Abstract
Background
Malaria and HIV-1 infection cause significant morbidity and mortality in sub-Saharan Africa. HIV-1 increases risk for malaria with the risk increasing as immunity declines. The effect of HIV-1 infection on antimalarial treatment outcome is still inconclusive.
Objective
To compare antimalarial treatment outcome among HIV-1 positive and negative patients with acute uncomplicated falciparum malaria treated with chloroquine plus sulfadoxine-pyrimethamine (CQ+SP).
Methods
Ninety eight HIV-1 positive patients aged 18 months or older with acute uncomplicated falciparum malaria were treated with CQ+SP and followed for 28 days to monitor outcome. Treatment outcome of HIV-1 positive patients was compared to that of 193 HIV-1 negative historical controls. The primary study outcome for both groups was treatment failure.
Results
HIV-1 positive patients older than 5 years of age were less likely to have treatment failure compared to HIV-1 negative patients in the same age group (RR 0.59 95% CI 0.4– 0.8, p < 0.001) and HIV-1 positive patients on routine cotrimoxazole prophylaxis were less likely to have treatment failure following CQ+SP treatment compared to HIV negative patients (RR 0.6 95% CI 0.43–0.92, p = 0.006). There was no difference in treatment outcome according to HIV-1 status for children younger than 5 years of age.
Conclusions
Adherence to cotrimoxazole prophylaxis should be reinforced in HIV positive patients and it should be reassessed if these patients present with acute episodes of malaria.
Keywords: malaria, HIV, Uganda, antimalarial treatment response
Running head: Effect of HIV infection on antimalarial treatment outcome
Introduction
In Sub Saharan Africa, malaria and HIV infections are endemic and responsible for significant morbidity and mortality. Malaria causes about 300–500 million clinical cases annually, 90% of which occur in sub-Saharan Africa1. The Joint United Nations Program on HIV/AIDS (UNAIDS) estimated that 29.4 Million Africans are infected with HIV (UNAIDS, December 2002).
Two recent studies on the effect of HIV-1 infection on malaria incidence have provided strong evidence for an increased risk of malaria among HIV-1 positive patients2–4. Because HIV-1 infection impairs cell-mediated immunity, some authors have argued that HIV-1 infected individuals may be at higher risk for or suffer poor outcomes to malaria infection. Only a few studies have examined the effect of HIV-1 infection on response to antimalarial treatment and these have yielded conflicting results5–13. Therefore the effect of HIV-1 infection on antimalarial treatment response is inconclusive. The objective of this study was to compare the treatment outcome among HIV-1 positive and negative patients with acute uncomplicated falciparum malaria treated with chloroquine plus sulfadoxine-pyrimethamine (CQ+SP) and followed for 28 days.
Materials and Methods
Study site
The study was conducted between November 2004 and June 2005 in Mulago Hospital, Kampala, Uganda. The HIV-1 positive patients were recruited from the paediatric and adult infectious diseases clinics and the HIV-negative patients were recruited from the outpatients' clinic.
Ethical consideration
The study was approved by the Makerere University Faculty Research and Ethics Committee and was conducted according to Good Clinical Practice standards. All participants gave written informed consent.
Population
HIV-1 positive patients
Consecutive HIV-1 positive patients with symptoms of acute uncomplicated falciparum malaria and a positive screening thick blood smear (stained with 10% Leishman's stain for 10 min) were referred for study enrolment. Patients were enrolled if they met the following inclusion criteria:1 age 18 months and above, 2 an elevated temperature at presentation (≥ 37.5°8C axillary) or a history of fever in the previous 24 hours, 3 P. falciparum mono-infection with ≥ 2,000 asexual parasites/ul, 4 absence of other causes of fever (based on the clinical judgment of the study physician), 5 absence of severe malaria (WHO, 2000) or danger signs (inability to stand or drink, recent convulsions, lethargy, or persistent vomiting in children less than five), 6 no history of an allergic reaction to sulphonamides, 7 willingness of the patient or an adult guardian to provide written informed consent, and 8 residence within the city of Kampala.
Patients enrolled in the study were evaluated by a study physician for symptoms and their duration, medication history with emphasis on use of cotrimoxazole prophylaxis, duration of use and adherence measured by self report. Weight was measured in kilograms and temperature was measured using an electronic axillary thermometer. Blood was collected by venipuncture on the day of enrolment and by finger-prick on follow-up days.
HIV-1 negative patients
HIV-1 negative historical controls were selected from an earlier antimalarial study conducted in the outpatients' clinic from December 2003 to August 2004. Selection criteria and followup schedule were similar to that for HIV-1 positive patients. A database containing information on patient's age, sex, weight, baseline temperature, pre-treatment parasite density, and treatment outcome was created. New random numbers were computer-generated and linked to original patient study numbers in the database and on filter paper blood samples to identify the corresponding data and blood sample for each patient. The original identification numbers were deleted from the database and the filter papers. HIV-1 testing was performed on dry filter paper blood samples. All participants in this study gave written informed consent to future use of biological specimens and the Faculty Ethics and Research Committee approved HIV-1 testing of the de-linked samples. HIV-1 testing of historical controls was performed on filter paper blood samples using two enzyme linked immunosorbent assays (ELISA) in parallel (Vironostika HIV-1 Plus O Microelisa System, BioMerieux, Inc. Durham, NC, U.S.A. and Genetic Systems rLAV EIA Bio-Rad Laboratories, Hercules, CA, USA). Patients were classified as HIV-1 positive if both enzyme immunoassays were positive and HIV-1 negative if both were negative. Western blots (Genetic Systems HIV-1 Western Blot, Bio-Rad Laboratories, Hercules, CA, USA) were performed on discordant samples, and the results were classified as positive, negative, or indeterminate. Indeterminate Western blot results were repeated and subsequently classified as positive or negative. Only HIV-negative subjects were selected as historical controls.
Treatment and follow-up
Both HIV-1 positive and negative patients were treated with 25 mg/kg of CQ (Avloclor, ZENECA, 10 mg/kg on days 0 and 1, 5 mg/kg on day 2) plus a single dose of 1.25 mg/kg pyrimethamine and 25 mg/kg sulfadoxine (Fansidar, Roche) on day 0. All doses were directly observed and if a patient vomited within thirty minutes of dosing, the medication was re-administered. Paracetamol was administered to all patients. Patients were followed on days 1, 2, 3, 7, 14, 21 and 28 and follow-up consisted of a brief history, clinical examination and a blood smear for malaria on each day. Patients were encouraged to come back to the clinic at any time if they felt ill, and they then received a full evaluation including examination of a blood smear. Patients who met criteria for clinical failure with CQ+SP were treated with intravenous or oral quinine (10 mg salt/kg every 8 hr for 7 days). If patients did not return for scheduled follow-up, they were visited and assessed at home. If the home health visitor could not locate patients, they were classified as lost to follow-up. Patients were excluded from the study for the following reasons: (1) administration of antimalarial drugs outside the study protocol, (2) emergence of another febrile illness which would interfere with classification of malaria treatment outcome, (3) movement away from the study area, or (4) withdrawal of informed consent.
Laboratory Tests
Thin blood smears (obtained on day 0) and thick blood smears (obtained on days 0, 3, 7, 14, 21, and 28) were stained with 2% Giemsa stain for thirty minutes, and parasite densities were calculated by counting the number of asexual parasites per 200 white blood cells (WBC) assuming a WBC count of 8,000/ul of blood.
Outcome Measurements
Treatment outcome over 28 days of follow-up was classified according to the WHO Treatment Outcome Classification (WHO, 2002). Patients were classified as Early Treatment Failure (ETF) if they developed danger signs or severe malaria on or before day 3, had fever and a day 2 parasite density greater than that on day 0, had fever and parasitemia on day 3, or had a day 3 parasite density ≥ 25% of that on day 0. Late Clinical Failure (LCF) was defined as parasitemia after day 3 with a documented temperature > 37.5°C (axillary), danger signs, or severe malaria. Late Parasitological Failure (LPF) was defined as presence of parasitemia on day 28 and temperature <37.5°C (axillary), without previously meeting any of the criteria of early or late treatment failure. All others were classified as Adequate Clinical and Parasitological Response (ACPR).
Statistical Analysis
Data were recorded on standardized case report forms, reviewed daily for accuracy and completeness, and entered into EpiInfo version 6.04® (Centers for Disease Control and Prevention, Atlanta, GA). Clinical treatment success was defined as an ACPR response and clinical treatment failure defined as either ETF or LCF or LPF. Data were summarized using frequencies, medians, and means. Analysis for malaria parasite density was done on log-transformed parasite density values. Continuous variables were compared using the Independent T-test. Association between HIV-1 infection and treatment outcome was determined by estimating relative risks and 95% confidence intervals (95% CI) using cross tabulation. A two sided p value < 0.05 was considered statistically significant.
Results
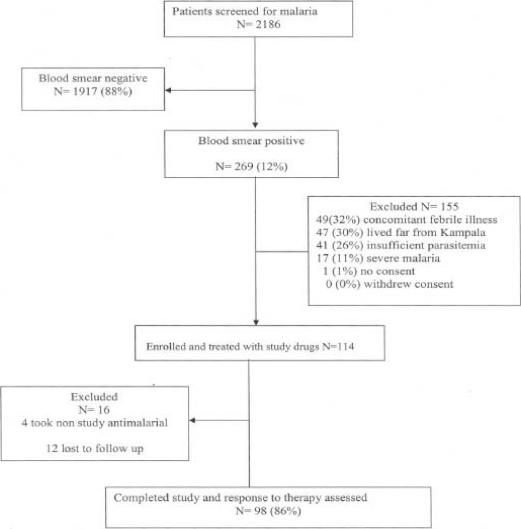
A total of 2186 HIV-1 positive patients with fever and axillary temperature ≥37.5°C were screened for malaria; 269 (12%) had a positive malaria blood smear and were referred for study inclusion, 114 fulfilled the inclusion criteria and were enrolled into the study (figure 1).
Figure 1.
Study profile of HIV-1 positive patients
The primary reasons for exclusion were: presence of concomitant febrile illness (49, 32%), residence outside the city of Kampala (47, 30%), insufficient parasitemia (41, 26%), severe malaria (17, 11%) and lack of consent (1, 1%). Of the 114 HIV-1 positive patients 16 were not included in the analysis because of either loss to follow up or use of additional antimalarial medication outside the study protocol.
From the historical cohort of 213 patients; 193 (90%) were HIV-1 negative and included as the comparison group, 8 (4%) were HIV-1 positive and 12 (6%) had discordant HIV test results.
The baseline characteristics of patients who completed the study and were included in the analysis are shown in Table 1. Baseline characteristics of HIV-1 positive and negative patients less than 5 years of age were comparable. HIV-1 positive patients older than 5 years were older than the HIV-1 negative patients in the same age group, they also weighed more (p<0.001), presented with higher temperature (p=0.009) and tended towards higher parasite density (p=0.2) compared to the HIV-1 negative patients in the same age group.
Table 1.
Baseline characteristics of patients who completed the study
| Characteristic | HIV status | |||
| HIV-1 negative | HIV-1 positive | |||
| Less than 5 years | 5 years and above | Less than 5 years | 5 years and above | |
| Percent female | 46% | 55% | 44% | 66% |
| Median age in years (IQR) | 3.0 (2–4) | 9 (6–12) | 4 (3–4) | 25 (10–34) |
| Mean temperature °C (SD) | 38.3 (1.2) | 37.7 (1.1) | 38.5 (0.9) | 38.0 (1.0) |
| Mean Parasite density per ul (SD) |
58,828 (74388) | 49,897 (65116) | 53,250 (67666) | 63,032 (107614) |
| Mean log parasite density (SD) | 4.4 (0.65) | 4.3 (0.62) | 4.3 (0.69) | 4.4 (0.6) |
| Median weight (IQR) | 13 (10–15) | 24 (19–35) | 13.1 (37.7–39.3) | 45.5 (24.6–54) |
Comparison of treatment outcome
Thirty three (34%) of the HIV-1 positive patients had CQ+SP treatment failure compared to 119 (62%) of the HIV-1 negative patients (RR 0.54 95% CI 0.4–0.7 p < 0.001).
Among patients younger than 5 years, 4 (44%) of the HIV-1 positive patients had treatment failure compared to 45 (76%) of the HIV-1 negative patients (RR 0.58 95%CI 0.2–1.2 p = 0.103). Among patients 5 years and older; 29 (33%) of the HIV-1 positive patients had treatment failure compared to 74 (55%) of the HIV-1 negative patients (RR 0.59 95% CI 0.4–0.8, p < 0.001) (Table 2).
Table 2.
Comparison of treatment outcome among HIV-1 positive and negative patients
| Treatment outcome | Age; below 5 years | Age; 5 years and above | ||
| HIV-1 negative | HIV-1 positive | HIV-1 negative | HIV-1 positive | |
| (N=59) | (N=9) | (N=134) | (N=89) | |
| Failure n (%) | 45 (76) | 4 (44) | 74 (55) | 29 (33) |
| ACPR n (%) | 14 (24) | 5 (56) | 60 (45) | 60 (67) |
| P=0.103 | P=0.001 | |||
Comparison of treatment outcome of HIV-1 positive patients on cotrimoxazole with that of HIV-1 negative patients (Table 3) showed that 18 (39%) of the HIV-1 positive patients on cotrimoxazole had treatment failure compared to 119 (62%) of the HIV-1 negative patients (RR 0.6 95% CI 0.43−0.9, p = 0.006).
Table 3.
Comparison of treatment outcome among HIV-1 positive patients on cotrimoxazole and HIV-1 negative patients
| Treatment outcome | HIV-1 positive on cotrimoxazole |
HIV-1 negative, not on cotrimoxazole |
| Failure n, (%) | 18 (39) | 119 (62) |
| ACPR n, (%) | 28 (61) | 74 (38) |
| RR = 0.6, 95% CI = 0.43 − 0.9, P = 0.006 | ||
Discussion
We compared CQ+SP treatment outcome for acute uncomplicated falciparum malaria among HIV-1 positive and negative patients over a 28 day period. We found that HIV-1 positive patients older than 5 years of age were less likely to have treatment failure compared to HIV-1 negative patients in the same age group. There was no difference in treatment outcome according to HIV-1 status among patients younger than 5 years of age; however, the sample size for this age group was very small.
Very few studies have examined the effect of HIV infection on response to antimalarial treatment outcome. Two studies done in Zaire found that there was no significant difference in the level of treatment failure among HIV positive and negative children on day 7 following treatment with quinine7. A few studies carried out in other areas have suggested a decreased response to antimalarial treatment in HIV infected patients. One of these was a retrospective study done in Uganda which suggested that co-infection with HIV-1 may render CQ less effective therapy for malaria in children6. However, this study used a single antimalarial drug with high resistance levels, on a very small sample size of children. Another study from Ethiopia, suggested decreased clearance of P.falciparum in HIV-1 positive patients after treatment with artemisinin10.
Our findings are similar to results from a few previous studies which showed that HIV-1 infection has no significant impact on malaria treatment outcome in children6–9, 11–14. The study done in Uganda found that HIV-1 infection increased the susceptibility for new malaria infections but not recrudescence in adults, and there was no increased risk of malaria among HIV-1 infected children14. The recent study from Zambia found that HIV-1 infection was not a risk factor for recrudescence or reinfection, although patients with a CD4 cell count <300cells/ul were more likely to have recurrent parasitemia, recrudescence and new infection13.
Response to antimalarial therapy is dependent on the abilities of both antimalarial drugs and host immune responses to inhibit infecting parasites15, 16. Malaria-specific immunity is acquired with repeated exposure to malaria parasites and this immunity increases with age 17–20. Similarly, response to antimalarial therapy improves as the level of acquired immunity increases18. Although the immunological consequences of HIV infection are well established, the interaction between HIV infection and P. falciparum, both widely co-distributed in sub-Saharan Africa is not fully understood21. The imunosuppression caused by HIV infection might be associated with the failure to protect against malarial infection and the development of clinical disease 22 but not treatment outcome. An older study suggested that some components of the specific immune responses to falciparum parasites may not be modified, despite the decrease in CD4 counts with HIV infection 21.
We classified patients into categories according to age with five years as the cut off age. Previous studies have shown that age is a predictor of antimalarial treatment response. A study on predictors of chloroquine treatment failure showed that patients under the age of five were more likely to fail therapy and fail early in the course of treatment23. This categorization of patients was also supported by data that shows that antimalarial immunity increases with age, and effectiveness of antimalarial drugs is affected by the immune status of the host24, 25.
The HIV-1 positive patients in our study were relatively older than the HIV negative patients. It is possible that the advantage of older age and therefore greater acquired immunity balanced out the relative disadvantage of HIV infection and reduced immunity among the HIV positive patients.
We also found that HIV-1 positive patients on routine cotrimoxazole prophylaxis were less likely to have treatment failure following CQ+SP treatment compared to HIV-1 negative patients. Daily cotrimoxazole prophylaxis has been shown to provide a beneficial effect in preventing malaria, and death in HIV-1 positive patients.26 Cotrimoxazole is 99.5% effective in preventing malaria while effectiveness with SP is 95%, and both have about 80% therapeutic efficacy for the treatment of malaria27. However, cross-resistance between cotrimoxazole and SP is a potential concern when cotrimoxazole prophylaxis is used in areas where SP is used for treatment of malaria. This cross resistance has been shown to occur between cotrimoxazole and SP (28–30), although analysis of malaria parasites from children in Mali who had received at least one month of cotrimoxazole prophylaxis detected no resistance-conferring mutations. Similarly, a study in Uganda31 found no significant difference between either the proportion of malarial episodes with resistant organisms or the incidence of SP-resistant malaria before and after cotrimoxazole prophylaxis was introduced.
Our study was done in a specialized HIV clinic, where comprehensive HIV care is provided, including health education, antiretroviral therapy and malaria preventive materials such as insecticide-treated bed nets. It is possible that patients at these clinics have developed better health-related behavior and higher rates of self-treatment compared to HIV-1 negative patients in the general population. In addition, some protease inhibitors used in the treatment of HIV infection may also be effective in the treatment or prevention of malaria.
Use of a historical cohort was a limitation of this study; however, this may not have significantly affected our study results because the HIV-1 positive cohort which was recruited latter had lower risk of treatment failure. We could have also introduced measurement bias in this study because the two groups of patients were followed up at different times and so there could have been differences in measurements as well as missing data in the historical database. We were unable to perform genotyping to distinguish re-infection from recrudescence. The study done in Uganda showed that HIV positive adults had higher risk for re-infection5 and the Zambian study found that patients with a CD4 cell count <300cells/ul were more likely to have recurrent parasitemia, recrudescence and new infection11.
In conclusion, our findings show that the HIV-1 positive patients older than 5 years of age were less likely to have treatment failure compared to the HIV-1 negative patients in the same age group and use of daily cotrimoxazole prophylaxis by the HIV positive patients was associated with reduced risk of CQ+SP treatment failure. Adherence to cotrimoxazole prophylaxis should be reinforced in HIV positive patients and it should be reassessed if these patients present with acute attacks of malaria.
Acknowledgements
We are grateful to Dr Ann Gasasira and Dr Heidi Hopkins for their invaluable input into this study. We thank the study team of doctors, nurses, laboratory technicians and the administrative staff. We sincerely thank all the patients who participated in the study.
Financial support was provided by the Fogarty International Centre grant for training malaria research in Uganda through the Makerere University-University of California San Francisco Malaria Research Collaboration and from the Uganda Global Fund to fight AIDS, TB and Malaria Project.
References
- 1.WHO, author. Malaria. Wkly Epidemiol Rec. 1982–1997;74:265–272. [Google Scholar]
- 2.French N, Nakiyingi J, Lugada E, et al. Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. Aids. 2001;15(7):899–906. doi: 10.1097/00002030-200105040-00010. [DOI] [PubMed] [Google Scholar]
- 3.Francesconi P, Fabiani M, Dente MG, et al. HIV, malaria parasites, and acute febrile episodes in Ugandan adults: a case-control study. Aids. 2001;15(18):2445–2450. doi: 10.1097/00002030-200112070-00013. [DOI] [PubMed] [Google Scholar]
- 4.Whitworth J, Morgan D, Quigley M, et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet. 2000;356(9235):1051–1056. doi: 10.1016/S0140-6736(00)02727-6. [DOI] [PubMed] [Google Scholar]
- 5.Kamya MR, Gasasira AF, Yeka A, et al. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. J Infect Dis. 2006;193(1):9–15. doi: 10.1086/498577. [DOI] [PubMed] [Google Scholar]
- 6.Kamya MR, Kigonya CN, McFarland W. HIV infection may adversely affect clinical response to chloroquine therapy for uncomplicated malaria in children. Aids. 2001;15(9):1187–1188. doi: 10.1097/00002030-200106150-00019. [DOI] [PubMed] [Google Scholar]
- 7.Colebunders R, Bahwe Y, Nekwei W, et al. Incidence of malaria and efficacy of oral quinine in patients recently infected with human immunodeficiency virus in Kinshasa, Zaire. J Infect. 1990;21(2):167–173. doi: 10.1016/0163-4453(90)91701-e. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg AE, Nsa W, Ryder RW, et al. Plasmodium Falciparum malaria and perinatally acquired human immunodeficiency virus type 1 infection in Kinshasa, Zaire. A prospective, longitudinal cohort study of 587 children. N Engl J Med. 1991;325(2):105–109. doi: 10.1056/NEJM199107113250206. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen-Dinh P, Greenberg AE, Mann JM, et al. Absence of association between Plasmodium falciparum malaria and human immunodeficiency virus infection in children in Kinshasa, Zaire. Bull World Health Organ. 1987;65(5):607–613. [PMC free article] [PubMed] [Google Scholar]
- 10.Birku Y, Mekonnen E, Bjorkman A, et al. Delayed parasite clearance of plasmodium falciparum in patients with human immunodeficiency virus co-infection treated with artemisinin. Ethiop Med J. 2002;40(1):17–26. [PubMed] [Google Scholar]
- 11.Chandramohan D GB. Is there an interaction between human immunodeficiency virus and Plasmodium falciparum? Int J Epidemiol. 1998;27:296–301. doi: 10.1093/ije/27.2.296. [DOI] [PubMed] [Google Scholar]
- 12.Simooya OO, Mwendapole RM, Sikateyo BM. Severe falciparum malaria and the acquired immunodeficiency syndrome (AIDS) in Zambia. Ann Trop Med Parasitol. 1991;85(2):269–270. doi: 10.1080/00034983.1991.11812556. [DOI] [PubMed] [Google Scholar]
- 13.Van geerturuyden Jean-Pierre, Mulenga Modest, Mwananyanda Lawrence, et al. HIV-1 Immune suppression and antimalarial Treatment Outcome in Zambian Adults with Uncomplicated Malaria. Journal of Infectious Diseases. 2006;194:917–925. doi: 10.1086/507310. [DOI] [PubMed] [Google Scholar]
- 14.Kamya MR, Gasasira AF, Yeka Adoke, et al. The effect of HIV on malaria treatment outcome among adults and children with uncomplicated falciparum malaria in Uganda. 2005 [Google Scholar]
- 15.Wernsdorfer WH. Epidemiology of drug resistance in malaria. Acta Tropica. 1994;56:143–156. doi: 10.1016/0001-706x(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 16.Wernsdorfer W. Epidemiology of drug resistance in malaria. Acta Tropica. 1994;56:143–156. doi: 10.1016/0001-706x(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 17.Rogier, author. Natural history of Plasmodium falciparum malaria and determinig factors of acquisition of antimalaria immunity in two endemic areas, Dielmo and Ndiop (Senegal) Bull Mem Acad R Med Belg. 2000;155:218–226. [PubMed] [Google Scholar]
- 18.Targett, author. Malaria: drug use and immune response. Parasitology. 1992;105:561–570. doi: 10.1017/s0031182000075363. [DOI] [PubMed] [Google Scholar]
- 19.Bloland PB, Boriga DA, Ruebush TK, et al. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission II. Descriptive epidemiology of malaria infection and disease among children. American Journal of Tropical Medicine and Hygiene. 1999;60(4):641–648. doi: 10.4269/ajtmh.1999.60.641. [DOI] [PubMed] [Google Scholar]
- 20.Rogier C, Tall A, Diagne N, et al. Plasmodium falciparum clinical malaria: lessons from longitudinal studies in Senegal. Parassitologia. 1999;41(1–3):255–259. [PubMed] [Google Scholar]
- 21.Migot F, Ouedraogo JB, Diallo J, et al. Selected P. falciparum specific immune responses are maintained in AIDS adults in Burkina Faso. Parasite Immunol. 1996;18(7):333–339. doi: 10.1046/j.1365-3024.1996.d01-116.x. [DOI] [PubMed] [Google Scholar]
- 22.van Eijk AM, Ayisi JG, ter Kuile FO, et al. HIV increases the risk of malaria in women of all gravidities in Kisumu, Kenya. Aids. 2003;17(4):595–603. doi: 10.1097/00002030-200303070-00015. [DOI] [PubMed] [Google Scholar]
- 23.Dorsey Grant, Kamya Moses R, Ndeezi Grace, et al. Predictors of chloroquine treatment failure in children and adults with falciparum malaria in Kampala, Uganda. Am J Trop Med Hyg. 2000;62(6):686–692. doi: 10.4269/ajtmh.2000.62.686. [DOI] [PubMed] [Google Scholar]
- 24.Target G. Malaria: drug use and the immune response. Parasitology. 1992;105(S):61–S70. doi: 10.1017/s0031182000075363. [DOI] [PubMed] [Google Scholar]
- 25.Baird JK, Jones TR, Danudirgo EW., et al. Age-dependent acquired protection against Plasmodium falciparum in people having two years exposure to hyperendemic malaria. Am J Trop Med Hyg. 1991;45:65–76. doi: 10.4269/ajtmh.1991.45.65. [DOI] [PubMed] [Google Scholar]
- 26.Grimwade KS. Cotrimoxazole prophylaxis for opportunistic infections in adults with HIV. Cochrane library. 2004;(2) doi: 10.1002/14651858.CD003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thera MA, Sehdev PS, Coulibaly D, et al. Impact of trimethoprim-sulfamethoxazole prophylaxis on falciparum malaria infection and disease. Journal of Infectious Diseases. 2005;192:1823–1829. doi: 10.1086/498249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitty CJ, Jaffar S. Plasmodium falciparum cross-resistance. Lancet. 2002;359(80) doi: 10.1016/S0140-6736(02)07300-2. [DOI] [PubMed] [Google Scholar]
- 29.Feikin DR, Dowell S, Nwanyanwu O. Increased carriage of trimethoprim/sulfamethoxazole-resistant S. pneumoniae in Malawian children after treatment for malaria with sufadoxine/pyrimethamine. J Infect Dis. 2000;181:1501–1515. doi: 10.1086/315382. [DOI] [PubMed] [Google Scholar]
- 30.Iyer JK, Milhous W, Cortese JF, et al. P.falciparum cross resistance between trimethoprim and pyrimethamine. Lancet. 2000;358:1006–1067. doi: 10.1016/S0140-6736(01)06201-8. [DOI] [PubMed] [Google Scholar]
- 31.Mermin J, Lule J, Ekwaru JP, et al. Cotrimoxazole prophylaxis by HIV-infected persons in Uganda reduces morbidity and mortality among HIV-uninfected family members. Aids. 2005;19(10):1035–1042. doi: 10.1097/01.aids.0000174449.32756.c7. [DOI] [PubMed] [Google Scholar]