and SA11‐30/19, both in its nucleotide sequence (552 residues) and in the amino‐acid sequence of its VP4 product (130 residues). In contrast, the other ten genes and protein products of SA11g4“O” and SA11‐30/19 differed in total from those of SA11‐H96 by no more than ten nucleotides and five amino acids (Table 1). The gene 4 RNAs of SA11g4“O” and SA11‐30/19 were identical in sequence, and nearly identical to the gene 4 RNA sequences reported previously for other SA11‐4F‐like viruses., e.g., SA11‐4F, SA11‐4fm (nucleotide difference of 1 and 6, respectively) (Fig. 2). Consistent with earlier suggestions (Nishikawa et al., 1988), phylogenetic analysis indicates that the gene 4 RNAs of the SA11‐4F‐like viruses are closely related to the gene 4 RNA of the bovine rotavirus NCDV (P6[1]:G6) (Fig. 2). For example, the SA11g4“O” gene 4 RNA and its VP4 product differ from those of NCDV by only 66 nucleotides (3%) and 10 amino acids (2%). Unlike the gene 4 RNA of the SA11‐4F‐like viruses, the gene 4 RNA of SA11‐H96 is nearly identical to those reported for the SA11 isolates TN‐S1 (Taniguchi et al., 1994), BN‐S4 (Taniguchi et al., 1994), SA11‐Both (Mitchell and Both, 1989), and SA11‐SEM (Nishikawa et al., 1988) (nucleotide difference of 1, 5, 9, 0, respectively), and shows considerably more similarity to the gene 4 RNA of rhesus rotavirus, RRV, than to the gene 4 RNA of bovine viruses (Nishikawa et al., 1988). These findings support a conclusion that the gene 4 RNA of SA11‐4F‐like viruses originated by the reassortment of SA11‐H96 with an unidentified bovine‐like rotavirus.
Figure 1.

mVISTA nucleotide alignment comparing the SA11‐H96 genome with those of other SA11‐derivative strains (A) and other non‐related strains (B). Percentage (right y‐axes) indicates sequence identity, and shading indicates level of conservation. In A, the number of nucleotide changes (amino acid changes) are indicated below each gene. Comparisons of the VP4 and NSP2 genes of the O agent with SA11 strains are also shown. GenBank accession numbers for the genome of SA11‐Both (SA11‐B) (see Table 2); lapine 30/96, DQ205221‐DQ205231; human B4106, AY740731‐AY740741; bovine RF, J04346, X14057, AY116592, U65924, M22308, K02254, Z21639, Z21640, X65940, AY116593, AF188126; bovine UK, X55444, X52589, AY300923, M22306, L11575, X53667, K02170, J02420, X00896, K03384, K03385; human KU, AB022765‐AB022773, AB222784, D16343; human TB‐Chen, AY787644‐AY787654; avian PO‐13, AB009625‐AB009633, D16329, D82979; and group C Bristol, NC007543‐NC007547, 007569‐007574. Accession numbers for gene 5 of SA11 strains (see Table 2).
Table 1.
Amino acid difference among SA11 strains.
| Gene | SA11‐H96 → SA11‐B | SA11‐H96 → SA11g4“O” | SA11‐H96 → SA11‐30/19 | SA11g4“O” → SA11‐30/19 | SA11g4“O” → SA11‐5S | SA11‐30/19 → SA11‐30‐1A | SA11g4“O” → ts mutant | O Agent → SA11 |
|---|---|---|---|---|---|---|---|---|
| VP1 | V* 276 → L | Y* 586 → F | R*680 → T | R* 680 → T | NC | T 680 → R* | tsC | ND |
| ALDQ* 343‐346 → CAGT | F 586 → Y* | L* 138 → P | ||||||
| VP2 | TEG* 219‐221 → PKb | NC | NC | NC | NC | Y* 311 → H | tsF | ND |
| DI* 270‐271 → VY | A* 387 → D | |||||||
| L* 536 → F | ||||||||
| NY* 555‐556 → IN | ||||||||
| VP3 | T* 25 → I | L 650 → H* | D* 83 → N L 650 → H* | D*83 → N | NC | NC | tsB | ND |
| D* 388 → H | G* 526 → D | |||||||
| V* 613 → I | ||||||||
| L 650 → H* | ||||||||
| VP4 | M 72 → T | 130Δ | 130Δ | NC | NC | NC | SA11‐5N: NC | |
| S 157 → P | SA11‐H96: 130Δ | |||||||
| G 187 → A | ||||||||
| S 332 → Y | ||||||||
| M 366 → V | ||||||||
| NSP1 | A 36 → E | NC | A 36 → E | A 36 → E | Rea: CTa Δ478‐496 | Rea: CTa Δ409‐496 | SA11g4“O”:314Δ | |
| NHYAKK 204‐209 → TIMQEb | ||||||||
| VP6 | F* 164 → Y | NC | NC | NC | V*385 → I | NC | tsG | ND |
| V* 385 → I | T* 10 → S | |||||||
| D* 13 → H | ||||||||
| A* 121 → G | ||||||||
| NSP3 | L* 63 → P | NC | NC | NC | V*23 → L | NC | ND | |
| A* 94 → P | ||||||||
| S* 214 → P | ||||||||
| NSP2 | 45Δ | NC | NC | NC | NC | NC | tsEd V* 200 → I | SA11‐B:NC SA11‐H96: 45Δ |
| A* 152 → V | ||||||||
| VP7 | F* 32 → C | T 65 → Ic | T 65 → Ic | Q*294 → Kc | NC | K 294 → Q* | ND | |
| F* 37 → L | Q* 294 → K | |||||||
| NSP4 | NC | NC | NC | NC | NC | NC | ND | |
| NSP5 | NC | NC | NC | NC | NC | NC | ND | |
| NSP6 | NC | NC | NC | NC | NC | NC | ND |
Residue typically found at the position among all rotavirus strains.
NC, no change; ND, not determined; Re, gene rearrangement; CT, carboxy truncation; Δ, total amino acid difference.
Includes a one amino acid deletion.
Nucleotide 194 of SA11‐H96 varies between C and T, resulting in amino acid 65 varying between I and T.
Figure 2.

Maximum parsimony trees generated from amino acid sequences of rotavirus VP4 (A), NSP2 (B), NSP1 (C), and VP7 (D). Numbers indicate branch lengths. GenBank accession numbers for VP4: Porcine (DQ061053), BRV‐UK (M22306), BRV‐NCDV (AB119636), BRV‐Sun9 (AB158430), BRV‐WC3 (AY050271), HRV‐Wa (L34161), HRV‐KU (AB222786), Lapine (U62150), SA11‐SEM (M23188), SA11 clone TNS1 (D16346), SA11 clone BNS4 (D16345), SA11‐4fm (Y00336), SA11‐4F (X57319), Lamb (L11599), SA11‐Both (X14204); NSP2: Porcine (X06722), BRV‐UK (L11599), BRV‐RF (Z21640), BRV‐NCDV (L04530), HRV‐Wa (L04534), HRV‐KU (AB022770), Lapine (DQ205227), SA11‐Both (J02353); NSP1: Lapine‐ALA (AF084549), Lapine‐C‐11 (AF130366), feline FRV64 (D78362), canine‐K9 (AF111946), Equine‐H2 (D38157), HRV‐MP409 (AF141916), BRV‐B641 (AF458087), Lapine‐30/96 (DQ205225), HRV‐B4106 (AY740735), SA11‐Both (X14914), RRV (Z32535); VP7: Canine‐RV52/96 (AF271090), HRV‐CMH222 (AY707792), Goat‐GRV (AB056650), Llama G8[P14] (AF545860), BRV‐Tokushima9503 (AB044293), BRV‐KAG87 (AB077054), BRV‐calf678 (L20877), BRV‐KAG75 (AB077054), Avian‐PO‐13 (D82979), Lapine‐30/96 (DQ205229), RRV (AF295303), HRV‐B4106 (AY740735), HRV‐Tb‐Chen (AY787646), HRV‐KU (D16343), BRV‐Rf (X65940), BRV‐UK (X00896), SA11‐Both (V01190).
In an attempt to identify the origin of the gene 4 RNA in SA11‐4F‐like viruses, we sequenced selected genes of the “O” agent, a rotavirus provided to this laboratory by Dr. Malherbe in 1975. Remarkably, the sequence of the “O”‐agent gene 4 RNA was identical to the gene 4 RNAs of SA11g4“O” and SA11‐30/19 (Fig. 1). Thus, the SA11‐4F‐like viruses most likely represent reassortant viruses originating from cross contamination of an SA11‐H96 preparation with the “O” agent. At least three of the SA11‐4F‐like viruses (SA11‐4F, SA11‐4fm, SA11‐FEM) have been defined as P‐type P6[1] (Ciarlet et al., 1998; Estes, 2001). Given the near or complete identity of the VP4 sequences of the SA11‐4F‐like viruses with the VP4 sequence of the “O” agent (Fig. 2), it follows that the P‐type of the O agent is P6[1].
Based on comparison of their gene 9 (VP7) sequences, the closest relative of the “O” agent gene 9 (VP7) RNA is a G8 bovine rotavirus isolated in Japan, differing by 45 nucleotides and 2 amino acids at the RNA and protein level, respectively (Okada and Matsumoto, 2002). In addition, based on its VP7 sequence, the “O” agent was also found to cluster with a number of other G8 rotaviruses (Fig. 2). Collectively, our analysis of the gene 4 and 9 sequences suggests that the “O” agent represents a P6[1]:G8 bovine rotavirus, consistent with its isolation from the sewage of a cattle‐sheep slaughterhouse.
mVISTA analysis also showed that the gene 8 (NSP2) RNA of SA11‐H96 differed significantly from that of the SA11‐Both strain both in nucleotide (203 residues) and amino acid (45 residues) sequence (Fig. 1). Although this indicates a fairly extensive number of amino acid changes between the NSP2 octamer of the SA11‐H96 and SA11‐Both strains, these changes generally represent charge‐neutral substitutions (shown in blue, Fig. 3) located outside the grooves of the octamer (Jayaram et al., 2002). Thus, the changes should not influence the ability of NSP2 to interact with NSP5 and RNA, since these activities are mediated by the grooves of NSP2 (Jiang et al., 2006).
Figure 3.
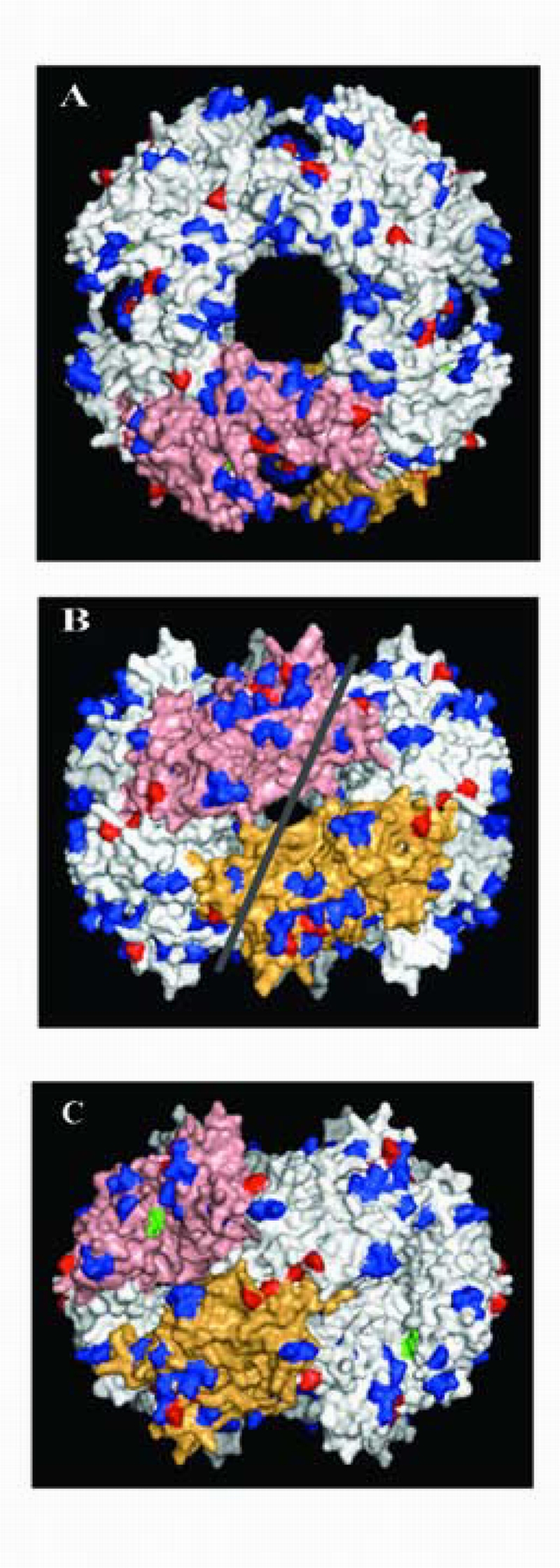
Comparison of surface exposed residues on the NSP2 octamers of SA11‐H96 and SA11‐Both. Top (A) and side (B and C) views of the octamer (4‐2‐2), with two monomers labeled (salmon and tan). The side views represent 90° rotations of each other. Amino acid differences not affecting charge (charge neutral) are shown in blue. Differences altering surface charge are shown in red. Residue K188 contained in the catalytic cleft of each NSP2 monomer is shown in green. The RNA‐binding groove is approximated with a white line.
Sequencing revealed that the “O”‐agent gene 8 RNA was identical to the gene 8 RNA of SA11‐Both. Thus, like the SA11‐4F‐like viruses, the SA11‐Both virus can be concluded to represent a reassortant virus, in this case originating from the introduction of the “O”‐agent gene 8 RNA into the SA11‐H96 genetic background. These results indicate that the only complete genome sequence purported for the SA11 virus (i.e., SA11‐Both) is actually that of a reassortant virus, and most likely does not represent the gene constellation or the sequence of the SA11 viruses recovered in 1958. Although the other ten genes of SA11‐Both differed minimally in total from those of SA11‐H96 (46 nucleotides, 35 amino acids), this difference was greater than the difference observed between the common background genes of SA11g4“O” or SA11‐30/19, and SA11‐H96 (7–10 nucleotides, 3–6 amino acids) (Table 1).
The discovery of “O”‐agent genes in the SA11‐Both and the SA11‐4F‐like viruses suggests that the size heterogeneity noted for other SA11 genes may likewise be due to reassortment events involving the “O” agent (Pereira et al., 1984; 1986). Whether this is the case awaits sequencing of SA11 strains not in our possession. It is important to note that the strains identified as SA11‐4F‐like viruses were assigned to this group only on the basis of the gene 4 characteristics. We cannot rule out the possibility that some of the SA11‐4F‐like viruses may have other genes (e.g., gene 8) originating from the “O” agent.
Gene 5 RNA of the SA11 strains
Comparison of the gene 5 (NSP1) sequences of the various SA11 strains reveals near perfect identity. However, the mVISTA comparison of the SA11 gene 5 sequences to those of other fully‐sequenced strains of rotaviruses recovered from a variety of animal species (human, bovine, lapine, avian) show a degree of nucleotide difference that is considerably greater than seen in similar comparisons with any other gene (Fig. 1B). One possible explanation for this is that the gene 5 RNA did not co‐evolve with the other genes of the SA11 strains, but instead originated by reassortment of a progenitor SA11 virus with a different virus. Phylogenic analysis indicates that the SA11‐H96 NSP1 protein is the single representative in a branch only distantly related to any other NSP1, including RRV and TUCH (Sestak et al., 2004), viruses recovered from rhesus monkeys (Fig. 2). Thus, there is little guidance concerning close viral relatives that might have been the source of the gene 5 RNA in the SA11 strains. However, based on sequence analysis, the “O” agent was not the source of the SA11 gene 5 RNA (Fig. 2). Instead, the NSP1 product of the “O” agent gene 5 RNA is more closely related to an Indian G8 human strain (MP409) of rotavirus than to SA11 NSP1 (Jagannath et al., 2000).
Determinants affecting growth
Earlier studies have noted differences in the growth properties of the SA11‐4F‐like viruses. For example, although the SA11‐5N and SA11‐30/19 strains grow to similar titers on MA104 cells, SA11‐5N produces a larger plaque (Patton et al., 2001). More recently, we determined that SA11g4“O” grows to a titer on CaCo2 and FRhL2 cells approximately one‐log greater than SA11‐30/19, and that SA11g4“O”, like SA11‐5N, produces a larger plaque on MA104 cells than does SA11‐30/19 (data not shown). Sequencing suggests that one to four amino acid changes (VP1 R680→T, F586→Y, VP3 D83→N, VP7 Q294→K) account for the differences in the growth phenotypes of the SA11g4“O” and SA11‐30/19 strains (Table 1). A previous study has pointed to the importance of NSP1 in promoting the cell‐to‐cell spread of rotavirus, and thus plaque‐size, through its subversion of interferon signaling pathways (Barro and Patton, 2005). However, because the NSP1 sequence of SA11g4“O” and SA11‐30/19 are identical, it cannot be this gene product that is responsible for the different phenotypes observed for these two strains.
A number of rotavirus variants have been isolated with gene 5 sequence rearrangements that alter the NSP1 ORF, such that the variants encode C‐truncated NSP1 species defective in the capacity to subvert interferon signaling (Barro and Patton, 2005; Graff et al., 2002; Patton et al., 2001). Characteristically, these variants have a smaller plaque phenotype in comparison to viruses encoding full‐length NSP1. Two such small‐plaque variants, SA11‐5S and SA11‐30/1A, were isolated from preparations of SA11‐4F‐like viruses (SA11‐4F (Periera et al., 1984) and SA11‐FEM (Nishikawa et al., 1988), respectively) that had undergone extensive serial passage (>26‐rounds) (Patton et al., 2001). In addition to SA11‐5S and SA11‐30/1A, sister viruses with non‐rearranged gene 5 segments were recovered from the high passage preparations (SA11‐5N and SA11‐30/19, respectively) (Patton et al., 2001). Comparison of the genome sequence of SA11g4“O” to that of SA11‐5S revealed two conservative amino acid substitutions, one in VP6 (V385→I) and the other in NSP3 (V23→L), in addition to the 18‐amino acid truncation of NSP1. Comparison of the genome sequence of SA11‐30/19 to that of SA11‐30/1A instead revealed three amino acid substitutions involving two different proteins (VP1 T680→R, Y311→H; VP7 K294→Q), in addition to the 87‐amino acid truncation of NSP1. This information indicates that the only single change or gene product that can be correlated with the small plaque phenotype of the SA11 variants is the NSP1 truncation, a finding that is consistent with an earlier report indicating that NSP1 can contribute to the cell‐to‐cell spread of rotavirus, and thus plaque size (Barro and Patton, 2005).
Temperature‐sensitive mutants
Collections of ts mutants have been described for several rotaviruses including SA11, UK, and RRV (Ramig, 1997). The collection generated for the SA11 strain consists of mutants that identify 10 of the 11 expected reassortment groups of the virus (Ramig and Petrie, 1984). The ts lesions of six of the SA11 collection have been mapped to specific genome segments: tsA, gene 4 (VP4); tsB, gene 3 (VP3); tsC, gene 1 (VP1); tsE, gene 8 (NSP2); tsF, gene 2 (VP2); tsG, gene 6 (VP6) (Gombold et al., 1985; Gombold and Ramig, 1987). Previous sequence analysis has identified one or two residue changes in VP2 (Mansell et al., 1994), VP6 (Mansell et al., 1994), and NSP2 (Taraporewala et al., 2002) suspected of causing the ts phenotype of tsF, tsG, and tsE, respectively (Fig. 4). The mutations introduced into tsF VP2 (A387→D) and tsG VP6 (T10→S and D13→H) were correlated with defects in the assembly of these inner capsid proteins into core and double‐layered virus particles (Mansell et al., 1994). Without an atomic structure for VP2, it is difficult to predict how the A387→D mutation affects capsid assembly. In contrast, structural analysis indicates that the ts mutations in tsG VP6 are contained in one of eight alpha‐helixes that collectively form a VP2‐interacting bundle at the base of VP6 (Fig. 4) (Charpilienne et al., 2002; Mathieu et al., 2001). The mutation in tsE NSP2 is positioned in a suspected hinge region between the N and C‐terminal domains of the protein (Fig. 4), and is correlated with the failure of the protein to support the formation of viral inclusion bodies (Ramig and Petrie, 1984).
Figure 4.

Temperature‐sensitive (ts) lesions in SA11 proteins. Atomic structures identifying mutation sites in the VP6 trimer (Thr10 and Asp13) (A) and the NSP2 monomer (Ala152) (B). C: Amino acid alignments of residues surrounding ts lesions. Genbank accession used in alignments that are not mentioned elsewhere are AF044358 and AF106319 (VP1), AY277916 and AY267335 (VP3), AF317127 and D00325 (VP6), and L04529 (NSP2). PDB Bank Numbers: VP6 (1QHD) and NSP2 (1L9Z).
By comparing the sequences of the gene 1 and 3 RNAs of tsC and tsB, respectively, with their SA11‐H96 counterparts, we have identified single amino acid mutations in the viral RNA polymerase VP1 and RNA‐capping enzyme VP3 that can be correlated with their ts phenotype (Fig. 4). In the case of tsC VP1, the amino acid change is of a residue (L138→P) conserved among the RNA polymerases of all group A rotaviruses. The effect of this mutation on the RNA‐polymerase function of VP1 at the restrictive temperature (39°C) is not known. However, given that L138 is positioned well upstream of the thumb‐palm‐finger domain of the polymerase (Patton et al., 2006), the mutation is probably not directly linked to its catalytic activity, but more likely tied to protein‐protein or protein‐RNA interactions necessary for forming functional complexes. The suspected ts lesion in tsB VP3 is the Gly→Asp mutation at residue 527. This glycine residue is conserved among both group A and C viruses, and is positioned immediately upstream of one of the few completely conserved lysines (L541) of the protein. During RNA capping, a lysine residue serves as the acceptor and donor of the GMP moiety transferred by the guanylyltransferase to the 5’‐end of the RNA (Cook and McCrae, 2004). Thus, the juxtapositioning of the G527→D ts lesion near what may be the catalytic lysine of VP3, could prevent the protein from appropriately functioning at the restrictive temperature, thereby leading to the ts phenotype. However, studies so far have indicated that the ts lesion does not undermine the capping activity of VP3, but instead promotes the formation of empty particles (Vasquez et al., 1993). Thus, VP3 may have a critical role in linking the processes of RNA packaging and capsid assembly.
Summary
Our study shows that derivative strains of the original SA11‐H96 isolate can vary markedly in their genetic make‐up, due to reassortment with the “O” agent, a P6[1]:G8 bovine rotavirus, and to point mutations. Both types of variations are associated with phenotype differences that impact virus growth. Based on our findings, the analysis of other widely used SA11 strains is warranted to assure the correct identification of all their genome segments (e.g., SA11 versus “O” agent). Without such information, conclusions reached on the behavior of one SA11 strain may be inappropriately generalized to the behavior of other SA11 strains. As revealed by this study, comparison of the complete genomes of rotavirus strains can contribute valuable information concerning the origins and relationships of their genes. Such information may have broad application to our understanding of the evolution of newly emerging strains of human rotaviruses.
Materials and Methods
Rotavirus strains
The SA11‐4F and SA11 ts‐mutant strains were kindly provided by Dr. R.F. Ramig, and the SA11‐30‐19, SA11‐5S, and SA11‐30‐1A strains were obtained as described elsewhere (Patton et al., 2001). Rotaviruses were propagated in MA104 cells, following standard protocols (Gray and Desselberger, 2000). SA11‐H96 (SVA178) and “O” (Offal) agent (SVA179) were provided to the LID in person by Dr. H.H. Malherbe in 1975, then with the Southwest Foundation for Research and Education (San Antonio, TX). After receiving the SA11‐H96 strain, the virus was passed 6‐times in bovine kidney cells and 4‐times in African green monkey kidney cells, then frozen. After receiving the “O” agent, the virus was passed 6‐times in bovine kidney cells and 10‐times in AGMK cells, then frozen. The rhesus TUCH rotavirus (Sestak et al., 2004) was provided by Dr. Karol Sestak (Tulane University School of Medicine).
Sequencing
Genomic dsRNAs were purified from rotavirus by Trizol LS (Invitrogen) extraction and ethanol precipitation. The SA11‐H96 and “O” agent samples had been stored at −70°C since 1975 and 1979, respectively. The extracted RNAs were denatured by adjusting to a final concentration of 50% dimethylsulfoxide followed by incubation for 10 min at 94°C. Viral RNA cDNAs were prepared by reverse transcriptase (RT)‐polymerase chain reaction (PCR) using Superscript II RT Platinum Taq Mix (Invitrogen). The initial RT step was carried out for 10 min at 42°C, then for 1 h at 50°C. The subsequent PCR step was carried out for 2 min at 94°C, followed by 40 cycles of amplification (30 s at 94°C, 30 s at 37°C, 5 min at 68°C). PCR products were gel purified with a QIAquick Gel Extraction Kit (Qiagen), and sequenced with an ABI Prism BigDye v.3.1 terminator cycle sequencing kit (Applied Biosystems Group). The 5’‐terminal sequence of gene 4 RNAs was determined using an Invitrogen 5’‐RACE system kit, version 2 (Patton et al., 2001). Viral‐specific primers used to generate and sequence cDNAs prepared by RT‐PCR and RACE are listed in Supplemental Table 1. Sequences were obtained with an ABI Prism 310 Genetic Analyzer. Rotavirus gene sequences were deposited in GenBank (Table 2).
Table 2.
GenBank accession numbers.
| Gene | SA11‐Both | SA11‐H96 | SA11g4“O” | SA11‐5S | SA11‐30/19 | SA11‐30/1A | O Agent | ts mutants | TUCH |
|---|---|---|---|---|---|---|---|---|---|
| VP1 | X16830 | *DQ838640 | *DQ838636 | *DQ838637 | *DQ838638 | *DQ838639 | ‐ | *DQ838601 (tsC) | ‐ |
| VP2 | X16831 | *DQ838635 | *DQ838631 | *DQ838632 | *DQ838633 | *DQ838634 | ‐ | AAA47349 (tsF) | ‐ |
| VP3 | X16387 | *DQ838645 | *DQ838641 | *DQ838642 | *DQ838643 | *DQ838644 | ‐ | *DQ838600 (tsB) | ‐ |
| VP4 | X14204 | *DQ841262 | *DQ838602 | *DQ838603 | *DQ838604 | *DQ838605 | *DQ838596 | ‐ | ‐ |
| NSP1 | X14914 | *DQ838599 | AF290881a | AF290884 | AF290881a | AF290882 | *DQ838595 | ‐ | *DQ838651 |
| VP6 | AY187029 | *DQ838650 | *DQ838646 | *DQ838647 | *DQ838648 | *DQ838649 | ‐ | AAA65638 (tsG) | ‐ |
| NSP3 | X00355 | *DQ838610 | *DQ838606 | *DQ838607 | *DQ838608 | *DQ838609 | ‐ | ‐ | ‐ |
| NSP2 | J02353 | *DQ838615 | *DQ838611 | *DQ838612 | *DQ838613 | *DQ838614 | *DQ838597 | AAA47303 (tsE) | ‐ |
| VP7 | V01190 | *DQ838620 | *DQ838616 | *DQ838617 | *DQ838618 | *DQ838619 | *DQ838596 | ‐ | ‐ |
| NSP4 | K01138 | *DQ838625 | *DQ838621 | *DQ838622 | *DQ838623 | *DQ838624 | ‐ | ‐ | ‐ |
| NSP5/6 | X07831 | *DQ838630 | *DQ838626 | *DQ838627 | *DQ838628 | *DQ838629 | ‐ | ‐ | ‐ |
Sequences determined by this study.
Previously reported sequence confirmed in this study (Patton et al., 2001).
Sequence and protein structure analysis
Sequencing files were analyzed using Sequencher 4.5 (Gene Codes Corporation) and MacVector 8.0 (Accelrys). Multiple sequence alignments were prepared using either ClustalW (EMBL‐EBI) or mVista ( http://genome.lbl.gov/vista/index.shtml) (Frazer et al., 2004). Searches for similar sequences were conducted using BLAST. Protein structures were generated using MacPyMol (Delano Scientific LLC). Phylogenetic and evolutionary analysis was performed using Paup* 4.0 (Sinauer Associates, Inc.) Unrooted, maximum parsimony trees were created, and bootstrap analysis was performed for VP4, NSP2, NSP1, and VP7.
Acknowledgements
We thank Jennifer DiMuro and Rodrigo Vasquez del Carpio for their help on this project. This research was supported by the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
References
- Barro M, Patton JT. Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3. Proc Natl Acad Sci U S A. 2005;102:4114–4119. doi: 10.1073/pnas.0408376102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brottier P, Nandi P, Bremont M, Cohen J. Bovine rotavirus segment 5 protein expressed in the baculovirus system interacts with zinc and RNA. J Gen Virol. 1992;73:1931–1938. doi: 10.1099/0022-1317-73-8-1931. [DOI] [PubMed] [Google Scholar]
- Burns JW, Chen D, Estes MK, Ramig RF. Biological and immunological characterization of a simian rotavirus SA11 variant with an altered genome segment 4. Virology. 1989;169:427–435. doi: 10.1016/0042-6822(89)90168-2. [DOI] [PubMed] [Google Scholar]
- Charpilienne A, Lepault J, Rey F, Cohen J. Identification of rotavirus VP6 residues located at the interface with VP2 that are essential for capsid assembly and transcriptase activity. J Virol. 2002;76:7822–7831. doi: 10.1128/JVI.76.15.7822-7831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarlet M, Estes MK, Barone C, Ramig RF, Conner ME. Analysis of host range restriction determinants in the rabbit model: Comparison of homologous and heterologous rotavirus infections. J Virol. 1998;72:2341–2351. doi: 10.1128/jvi.72.3.2341-2351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JP, McCrae MA. Sequence analysis of the guanylyltransferase (VP3) of group A rotaviruses. J Gen Virol. 2004;85:929–932. doi: 10.1099/vir.0.19629-0. [DOI] [PubMed] [Google Scholar]
- Estes MK. In: Rotaviruses and their replication. 4th edition. Knipe D, Howlet M, et al., editors. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 1747–1785. [Google Scholar]
- Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombold JL, Estes MK, Ramig RF. Assignment of simian rotavirus SA11 temperature‐sensitive mutant groups B and E to genome segments. Virology. 1985;143:309–320. doi: 10.1016/0042-6822(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Gombold JL, Ramig RF. Assignment of simian rotavirus SA11 temperature‐sensitive mutant groups A, C, F, and G to genome segments. Virology. 1987;161:463–473. doi: 10.1016/0042-6822(87)90140-1. [DOI] [PubMed] [Google Scholar]
- Graff JW, Mitzel DN, Weisend CM, Flenniken ML, Hardy ME. Interferon regulatory factor 3 is a cellular partner of rotavirus NSP1. J Virol. 2002;76:9545–9550. doi: 10.1128/JVI.76.18.9545-9550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Desselberger U. Methods in Molecular Medicine. Vol. 34. Totowa, NJ: Humana Press; 2000. Rotaviruses: Methods and Protocols. [Google Scholar]
- Ito H, Sugiyama M, Masubuchi K, Mori Y, Minamoto N. Complete nucleotide sequence of a group A avian rotavirus genome and a comparison with its counterparts of mammalian rotaviruses. Virus Res. 2001;75:123–138. doi: 10.1016/s0168-1702(01)00234-9. [DOI] [PubMed] [Google Scholar]
- Jagannath MR, Vethanayagam RR, Reddy BS, Raman BS, Rao CD. Characterization of human symptomatic rotavirus isolates MP409 and MP480 having ‘long’ RNA electropherotype and subgroup I specificity, highly related to the P6[1],G8 type bovine rotavirus A5, from Mysore, India. Arch Virol. 2000;145:1339–1357. doi: 10.1007/s007050070094. [DOI] [PubMed] [Google Scholar]
- Jiang X, Jayaram H, Ludtke S, Estes M, Prasad BV. Cryo‐EM structures of rotavirus NSP2‐NSP5 and NSP2‐RNA complexes: Implications for genome replication. J Virol. 2006 doi: 10.1128/JVI.01347-06. (in press; Aug 24, Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram H, Taraporewala Z, Patton JT, Prasad BV. Rotavirus protein involved in genome replication and packaging exhibits a HIT‐like fold. Nature. 2002;417:311–315. doi: 10.1038/417311a. [DOI] [PubMed] [Google Scholar]
- Kantharidis P, Dyall‐Smith ML, Tregear GW, Holmes IH. Nucleotide sequence of UK bovine rotavirus segment 4: possible host restriction of VP3 genes. Virology. 1988;166:308–315. doi: 10.1016/0042-6822(88)90501-6. [DOI] [PubMed] [Google Scholar]
- Kapikian AZ, Cline WL, Kim HW, Kalica AR, Wyatt RG, Vankirk DH, Chanock RM, James HD, Jr, Vaughn AL. Antigenic relationships among five reovirus‐like (RVL) agents by complement fixation (CF) and development of new substitute CF antigens for the human RVL agent of infantile gastroenteritis. Proc Soc Exp Biol Med. 1976a;152:535–539. doi: 10.3181/00379727-152-39434. [DOI] [PubMed] [Google Scholar]
- Kapikian AZ, Hoshino Y, Chanock RM. Rotaviruses. In: Knipe D, Howlet M, et al., editors. Fields Virology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 1787–1833. [Google Scholar]
- Kapikian AZ, Kim HW, Wyatt RG, Cline WL, Parrott RH, Chanock RM, Arrobio JO, Brandt CD, Rodriguez WJ, Kalica AR, Van Kirk DH. Recent advances in the etiology of viral gastroenteritis. Ciba Found Symp. 1976b;(42):273–309. doi: 10.1002/9780470720240.ch16. [DOI] [PubMed] [Google Scholar]
- Lopez S, Arias CF. The nucleotide sequence of the 5′ and 3′ ends of rotavirus SA11 gene 4. Nucleic Acids Res. 1987;15:4691. doi: 10.1093/nar/15.11.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe H, Harwin R, Ulrich M. The cytopathic effects of vervet monkey viruses. S Afr Med J. 1963;37:407–411. [PubMed] [Google Scholar]
- Malherbe HH, Strickland‐Cholmley M. Simian virus SA11 and the related O agent. Arch Gesamte Virusforsch. 1967;22:235–245. doi: 10.1007/BF01240518. [DOI] [PubMed] [Google Scholar]
- Mansell EA, Ramig RF, Patton JT. Temperature‐sensitive lesions in the capsid proteins of the rotavirus mutants tsF and tsG that affect virion assembly. Virology. 1994;204:69–81. doi: 10.1006/viro.1994.1511. [DOI] [PubMed] [Google Scholar]
- Mathieu M, Petitpas I, Navaza J, Lepault J, Kohli E, Pothier P, Prasad BV, Cohen J, Rey FA. Atomic structure of the major capsid protein of rotavirus: implications for the architecture of the virion. EMBO J. 2001;20:1485–1497. doi: 10.1093/emboj/20.7.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Rahman M, Martella V, Xuelei Y, De Vos S, De Leener K, De Lenner K, Ciarlet M, Buonavoglia C, Van Ranst M. Full genomic analysis of human rotavirus strain B4106 and lapine rotavirus strain 30/96 provides evidence for interspecies transmission. J Virol. 2006;80:3801–3810. doi: 10.1128/JVI.80.8.3801-3810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattion NM, Estes MK. Sequence of a rotavirus gene 4 associated with unique biologic properties. Arch Virol. 1991;120:109–113. doi: 10.1007/BF01310953. [DOI] [PubMed] [Google Scholar]
- Mitchell DB, Both GW. Complete nucleotide sequence of the simian rotavirus SA11 VP4 gene. Nucleic Acids Res. 1989;17:2122. doi: 10.1093/nar/17.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DB, Both GW. Completion of the genomic sequence of the simian rotavirus SA11: nucleotide sequences of segments 1, 2, and 3. Virology. 1990;177:324–331. doi: 10.1016/0042-6822(90)90487-c. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Taniguchi K, Torres A, Hoshino Y, Green K, Kapikian AZ, Chanock RM, Gorziglia M. Comparative analysis of the VP3 gene of divergent strains of the rotaviruses simian SA11 and bovine Nebraska calf diarrhea virus. J Virol. 1988;62:4022–4026. doi: 10.1128/jvi.62.11.4022-4026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N, Matsumoto Y. Bovine rotavirus G and P types and sequence analysis of the VP7 gene of two G8 bovine rotaviruses from Japan. Vet Microbiol. 2002;84:297–305. doi: 10.1016/s0378-1135(01)00445-x. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JT, Taraporewala Z, Chen D, Chizhikov V, Jones M, Elhelu A, Collins M, Kearney K, Wagner M, Hoshino Y, Gouvea V. Effect of intragenic rearrangement and changes in the 3′ consensus sequence on NSP1 expression and rotavirus replication. J Virol. 2001;75:2076–2086. doi: 10.1128/JVI.75.5.2076-2086.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JT, Vasquez‐Del Carpio R, Taraporewala ZF, Tortorici MA. Coupling of rotavirus genome replication and capsid assembly. In: Maramorosch K, Shatkin AJ, editors. Advances in Virus Research. San Diego, CA: Academic Press; 2006. (in press) [DOI] [PubMed] [Google Scholar]
- Pereira HG, Azeredo RS, Fialho AM, Vidal MN. Genomic heterogeneity of simian rotavirus SA11. J Gen Virol. 1984;65:815–818. doi: 10.1099/0022-1317-65-4-815. [DOI] [PubMed] [Google Scholar]
- Pereira HG, Gouvea VS, Fialho AM. A comparison of simian rotavirus SA11 preparations maintained in different laboratories. Mem Inst Oswaldo Cruz. 1986;81:389–393. doi: 10.1590/s0074-02761986000400005. [DOI] [PubMed] [Google Scholar]
- Ramig RF. Genetics of the rotaviruses. Annu Rev Microbiol. 1997;51:225–255. doi: 10.1146/annurev.micro.51.1.225. [DOI] [PubMed] [Google Scholar]
- Ramig RF, Petrie BL. Characterization of temperature‐sensitive mutants of simian rotavirus SA11: protein synthesis and morphogenesis. J Virol. 1984;49:665–673. doi: 10.1128/jvi.49.3.665-673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak K, McNeal MM, Choi A, Cole MJ, Ramesh G, Alvarez X, Aye PP, Bohm RP, Mohamadzadeh M, Ward RL. Defining T‐cell‐mediated immune responses in rotavirus‐infected juvenile rhesus macaques. J Virol. 2004;78:10258–10264. doi: 10.1128/JVI.78.19.10258-10264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, Nishikawa K, Kobayashi N, Urasawa T, Wu H, Gorziglia M, Urasawa S. Differences in plaque size and VP4 sequence found in SA11 virus clones having simian authentic VP4. Virology. 1994;198:325–330. doi: 10.1006/viro.1994.1035. [DOI] [PubMed] [Google Scholar]
- Taraporewala ZF, Schuck P, Ramig RF, Silvestri L, Patton JT. Analysis of a temperature‐sensitive mutant rotavirus indicates that NSP2 octamers are the functional form of the protein. J Virol. 2002;76:7082–7093. doi: 10.1128/JVI.76.14.7082-7093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez M, Sandino AM, Pizarro JM, Fernandez J, Valenzuela S, Spencer E. Function of rotavirus VP3 polypeptide in viral morphogenesis. J Gen Virol. 1993;74:937–941. doi: 10.1099/0022-1317-74-5-937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


