Abstract
Small intestinal brush-border hydrolases usually are assayed in intestinal mucosal homogenates resuspended in solutions of unphysiological ionic composition. Thus, extrapolation of measured Vmax values (maximal reaction rates at high substrate concentrations) to in vivo conditions, hence comparison with physiological substrate loads, is uncertain. We therefore have developed a sucrase assay in an intact preparation of mouse small intestine, an everted intestinal sleeve incubated in a physiological Ringer’s solution. As in homogenate studies, sucrase is assayed by glucose production measured colorimetrically, but uptake of liberated glucose into the intestinal sleeve is prevented by the transport inhibitor phlorizin. The coefficient of variation of Vmax is 16% for sleeves from the same mouse and 8% for mean values from different mice. Sleeve sucrase activity is abolished by the inhibitor castanospermine. Activity in sleeves and homogenates proves to be the same when measured under identical solution conditions, but variations in assay conditions cause large activity changes from values measured in physiological solutions.
Two basic problems of biochemistry concern the relation, within a series metabolic pathway, of enzyme or transporter Vmax values (maximal reaction rates at high substrate concentrations) to each other, and their relation to actual physiological reaction rates in the pathway. First, do enzymes in series differ considerably from each other in their Vmax values, such that amounts of one enzyme are rate-limiting while other enzymes are present in unused excess quantities? Or do enzymes in series tend instead to have similar Vmax values, such that resistance is distributed over the pathway and no single step is rate-limiting? Second, are quantities of enzymes “enough but not too much,” such that their Vmax values are little greater than reaction rates occurring under natural conditions, or are enzymes instead present in superabundance such that their Vmax values far exceed natural reaction rates?
Although belief in the existence of single rate-limiting steps is widespread, there has in fact been a lengthy and still unresolved debate over that conclusion (1). Perhaps the main obstacle to resolution is the difficulty in measuring enzyme Vmax values under in vivo conditions. There are three obvious reasons why the usual enzyme assays in tissue homogenates may yield values differing by an unknown and possibly large factor from physiological values: recovery of enzyme after homogenization may be less than 100%; enzyme turnover numbers may be altered by disruption of in vivo structural organization; and differences in solution conditions (pH, osmolarity, ionic strength, and ionic composition) between in vitro assay conditions and in vivo conditions also may alter turnover numbers.
Potentially, an advantageous system for overcoming these problems is the brush-border membrane of the small intestine. That membrane contains numerous hydrolases for dietary nutrients dissolved in the intestinal luminal fluid, as well as numerous transporters conveying the reaction products of those hydrolases out of the lumen across the brush border. Familiar pairs of brush-border hydrolases and transporters in series include disaccharidases and monosaccharide transporters, or peptidases and amino acid transporters. Those hydrolases and transporters are not intracellular but are part of a membrane facing the luminal extracellular compartment. Hence physiological values of those enzyme and transporter activities could be measured in an intact tissue facing a bulk bathing solution mimicking the normal luminal contents. Thus, in vitro Vmax measurements could be straightforwardly extrapolated to in vivo conditions, without the uncertainties introduced by tissue homogenization.
In practice, small intestinal brush-border sucrase, isomaltase, maltase, and lactase are customarily assayed in tissue homogenates by modifications or extensions of methods developed by Dahlqvist (2–4). Those assays are subject to the usual uncertainties of extrapolation to in vivo conditions. For example, the assays for the disaccharidases not only homogenize the intestinal mucosa but use as the incubation solution a pH 6.0 buffer of 0.05 M Na+ phosphate or 0.1 M Na+ maleate, which differs greatly from the luminal contents of the small intestine (e.g., in having very high phosphate or maleate concentration, lower ionic strength and osmolarity, and lack of Ca2+, Mg2+, HCO3−, K+, and other physiologically important ions).
Our laboratory previously developed a physiologically realistic preparation of intestinal brush border, termed an everted sleeve, for measuring the Vmax of glucose and amino acid transporters in intact tissue (5). Hence the present paper describes and validates a method for assaying sucrase in intact intestinal tissue by means of the same everted sleeve preparation. In the next paper we shall compare the resulting Vmax values for sucrase and the glucose transporter, assayed under relatively physiological conditions, to each other and to the dietary substrate load consumed by the whole animal (6).
MATERIALS AND METHODS
Animals and Diets.
Virgin female Swiss–Webster mice 80–120 days old, obtained from Charles River Breeding Laboratories, were caged individually in a room at 24°C on a constant 12 light/12 dark light schedule. They were supplied ad libitum with a pelleted high-sucrose ration manufactured by ICN (55% sucrose, 15% casein, 16% cellulose, 7% vegetable oil, 4% USP XIV salt mixture, 2% brewer’s yeast, 1% Vitamin Diet Fortification Mixture).
Glucose Transporter Activity.
We measured activity of the brush-border Na+/glucose cotransporter by the everted sleeve preparation described in detail elsewhere (5, 7). Briefly, a mouse was anesthetized with 0.07–0.10 ml Nembutal, and the small intestine was rinsed out with Ringer’s solution, excised, cut into thirds of equal length, and everted so that the brush-border membrane facing the lumen in vivo now faced the outside. An everted sleeve 1.3–1.5 cm in length (cut so as to avoid Peyer’s patches) was mounted on a metal rod 20 cm long, 2–4 mm in diameter, and with two grooves 1 cm apart, 1 and 11 mm, respectively, from the bottom of the rod. The sleeve was secured by ligatures over the grooves, and excess tissue beyond the ligatures was cut away. Next, the sleeve was preincubated at 37°C for 5 min in Ringer’s solution (see ref. 5 for composition) at pH 7.3, aerated with 95% O2/5% CO2. The sleeve then was incubated for 2 min (also at 37°C, pH 7.3, aerated, and stirred at 1,200 rpm) in 10 ml of Ringer’s solution containing 50 mM d-glucose isosmotically replacing a corresponding amount of NaCl in Ringer’s solution. That glucose concentration is far above the Km value (Michaelis constant) of the glucose transporter (6 mM in this preparation: ref. 5). Also incorporated into the incubation solution were trace concentrations of 14C d-glucose and 3H l-glucose. The incubation solution was stirred at 1,200 rpm with a stirring bar to minimize the influence of unstirred layers. At the end of the 2-min incubation the sleeve was lightly blotted, cut over the grooves to a 1-cm length, and weighed and removed for liquid scintillation counting.
Carrier-mediated d-glucose uptake was calculated as the uptake of 14C d-glucose, corrected both for passive glucose uptake and for glucose in the adherent fluid by subtracting the simultaneously measured uptake of the stereoisomer 3H l-glucose, which is not subject to carrier-mediated transport. Uptake was normalized to mg wet weight of the tissue. We multiplied the resulting uptake value by 1.12, the factor calculated from the Michaelis–Menten equation to convert uptake measured at 50 mM into a Vmax value, given the Km of 6 mM.
Thus, this method yields glucose transporter activity in an intact intestinal preparation with good control of unstirred layers.
Sucrase Activity.
Brush-border sucrase activity was measured in the same everted sleeve preparation, by measuring colorimetrically the glucose released into the adjacent solution by the action of sucrase on sucrose. The assay conditions that we shall now describe were selected on the basis of measurements, to be described in the Results section, of variations in these conditions.
Because the glucose released by action of sucrase on sucrose normally would be taken up into the tissue by the glucose transporter, we inhibited the transporter by preincubating the sleeve for 20 min at 37°C in Ringer’s solution containing the transport inhibitor phlorizin at 0.5 mM. The tissue then was incubated for 16 min at 37°C and pH 7.3 in 10 ml of Ringer’s solution containing 50 mM sucrose (isosmotically replacing NaCl) as well as 0.5 mM phlorizin and stirred at 1,200 rpm. At the end of the incubation we cut the ligatures securing the sleeve to the rod, slipped the sleeve off the rod, and weighed it wet, while two 250-μl replicate samples of the incubation solution were added to tubes containing 750 μl of Glucostat solution: 250 mM Tris buffer, 200 units/liter horseradish peroxidase, 10 mM p-hydrobenzoic acid, 0.2 mM aminoantipyrine, and 4,442 units/liter glucose oxidase (Sigma) (3, 8). The Tris halts sucrose hydrolysis immediately (3). After a 30-min incubation at 37°C, allowing the colorimetric reaction to proceed to completion, absorbance at 500 nm was read in a Beckman DV-200 spectrophotometer. Like glucose transporter activity, sucrase activity was normalized to mg wet weight of the intestinal sleeve. Unlike sleeves weighed for glucose transporter assays, sleeves for sucrase assays were weighed without cutting the sleeve itself over the rod grooves to a length of exactly 1 cm, because the slight extensions of the sleeve beyond the grooves also had been hydrolyzing sucrose.
Two slight corrections were subtracted from the measured absorbance. One correction (“reagent blank”), to take account of impurities in the sucrose solution, was the absorbance (measured in two replicates) of a 250-μl aliquot of 50 mM sucrose-containing Ringer’s solution treated identically (i.e., incubated for 30 min with the colorimetric reagents), except that previously no intestinal sleeve had been incubated in it. The other correction (“tissue blank”), to take account of glucose or other materials leaking out of the intestinal sleeve, was the absorbance of a 250-μl aliquot from a Ringer’s solution that contained 0.5 mM phlorizin and no sucrose but in which an intestinal sleeve had been incubated for 16 min; the aliquot then was incubated for 30 min with colorimetric reagents before measuring its absorbance. The tissue blank absorbance was divided by mg wet weight of the tissue blank and multiplied by mg wet weight of the sample tissue to take account of slight differences in sleeve masses. We measured a tissue blank and reagent blank for each intestinal region (one-third of intestinal length) used for sucrase assays. The resulting corrected absorbance was translated, by means of absorbance measured for glucose standards, into a preliminary sucrase activity, in units of nmol glucose released per min per mg tissue. Two further corrections were applied to this preliminary activity: it was multiplied by 1.11 to take account of the slight inhibition of sucrase by 0.5 mM phlorizin; and it was multiplied by 1.36 to take account of sucrase’s Km value (18 mM) by converting activity measured at 50 mM sucrose into a Vmax value for sucrose.
Passive Glucose Uptake.
Uptake of the nontransported isomer l-glucose was measured in everted sleeves as described elsewhere (5). Briefly, sleeves were incubated for 2, 4, 8, or 16 min at 37°C in Ringer’s solution containing trace concentrations of 3H l-glucose and 14C polyethylene glycol (PEG), plus 50 mM sucrose and 0.5 mM phlorizin. The PEG serves as an extracellular marker of incubation solution adhering to the tissue, hence is used to calculate l-glucose in adherent fluid and to subtract it from total l-glucose associated with the sleeve, thereby obtaining l-glucose taken up by the tissue.
Sucrase Assay in Tissue Homogenates.
To compare our everted sleeve assay of sucrase with conventional assays using tissue homogenates, we also used the latter assay according to the classical procedure of Dahlqvist (3), slightly modified. The small intestine was rinsed and excised as described above, then a 4-cm length of mid-intestine was cut out, diluted 1:10 by weight with Hepes buffer (290 mM mannitol in 1 mM Hepes/KOH at pH 7.4), and homogenized with a Brinkmann homogenizer at 0°C. An aliquot of the homogenate was further diluted 1:15 with the Hepes buffer and vortexed, then 50 μl of the resulting diluted homogenate (the so-called tissue sample) was pipetted into an ice-cold culture tube. A 200-μl volume of 62.5 mM sucrose in pH 6.0 0.1 M Na+ maleate/NaOH was added to the tissue sample, yielding a final reaction concentration of 50 mM sucrose. To initiate the reaction, we then placed the sample tube in a water bath at 37°C for 30 min, after which 750 μl of Glucostat solution (described above) was added to halt the enzymatic reaction and the liberated glucose was measured just as in our everted sleeve assay. We also incubated a tissue blank (the same 50 μl of homogenate sample and 200 μl of maleate buffer but no sucrose), reagent blank (50 μl of Hepes buffer and 200 μl of maleate buffer but no homogenate or sucrose), and substrate blank (50 μl of Hepes buffer and 200 μl of sucrose-containing maleate buffer but no homogenate). From the measured value of liberated glucose for the tissue sample, we subtracted the slight corrections caused by the substrate blank and the tissue blank minus the reagent blank.
Statistics.
Statistical significance was assessed by t tests and (in the study of pH effects) two-way ANOVA, with the P < 0.05 level taken as significant. Values are reported as means ± SEM, with sample size in parentheses.
RESULTS
We first describe results of experiments aimed at selecting optimal conditions for our sucrase assay in everted sleeves. We then describe measurements serving to validate the resulting assay procedure and to compare it with the classical homogenate assay procedure.
Selection of Assay Conditions.
Inhibiting the glucose transporter. We wished to assay sucrase activity by measuring the glucose accumulation in the bathing solution resulting from hydrolysis of sucrose. To prevent tissue glucose uptake out of the bathing solution from introducing an underestimate into our sucrase assay, we compared three means of inhibiting the brush-border glucose transporter. Preincubation of sleeves in Ringer’s solution containing 2.4 mM NaF for 5 or 10 min inhibited carrier-mediated glucose uptake by only 8% or 19%, respectively, compared with uptake by adjacent control sleeves preincubated without NaF. Preincubation in Na+-free solutions (all Na+ replaced with choline) inhibited glucose uptake in sleeves by 95%, but unfortunately it proved to stimulate sucrase activity by 160%, as measured in homogenates. Hence we turned to the glucose transporter inhibitor phlorizin (9).
Preincubation of sleeves for 5 or 10 min, in Ringer’s solution containing 50 mM d-glucose plus various phlorizin concentrations from 0.1 mM upward, showed that inhibition of glucose uptake (as compared with adjacent control sleeves from the same mouse, preincubated without phlorizin) reached an asymptotic value at 0.5 mM phlorizin. At 0.5 mM d-glucose, which was the average glucose concentration prevailing in our incubation tubes at the end of our 16-min incubations of sleeves for sucrase assays, a 10-min or 20-min preincubation with 0.5 mM phlorizin inhibited glucose uptake by 98.4% or 99.5%, respectively. (At 50 mM d-glucose and otherwise identical preincubation conditions, inhibition of glucose uptake was only by 94%, because the higher glucose concentration overcame some of the competitive inhibition.) The 20-min preincubation with 0.5 mM phlorizin had no effect on the tissue l-glucose space, a measure of passive glucose uptake.
To test whether phlorizin also inhibited sucrase itself, we turned to homogenates, because the sucrase assay in sleeves requires the presence of phlorizin and thus we could not compare sleeve sucrase activity in the presence and absence of phlorizin. A 20-min preincubation without phlorizin had no effect (as compared with no preincubation) on sucrase activity measured in homogenates, hence sucrase did not deteriorate during the extra 20 min required for the preincubation. However, a 20-min preincubation in 0.5 mM phlorizin inhibited by 10% the sucrase activity measured in homogenates. We therefore settled on preincubating for 20 min with 0.5 mM phlorizin to inhibit the glucose transporter nearly completely (by 99.5%), and multiplying the resulting measured sucrase activities by 1.0/0.9 = 1.11 to correct for the slight inhibition of sucrase itself.
Passive glucose uptake.
Like carrier-mediated glucose uptake, passive glucose uptake would tend to cause our sucrase assay to yield underestimates of true values, because some glucose liberated into the incubation solution would diffuse into the tissue and thus escape detection. However, in all three intestinal regions of three mice we found l-glucose uptake at 2, 4, 8, and 16 min not to differ significantly from zero (i.e., no difference between the l-glucose and polyethylene glycol spaces). Previous studies found measurable but very small l-glucose uptake (5, 10).
Incubation time.
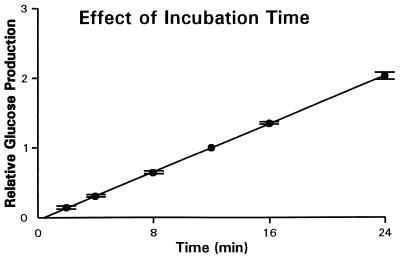
The incubation time for everted sleeves in sucrose-containing solutions should be long enough that the amount of released glucose is large and accurately measurable, but not so long that the sucrose substrate is becoming exhausted and hence that the curve of glucose production against time is becoming sublinear. Hence we incubated two sleeves from each of the three intestinal regions of each of three mice, and from each incubation solution we drew two duplicate samples of 250 μl each at 2, 4, 8, 12, 16, and 24 min. To permit comparison among mice, we normalized measured values of glucose production at any given time to the value measured at 12 min in the same sleeve. It turned out that, in each intestinal region, glucose production increased virtually linearly with time (R2 = 1.00 for linear regression) over the span of up to 24 min, with a y-intercept near 0 (Fig. 1). This linearity implies that sucrase properties are not changing over the incubation time and that sucrose substrate is not becoming exhausted. It also implies that inhibition of sucrase by liberated glucose (11) is negligible under our assay conditions (liberated glucose concentration only ca. 0.5 mM). Hence we settled on a 16-min incubation, well within the linear phase of glucose production.
Figure 1.
Relative glucose production as a function of incubation time, by everted sleeves from the proximal region of the intestine of six mice. Sleeves were incubated at 37°C in Ringer’s solution containing 50 mM sucrose (by isosmotic replacement of NaCl), and sequential samples were drawn at 2, 4, 8, 12, 16, and 24 min and assayed for glucose produced by action of brush-border sucrase on the sucrose. Cumulative glucose production up to a given time was divided by production up to 12 min by the same sleeve, to account for individual variation among mice. Points are average values for the six mice, and bars denote standard errors (omitted if the bar is smaller than the size of the data symbol). Note the good fit (R2 = 1.00) to a straight line passing close to the origin. Results for mid- and distal intestine give identically good fits (R2 = 1.00) to very similar straight lines.
Incubation concentration.
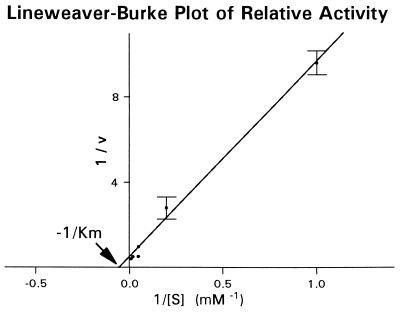
Measurements of glucose production as a function of sucrose concentration, when fitted to the Michaelis–Menten equation, yielded a Km value (Michaelis constant) of 18 mM for mouse intestine (Fig. 2). This value is close to the Km values of 20 mM determined for purified sucrase from Sprague–Dawley rats (12), and 13 mM for intestinal homogenates from Wistar rats (13). Hence we used an incubation concentration of 50 mM sucrose and multiplied the resulting activity by 1.36 (the factor calculated from the Michaelis–Menten equation with Km = 18 mM, C = 50 mM) to obtain the Vmax value. We decided not to use an incubation concentration above 50 mM because that would have required an excessive reduction in NaCl concentration to maintain solution osmolarity constant.
Figure 2.
Lineweaver–Burke plot of the sucrose concentration dependence of relative sucrase activity of everted sleeves of mouse intestine. From intestines of each of three mice, 15 consecutive sleeves centered around the mid-intestine were cut and incubated in Ringer’s solution containing 5, 20, or 50 mM sucrose (isosmotically replacing NaCl). To correct for variation in sucrase activity with intestinal position, all sleeves incubated in 5 or 50 mM sucrose were bracketed by adjacent sleeves incubated in 20 mM sucrose, in the sequence (from proximally to distally) 20, 5, 50, 20, 5, 50, 20, 5 … 20 mM. From each of three other mice, 15 sleeves were similarly incubated in Ringer’s solution containing 1, 20, or 100 mM sucrose, in the sequence (from proximally to distally) 20, 100, 1, 20, 100, 1, 20, 100 … 20 mM. For each mouse, we then calculated the average value of the ratio of activity at 5, 50, 1, or 100 mM to activity in the bracketing pair of sleeves at 20 mM. The figure is a Lineweaver-Burke plot of the fit of the resulting data to the Michaelis–Menten equation: i.e., the ordinate is (1/V), the reciprocal of activity (relative to activity at 20 mM in the same mouse), and the abscissa is (1/[S]), reciprocal sucrose concentration (mM−1). Values are means for three mice, and error bars are standard errors. Note the good fit (R2 = 1.00) to the straight line (1/V) = 9.1 (1/[S]) + 0.5. The negative inverse of the x-intercept is Km, the effective Michaelis constant of sucrase in everted sleeves of mouse intestine: 18 mM.
Reagent and tissue blanks.
Samples of Ringer’s solution containing 50 mM sucrose, carried through our normal experimental procedure (16-min incubation) except for not having an intestinal sleeve incubated in them, yielded an apparent glucose production of 0.003 ± 0.000 μmol (n = 36). This finding may reflect glucose or glucose-like impurities in reagents and/or nonenzymatic hydrolysis of sucrose. We measured this value in each experiment as a “reagent blank.” Similarly, sleeves carried through our normal experimental procedure except for omitting sucrose from the incubation solution yielded an apparent glucose production of 0.007 ± 0.000 μmol (n = 24) in the proximal and mid-intestine, 0.002 ± 0.000 μmol (n = 12) in the distal intestine. These values may reflect leakage of glucose or glucose-like substances out of the tissue. We measured this value for each intestinal region in each experiment as a “tissue blank.” We subtracted these reagent and tissue blanks from measured apparent glucose production values of every sleeve in all experiments. The reagent and tissue blanks represented on the average, respectively, 4.1 and 2.5%, 7.0 and 3.1%, and 18.4 and 22.7% of measured apparent glucose production in the proximal, mid-, and distal intestine, respectively. The percentage increases distally because sucrase activity itself (the major source of measured apparent glucose production) declines distally.
The foregoing results were the ones used to design details of our everted sleeve assay of sucrase activity, as described in Materials and Methods.
Validation of Everted Sleeve Sucrase Assay.
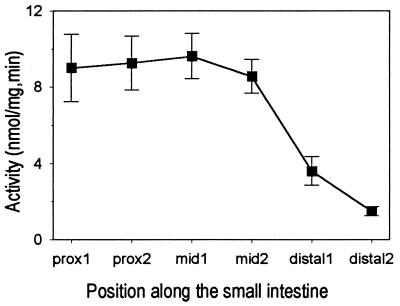
Reproducibility. The apparent coefficients of variation (CVs) of sucrase activity and of glucose transporter activity for 4–6 adjacent mid-intestinal sleeves from the same mouse were 16% and 9%, respectively. We refer to these as apparent CVs, because part of the variation represents a real proximal-to-distal gradient in sucrase activity along the intestine (Fig. 3); actual reproducibility is evidently better than 16% or 9%. When we calculated the mean sucrase activity for mid-intestinal sleeves of each of six mice, the inter-animal CV of those six mean values was 8%.
Figure 3.
Gradient of brush-border sucrase activity, measured in everted intestinal sleeves at six locations from proximally to distally along mouse small intestine. Each point represents the mean value, with standard error bars, for six mice. Ordinate units are nmol glucose produced by sucrase from sucrose, per mg wet weight of intestinal sleeve per min.
Next, we tested whether our everted sleeve assay of sucrase exhibited two characteristics already demonstrated for sucrase by other assay methods: inhibition by castanospermine and variation of activity with intestinal position.
Selective inhibition.
Castanospermine is an alkaloid that, at very low concentrations, quasi-irreversibly inhibits rat sucrase either in vitro in intestinal homogenates or on oral administration to rats (14, 15). We added castanospermine at 10−7 M to both the preincubation and the incubation solution for sleeves from each third of the intestine of each of four mice. The resulting very low values of apparent glucose production were not significantly different (P > 0.2 by t test) from the tissue blank measured in an adjacent sleeve for the mid- and distal intestine and were even lower (P < 0.01) than the tissue blank in the proximal intestine. Thus, castanospermine inhibition of sucrase in everted sleeves is complete.
Effect of intestinal position.
Sucrase activity assayed in sleeves sampled along the length of the small intestine exhibited a broad plateau over the proximal and mid-intestine, then declined steeply toward the distal intestine (Fig. 3). Essentially the same effect of position has been observed for sucrase activity assayed in intestinal homogenates (e.g., table 1 of ref. 13).
Comparison of sleeve and homogenate assays of sucrose.
We measured reproducibility of the classical Dahlqvist assay of sucrase in intestinal homogenates in our hands. Our experimental design was essentially the same as the design described above for measuring reproducibility in sleeves: we assayed sucrase in homogenates of six adjacent mid-intestinal tissues from each of five mice. The CVs proved to be, on the average, 16% between adjacent tissues of the same mouse, and 19% between tissues from corresponding positions of different mice. These CVs are the same as or somewhat higher than the corresponding CVs reported above for sleeves: 16% and 8%, respectively.
We then compared results of sucrase activity determinations by the sleeve and homogenate methods, in adjacent mid-intestinal tissues of the same mouse. Both determinations were at the same sucrose concentration (50 mM) and same incubation temperature (37°C). However, incubation solution pH, buffer, and ionic strength and composition differed. The homogenate determinations were under the classical Dahlqvist conditions (pH 6, 0.1 M Na+ maleate), whereas the sleeve determinations were under a slight modification of our usual conditions (pH 7.4, a balanced salt solution of ionic strength equivalent to 0.16 M NaCl, 20 mM Hepes buffer). [These conditions differ from our usual conditions only in replacement of our usual aerated 20 mM HCO3− buffer with 20 mM Hepes, to facilitate direct comparison with homogenate determinations under identical solution conditions, because homogenates cannot be aerated. In a separate experiment we established that Hepes and HCO3− buffers at pH 7.4 yield statistically indistinguishable sucrase values in adjacent sleeves of the same mouse: ratio of the former to the latter 1.05 ± 0.04 (n = 3).] We found that the classical homogenate method yielded sucrase activities twice as high as values from our sleeve method: ratio of the former to the latter 2.01 ± 0.09 (n = 4).
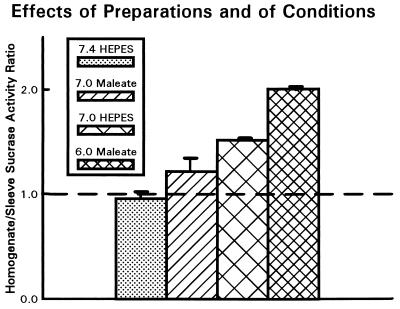
Fig. 4 resolves the factors underlying this difference, which could potentially involve an effect of the differing preparations (homogenate vs. sleeve), differing pHs (6.0 vs. 7.4), and/or differing solution composition including choice of buffer, ionic strength, and ionic composition (0.1 M Na+ maleate vs. 0.15 M Ringer’s solution with Hepes buffer). It turns out that there is no effect of preparation: under identical solution conditions (pH 7.4 Ringer’s solution with Hepes) the ratio of homogenate to sleeve activities [0.96 ± 0.05 (n = 4)] is statistically indistinguishable from 1.0. However, at identical pH (7.0) there is an effect of solution composition: sucrase activity of homogenates in Ringer’s solution with Hepes is 1.26 ± 0.04 (n = 4) times activity of homogenates in 0.1 M Na+ maleate. In solutions of otherwise identical composition there is an even larger effect of pH: activity of homogenates in Ringer’s solution with Hepes at pH 7.0 is 1.57 ± 0.04 (n = 4) times that at pH 7.4, and activity in 0.1 Na+ maleate at pH 6.0 is 1.68 ± 0.10 (n = 4) times that at pH 7.0.
Figure 4.
Comparison of sleeve and homogenate sucrase assays. Sucrase activity of a homogenate of a 2-cm length of mid-intestine was compared with activities measured from sleeves of the two adjacent pieces of intestine of the same mouse. All incubations were at 37°C and 50 mM sucrose, and solution conditions were always pH 7.4 Ringer’s solution with 20 mM Hepes for the sleeves, but solution conditions for the homogenates varied: either the same condition as for the sleeves (pH 7.4 Ringer’s solution with 20 mM Hepes), or else 0.1 M Na+ maleate at pH 7.0, pH 7.0 Ringer’s solution with 20 mM Hepes, or 0.1 M Na+ maleate at pH 6.0 (see symbols on figure). Ordinate values are mean values of ratios of homogenate activities to pH 7.4 Ringer’s solution/20 mM Hepes sleeve activity in the same individual mouse, with error bars denoting the SEM. (n = 4 mice). Note that sleeves and homogenates yield identical sucrase activities under identical solution conditions (ratio near 1.0 for homogenates in pH 7.4 Hepes); and that the classical Dahlqvist assay in homogenates (pH 6.0 maleate) yields a ratio of 2.0 (i.e., activities double our sleeve values measured in pH 7.4 Ringer’s solution), because sucrase activity increases considerably with decreasing pH (compare pH 6.0 and 7.0 maleate, or pH 7.0 and 7.4 Hepes) and despite the somewhat lower sucrase activity in maleate than in Hepes at the same pH (compare pH 7.0 maleate and pH 7.0 Hepes).
Thus, our sleeve assay under our experimental conditions yields half the sucrase activity of the Dahlqvist homogenate assay under its usual conditions. This result is because the Dahlqvist assay is conducted at a lower pH (6.0 instead of 7.4), close to the pH optimum that we find for mouse intestinal sucrase. Rat sucrase also exhibits a pH optimum near 6 (13). This large effect of pH offsets the smaller (21%) decrease in sucrase activity that the Dahlqvist assay incurs by using 0.1 M Na+ maleate instead of the HCO3− (or Hepes) Ringer’s solution of our sleeve assay. Under conditions of identical pH and solution composition, the sleeve and homogenate methods yield statistically indistinguishable results.
DISCUSSION
We have described a method for assaying intestinal brush-border sucrase in an intact intestinal preparation. The preparation is identical to the one used in many previous papers from this laboratory for assaying brush-border transporters (e.g., refs. 5, 10, and 16). However, sucrase usually has been assayed instead in intestinal homogenates. The previous sucrase assay most nearly similar to ours was that of Robinson et al. (15), which differed in three respects: it inhibited glucose uptake with NaF, which we found much less effective than phlorizin; it incubated for 45 min instead of 16 min; and it stirred by shaking at 215 rpm instead of by using a stir bar rotating at 1,200 rpm.
With the everted sleeve preparation, we have confirmed three familiar properties of sucrase established in homogenate studies: proximal-to-distal activity gradient, inhibition by castanospermine, and (in the following paper: ref. 6) substrate-dependent induction. The sleeve preparation yields the same value of sucrase activity as does the more familiar homogenate preparation when solution pH and composition are the same. This finding is not surprising, because sucrase is an external membrane-bound enzyme whose activity one might expect to be much the same whether its membrane is intact or disrupted. It also is not surprising that homogenization, by introducing an additional source of variability, causes the coefficient of variation to be slightly higher for homogenate assays than for sleeve assays.
Most scientists assaying intestinal sucrase do so for reasons other than its relation to the glucose transporter. Our results give such scientists every reason to continue using the familiar Dahlqvist assay (but they should be aware that the 0.1 M Na+ maleate buffer used in the Dahlqvist assay yields sucrase activities 21% lower than those measured in a more physiological HCO3− Ringer’s solution). Instead, the advantage of our assay lies in its suitability for quantitatively comparing hydrolase and transporter activities, measured in the same preparation under the same conditions. The following paper (6) applies this principle to sucrase and the glucose transporter.
Acknowledgments
It is a pleasure to acknowledge our debts to Ricardo Castillo, Kimberly Hammond, Susan Jackson, Ann Reisenauer, and Kenneth Tsuboi for generous help or suggestions; to David Alpers, Carlos Martinez del Rio, and Ernest Wright for valuable criticism of an earlier draft of the manuscripts, stimulating us to do further experiments; to Timothy O’Connor for criticism of a later draft; and to Drs. Ekkehard Bohme and Keith Robinson of Marion Merrell Dow Research Institute for their generous gift of castanospermine. The work for this and the following paper (6) was supported by National Institutes of Health Grants GM14772, HD30745, and DK17328 (UCLA Center for Ulcer Research and Education).
ABBREVIATION
- CV
coefficient of variation
References
- 1.Cornish-Bowden A, Cárdenas M L. Control of Metabolic Processes. New York: Plenum; 1990. [Google Scholar]
- 2.Dahlqvist A. Anal Biochem. 1964;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- 3.Dahlqvist A. Anal Biochem. 1968;22:99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- 4.Dahlqvist A. Scand J Clin Lab Invest. 1984;44:169–172. doi: 10.3109/00365518409161400. [DOI] [PubMed] [Google Scholar]
- 5.Karasov W H, Diamond J M. J Comp Physiol. 1983;152:105–116. [Google Scholar]
- 6.Weiss S L, Lee E A, Diamond J M. Proc Natl Acad Sci USA. 1998;95:2117–2121. doi: 10.1073/pnas.95.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond J M, Karasov W H. J Physiol. 1984;349:419–440. doi: 10.1113/jphysiol.1984.sp015165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuboi K K, Schwartz S M, Burrill B H, Kwong L K, Sunshine P. Biochim Biophys Acta. 1979;554:234–248. doi: 10.1016/0005-2736(79)90021-x. [DOI] [PubMed] [Google Scholar]
- 9.Alvarado F, Crane R K. Biochem Biophys Acta. 1964;93:116–135. doi: 10.1016/0304-4165(64)90266-1. [DOI] [PubMed] [Google Scholar]
- 10.Karasov W H, Solberg D H, Diamond J M. Am J Physiol. 1985;249:G271–G283. doi: 10.1152/ajpgi.1985.249.2.G271. [DOI] [PubMed] [Google Scholar]
- 11.Gray G M, Ingelfinger F J. J Clin Invest. 1966;45:388–398. doi: 10.1172/JCI105354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conklin K A, Yamashiro K N, Gray G M. J Biol Chem. 1975;25:5735–5741. [PubMed] [Google Scholar]
- 13.Blair D G R, Tuba J. Can J Biochem Physiol. 1963;41:905–916. [PubMed] [Google Scholar]
- 14.Rhinehart B L, Robinson K M, Payne A J, Wheatley M E, Fisher J L, Liu P S, Cheng W. Life Sci. 1987;41:2325–2331. doi: 10.1016/0024-3205(87)90546-7. [DOI] [PubMed] [Google Scholar]
- 15.Robinson K M, Heineke E W, Begovic M E. J Nutr. 1990;120:105–111. doi: 10.1093/jn/120.1.105. [DOI] [PubMed] [Google Scholar]
- 16.Hammond K A, Diamond J M. Physiol Zool. 1994;67:282–303. [Google Scholar]