Abstract
Background
Effective control and management of severe malaria cases depends on a clear understanding of the local epidemiological factors and specific clinical manifestations of the disease in the different endemic regions.
Objectives
To determine the prevalence of severe malaria and epidemiological factors that affect the development of malaria anaemia.
Methods
A cross-sectional survey was carried out among children below 5 years of age, at the Adeoyo State Maternity Hospital, Ibadan, Nigeria. Questionnaires and case histories were taken from patients clinically diagnosed of malaria. Thus, 372 volunteers were recruited into the study from the 3131 paediatric cases that reported over the 10-week period to the out-patient department (OPD) of the hospital. 229 (61.6%) of the recruited volunteers presented with fever (>37.5 °C) at consultation. These had malaria parasite and PCV tests done.
Results
Clinical diagnosis was confirmed microscopically in 78% (290/372) for Plasmodium infection using thick film slides. Anaemia (PCV <28%) prevalence was 28.2%. Factors that contributed to the rapid progression of uncomplicated malaria to severe status included: age of the child, level of parasitaemia, careless response and attitude of parents or guardians to fever in the children; parents' preoccupation with their jobs or other healthy children and unwillingness to use available health facilities.
Conclusion
The study underscores the need for community involved partnership for malaria control especially through health education for the home management of malaria, espeically among those experiencing some form of inequity in access to healthcare.
Keywords: malaria, epidemiology, development, anaemia children
Running title: Epidemiological factors that promote severe malaria in children
Introduction
The clinical manifestation of severe malaria in children below 5years in Africa is characterized by two predominant outcomes, malaria anaemia and cerebral anaemia 1 and other associated outcomes such as cough, vomiting and diarrhea have been observed 2. The actual pathophysiological mechanisms leading to these outcomes in children, though poorly understood, are known to vary from one geographical location to another and within populations, and are believed to be influenced by a number of immunological 3, 4, 5, 2 and epidemiological factors 6, 1.
The human behavioural pattern is a major epidemiological factor that impacts on disease transmission and progression in Africa and there is growing evidence that with appropriate awareness, education, attitude, attention to and chemotherapy of, the key symptoms of malaria, the incidence of severe malaria can be drastically reduced especially in the rural and urban areas where most of the estimated 2–3 million deaths per year from malaria occur 7, 8.
Since there is not yet a vaccine for malaria, prospects of curtailing the mortality and morbidity of malaria especially amongst children lie in the effective control and management of cases through a clear understanding of the local epidemiological factors and peculiar clinical manifestations of this disease in different regions where it is endemic.
Studies in areas of high and moderate transmission have shown that relative frequencies of severe anaemia and cerebral malaria (the two main presentations of severe malaria in African children) vary with the level of transmission and age 8, 9, 10, 1. In Nigeria, reports on the clinical manifestation of malaria in children showed that cerebral malaria sequelae are a more likely outcome of severe malaria infection than severe anaemia in the north 10 whereas severe malaria anaemia is a more likely outcome in the south 2.
In this paper we report the peculiar epidemiological factors specific to Ibadan that affect the rapid development of severe malaria anaemia from uncomplicated infection in children resident in the southwestern part of Nigeria.
Materials and Methods
From patients attending the out-patient department (OPD) of the Adeoyo State Maternity Hospital, Yemetu Ibadan, 372 children (4mths– 5yrs old) were recruited during the high malaria transmission season (April–June, 2001). The hospital serves the central Ibadan area and the inhabitants of this area are mostly peasant farmers and petty traders. The houses are closely packed; nutrition and physical hygiene are generally poor. Inclusion criteria were based on doctors' diagnosis of malaria and age ≤5yrs. After obtaining informed consent from parents or guardians, 200µl of blood was collected by venous puncture and centrifuged. Sera were stored at <-20 °C before use in immunological assays.
Malaria infection in the children was treated with Chloroquine (CQ). Other drugs, such as Quinine and Halofantrine were used in cases of CQ failure. Parasites were detected using thick Giemsa-stained blood films in 100 high power fields. Malaria parasites were counted against leukocytes, assuming a constant leukocyte count of 8000/µl blood.
Questionnaires were administered to the parents of the volunteers, medical personnel and care givers to obtain demographic information, case history, signs and symptoms. They provided information about the care-giver's knowledge and experience with previous malaria infection and their ability to make a quick presumptive diagnosis of the current infection prior to receiving help from the hospital or health center. Also, we obtained information about factors such as availability of funds, nearness and willingness to the help of a trained clinician, perception of the reception received at the health centers that may have affected the decision to seek treatment when the child was ill.
We were also interested in the choice of medication used during the home care period, the knowledge of the care giver about such medication and their compliance of with the recommended drug dosage of the drug used, during the home care period and after they had received prescribed drugs from the health centers.
Essentially the information from the case history and hospital records was used to corroborate some of the information that was supplied in the questionnaire and to fill up any other missing information that might have been asked by the clinician in the course of consultation with the care-giver.
To obtain the age-specific prevalence of the Plasmodium parasite the children were grouped by age 0–6 months, >0.5–1yrs, >1–2yrs, >2–5yrs and >5yrs. The response of parent or guardian to the presentations of sickness in their wards was tested for differences using SPSS 11.0 software package (SPSS Inc, 2001). Malaria parasite density was defined as log10 of the number of asexual malaria parasites/µl blood in the thick blood films of malaria positive volunteers to approximate normality. Confidence level was set at P=0.05
Results
A total number of 3131 cases were seen in the hospital during the period of study. Eighty two percent (2592) were below the age of six years. Eleven percent (372) of the volunteers (Table 1) that visited the OPD were recruited into the study. Fifty five percent (205) of the children were male and the remaining 167 (45%) were female. On the basis of information provided by the mothers of the recruited children, 290/372 (78%) mothers claimed either not to have studied or did not study beyond primary six level, 79/372 (21%) studied beyond primary six and 3/372 (<1%) studied above secondary school.
Table 1.
Age group and sex distribution of the volunteers recruited in the cross-sectional survey
| Age (years) | Male | Female | Total | % of Total |
| 0–0.5 yrs | 4 | 6 | 10 | 2.7 |
| >0.5–1 yrs | 67 | 51 | 118 | 31.7 |
| >1–2 yrs | 70 | 50 | 120 | 32.3 |
| >2–5 yrs | 55 | 50 | 105 | 28.2 |
| >5 yrs | 9 | 10 | 19 | 5.1 |
| Total | 205 | 167 | 372 | 100 |
Haematology
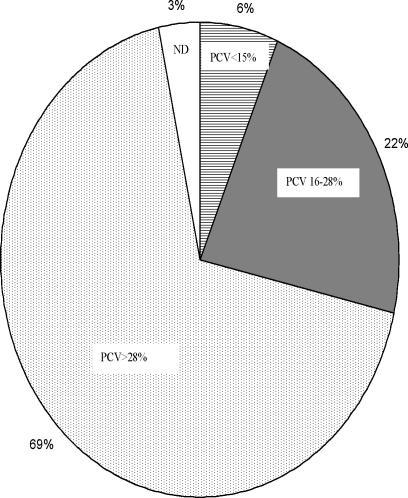
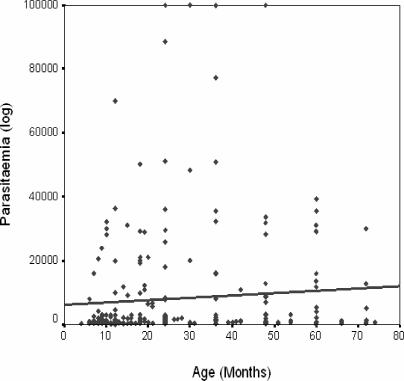
Microscopic examination of the thick blood film slides confirmed that 290/372 (78%) of the 372 patients were positive for Plasmodium. Twenty four (8.3%) of the parasitaemic 290 children had acute severe anaemia (PCV <15%), while another 82 volunteers had mild malaria anaemia (PCV 16–28%, (Fig 1) according to the WHO classification and 6 volunteers presented with altered consciousness (≥7 of 15 points on the Blantyre scale). Parasite density ranged from 80 to 100, 000 parasites/µl blood and was highest in children in the age group 2–5 years. Two Plasmodium species were observed within the population: Plasmodium falciparum, 273 (94.1%) and Plasmodium malariae, seen in only 17 (5.9%) cases. There was a positive correlation between age and parasitaemia, indicating that older children tolerate higher parasite loads than the younger children (Figure 2). There was a negative correlation between the PCV and parasitaemia; indicating that PCV decreased with increasing parasite density in the children (Figure 3).
Figure 1.
The distribution of percentage packed cell volume (PCV) the study population.
Figure 2.
Correlation between parasitaemia and age.
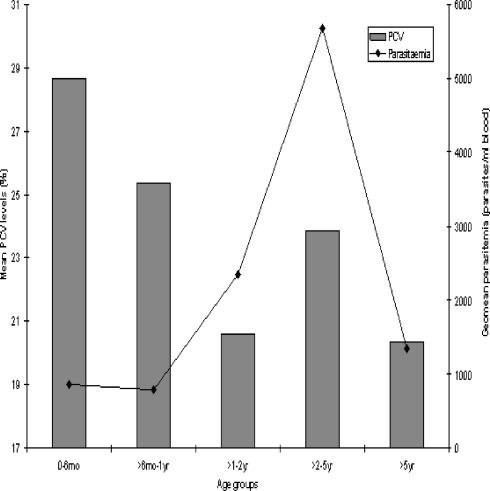
Figure 3.
Age group and mean PCV
Parasitaemia increased with increasing age among the children in this study
The mean hemoglobin levels (PCV) and geometric mean parasite density levels in the 290 individuals that were positive for Plasmodium. There is a consistent decrease in the hemoglobulin levels from the birth till the 2yrs of age whereas parasite density in the blood increases particularly between 2–5yrs of age.
Epidemiological Factors
We studied the pattern and behaviour of parents or care givers in response to infection in their children or wards, using questionnaires and other information from the hospital records about the history of infection. A number of factors were observed (Table 3), which had important implications for the disease progression and development of severe malaria anaemia. First, the low priority given by the parents to their child's health against other personal commitments - less than 20% of parents had their children staying with their grandparents who were quite aged (the least being 60 years). Secondly, many parents (70%) did not show prompt response to the early symptoms presented in the children; there was at least a 3-day history of fever before attending the hospital. Thirdly, inadequate knowledge of the early symptoms presented in children for diagnosing malaria or any other infection; 90% of the parents or care givers of children between the ages of 6mths and 2yrs thought that fever in their children meant teething problems. Fourthly, most parents were not willing to bring their childrend to the health centers since they felt that the hospital protocol was tedious and too long and did so only when other remedies had failed. Lastly, very few parents (less than 9%) used the right medication, neither in using the right drug for the illness suspected nor in enforcing the right dosage after leaving the hospital. The care givers did not demonstrate good knowledge of home management skills of malaria, especially in the children. However, drug treatment and dosaging may have been complicated by the effect of fake drugs that were purchased and used by some parents. Three parents complained that the CQ they had previously administered at home was repeated in less than a week of the initial administration at the hospital before their children got better. There were also news reports of confiscation of antipyretics and antimalarial drugs by the National Agency for Food and Drug Administration (NAFDAC) from drug importers during this period.
Table 3.
Factors that promote the development of severe malaria in the children
| Epidemiological Factors | Odds Ratio (CI = 95%) |
p-value |
| Priority of other personal commitments over child's health. | 6.6 | 0.01 |
| Lack of prompt response to the early symptoms in children | 8.2 | 0.002 |
| Lack of knowledge of the early malaria symptoms | 12.8 | 0.005 |
| Use of wrong malaria medication and dosage. | 7.0 | 0.01 |
| Closeness and accessibility of health centers. | nd | nd |
Spearman rank correlation test was used (it is usually used for non-parametric test) and our PCV values were ranked as either normal (>28%), mild anemia (16–28%) or severe anemia (>16%). Thus the correlation between parasitemia and PCV (Spearmans coefficient) R = −0.306 (p = 0.005). This was done using the SPSS statistical software.
To calculate odds ratio there would need to be a control group, but in our study there was no control study group (since it was an epidemiological survey and not a case control study). But what we can do is to control for affected subjects (those with severe outcomes) using the unaffected (subjects without complicated outcomes) within the study group.
Discussion
The development of severe malaria from uncomplicated malaria and the eventual progression of the two most common fatal outcomes (malaria anaemia and severe malaria) of this infection depend not only on immunological, but also on some epidemiological factors. In the absence of any form of immune compromise and parasite resistance to choice drugs, the frequency of malaria infection will be curtailed and the development of severe anaemia and its outcomes checked if caregivers have the right knowledge of symptoms and give prompt attention to these symptoms with appropriate chemotherapy.
We found that there was a very strong correlation between the attitudes and response of parents to the initial symptoms of malaria in their wards and development of severe malaria status, especially anaemia.
The inability of the caregivers to correctly recognize malaria has contributed substantially to child morbidity and mortality due to malaria. Due to lack of basic education many parents depend on the pharmacist to indicate the drug dosage. Among this cohort, the nature of the family is such that women in polygamous homes are more or less the bread-winners for their offspring. They spend long hours working outside the home in such occupations as peasant farming and petty trading, and often childcare is not thorough; someone else is entrusted with the care of the sick child. It is known that in an holoendemic area such as ours, the age group 1 – 5 years is the most vulnerable to severe and complicated malaria since their immunity to malaria is still very poor12, 13, 14 and children may not be able to speak their discomfort at the early stages of the infection when the infection could have been stopped in time. Parents and caregivers need to be taught possibly from the time they start attending the maternity clinic, and in a community health programme, how to recognize and treat malaria symptoms and to act quickly, especially since fever has been proven to be the interactive clinical indicator of malaria infection and perhaps any other infection that may have affected the children.
Previous work showed high titres of the anti-MSP1 antibodies in the serum of the anaemic volunteers, suggesting that the co-existence of these high titres with anaemia is either as result of slow immune response to infection or that the antibodies lacked the protective efficacy to abrogate disease progression and development of anaemia or a combination of both. Non-efficient humoral response11, 15 can be elicited by the parasite as has been previously suggested 16 and lack of correlation between antibody titres and parasitaemia has been reported11. Consequently there is a need to further study the binding specificity of these antibodies and how modifications made on the epitopes can influence the development of lasting vaccine and elicit good humoral response. The results obtained in this study with respect to malaria and PCV levels were very similar to those of infants in Igbo-Ora 14. The degree of anaemia correlated with parasitaemia. All the children who had acute severe anaemia had PCV less than 15% (Fig 3). Increasing malaria parasitaemia correlated with decreasing PCV particularly in the age group 4–10months14. Our data showed the same correlation across all the age groups (Fig 2)
In this study, effort has been made to establish the prevalent symptoms and clinical manifestations in severe malaria amongst children in the south-western zone of Nigeria. This has been compared with results from other studies in Nigeria 10. Anaemia has been shown in this study to be a major clinical outcome of malaria infection that progresses from an initial uncomplicated infection to complicated and ultimately to the severe status in children.
Among the Bwatiye people, north-eastern Nigeria, most febrile children are thought to have “zazzabi”(an ordinary illness that cannot kill) and if fever persists in children it is thought to be an act of evil-doer and if death results, it could not have been malaria17. This type of belief was not common among the study group; rather there was a traditional acceptance of the unproven association of teething in children with fever and diarrhea. This could be addressed during the maternity clinics because this period in the life of a child (at about 6 months) is believed 11, 18 to correspond with the experience of the first clinical malaria infection. If there will be any control measure that will greatly impact the quick deterioration of sick children and reduce the rapid development of malaria related anaemia or other severe malaria outcomes, serious thoughts should be given to, a) empowerment of mothers with the capability to recognize and treat malaria, b) education of mothers especially in endemic regions to put malaria always on top of the list of suspected illnesses in every case of febrile illness and c) identification and exploitation of indigenous traditional beliefs of different cultures on the etiology of infection as a means to educate the people than insistence on their replacement with modern knowledge19. The importance of teaching home management and care of malaria in the tropics especially Africa cannot be over emphasized.
Table 2.
Home treatment of children by care-givers and the medication used before coming to the hospital.
| Type of Medication | No. of individuals |
% of total |
| No medication235 | 63.2 | |
| Chloroquine | 65 | 17.5 |
| Antipyretics (Paracetamol) | 36 | 9.7 |
| Antibiotics (Septrin) | 20 | 5.4 |
| Drug concoction | 14 | 3.8 |
| Herbal concoction | *2 | 0.5 |
This figure is so low because some care-givers denied the use of local herbs in home care since they were often rebuked in the hospital for taking such measures instead of coming to the health center.
Acknowledgement
We acknowledge with deep gratitude the cooperation and endurance of the children who participated in this study. We are grateful to Drs. Popoola, Bakare, Akinyemi, Tolu Bella and the OPD staff members of the Adeoyo State Maternity Hospital, Ibadan. This work was supported by Research Capability Strengthening grant from the MIM/TDR for Research and Training in the Tropics.
References
- 1.Greenwood BM. The Epidemiology of Malaria. Ann Trop Med Parasitol. 1997;91(7):763–769. doi: 10.1080/00034989760518. [DOI] [PubMed] [Google Scholar]
- 2.Anumudu CI, Okafor CMF, Ngumohaike V, Afolabi KA, Nwuba RI, Nwagwu M. Clinical Manifestation and Immunological Response in Severe Paediatric Malaria in Adeoyo hospital, Ibadan. Afr J Med Med Sci. 2004;33:57–63. [PubMed] [Google Scholar]
- 3.Grau GE, De Kossodo S. Cerebral Malaria: Mediators, Mechanical Obstruction or More. Parasitology. 1994;10(10):408–411. doi: 10.1016/0169-4758(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 4.Clark IA, Rockett KA. Nitric Oxide and Parasite Disease. Parasitology. 1996;37:51–56. doi: 10.1016/s0065-308x(08)60218-3. [DOI] [PubMed] [Google Scholar]
- 5.Berendt AR, Ferguson DJP, Garduar J, Turnar G, Rowe A, Mc Cormick C, Roberts D, Craig A, Pinches R, Elford BC, Newbold CI. Molecular Mechanisms of Sequestration in Malaria. Parasitology. 1994;108(Suppl):519–528. doi: 10.1017/s0031182000075685. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood BM. What can the residents of malaria endemic countries do to protect themselves against malaria? Parassitologia. 1999;41:295–299. [PubMed] [Google Scholar]
- 7.Miller LH, Good MF, Milon G. Malaria Pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization Severe falciparum Malaria. Trans Roy Soc Trop Med Hyg. 2000;(94 Suppl. 1) [PubMed] [Google Scholar]
- 9.Snow RW, Craig M, Diechmann U, Marsh K. Estimating Mortality, Morbidity and Disability due to Malaria among African Non-pregnant Population. Bull WHO. 1999;77(8):624–640. [PMC free article] [PubMed] [Google Scholar]
- 10.Angyo JA, Pam SD, Szlachetka R. Clinical Pattern and Outcome in Children with Acute Severe falciparum Malaria at Jos University Teaching Hospital, Nigeria. East Afr Med J. 1996;72(12):823–826. [PubMed] [Google Scholar]
- 11.Nwuba R, Sodeinde O, Anumudu C, Omosun Y, Odaibo A, Holder A, Nwagwu M. The human immune response to Plasmodium falciparum includes both antibodies that inhibit merozoite surface protein 1 secondary processing and blocking antibodies. Infect Immun. 2002;70:5328–5331. doi: 10.1128/IAI.70.9.5328-5331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chongsuphajaisiddh T. Malaria. In: Stanfield P, Breuton M, Chan M, Waterson A, editors. Diseases of Children in the Subtropics and Tropics. London: Edward Arnolds; 1991. pp. 657–674. [Google Scholar]
- 13.Hendrickse RG. Parasitic Diseases. In: Hendrickse RG, Barr DBG, Matthew TS, editors. Pediatrics in the Tropics. Blackwell Scientifics Publications; 1991. pp. 695–710. [Google Scholar]
- 14.Achidi EA, Salimonu LS, Asuzu MC, Berzins K, Walker O. Studies on Plasmodium falciparum Parasitaemia and Development of Anaemia in Nigerian Infants in the First Year of Life. Am J Trop Med and Hyg. 1996;55(2):138–143. doi: 10.4269/ajtmh.1996.55.138. [DOI] [PubMed] [Google Scholar]
- 15.Uthaipibull C, Aufiero B, Syed S, Hansen B, Guevara Patino J, Angov E, Ling I, Fegeding K, Morgan, Ockenhouse C, Birdsall B, Feeney J, Lyon J, Holder A. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J Mol Biol. 2001;307:1381–1394. doi: 10.1006/jmbi.2001.4574. [DOI] [PubMed] [Google Scholar]
- 16.Guevara-Patino JA, Holder AA, McBride JS, Blackman MJ. Antibodies that Inhibit the Merozoite Surface Protein-1 Processing and Erythrocyte Invasion are Blocked by Naturally Acquired Human Antibodies. J Exp Med. 1997;186:1689–1699. doi: 10.1084/jem.186.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oladele BA, Kauna K. Illness-related practices for the management of childhood malaria among the Bwatiye people of north-eastern Nigeria. Malaria Journal. 2005;4(13):1–6. doi: 10.1186/1475-2875-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achidi EA, Perlmann H, Salimonu LS, Asuzu MC, Perlmann P, Walker O. A longitudinal study of seropositivity to Plasmodium falciparum antigen in Nigerian Infants during their first year of life. Acta Tropica. 1995;59:173–183. doi: 10.1016/0001-706x(95)00076-q. [DOI] [PubMed] [Google Scholar]
- 19.Baume C, Helitzer D, Kachur SP. Patterns of care for childhood malaria in Zambia. Soc Sci Med. 2000;51:1491–1503. doi: 10.1016/s0277-9536(00)00049-6. [DOI] [PubMed] [Google Scholar]