Abstract
OBJECTIVE
To validate a rat model of endometriosis using cDNA microarrays by identifying common gene expression patterns beween experimental and natural disease.
DESIGN
Autotransplantation rat model.
SETTING
Medical school department.
ANIMALS
Female Sprague-Dawley rats.
INTERVENTIONS
Endometriosis was surgically-induced by suturing uterine horn implants next to the small intestine’s mesentery. Control rats received sutures with no implants. After 60 days, endometriotic implants and uterine horn were obtained.
MAIN OUTCOME MEASURES
Gene expression levels determined by cDNA microarrays and QRT-PCR.
METHODS
Cy5-labeled cDNA was synthesized from total RNA obtained from endometriotic implants. Cy3-labeled cDNA was synthesized using uterine RNA from a control rat. Gene expression levels were analyzed after hybridizing experimental and control labeled cDNA to PIQOR™ Toxicology Rat Microarrays (Miltenyi Biotec) containing 1,252 known genes. Cy5/Cy3 ratios were determined and genes with >2-fold higher or <0.5-fold lower expression levels were selected. Microarray results were validated by QRT-PCR.
RESULTS
We observed differential expression of genes previously shown to be upregulated in patients, including growth factors, inflammatory cytokines/receptors, tumor invasion/metastasis factors, adhesion molecules, and anti-apoptotic factors.
CONCLUSIONS
This study presents evidence in support of using this rat model to study the natural history of endometriosis and test novel therapeutics for this incurable disease.
Keywords: endometriosis, animal model, rat, validation, cDNA microarrays
INTRODUCTION
Endometriosis is a gynecological disease associated with severe pelvic pain and infertility, thus affecting the reproductive health and quality of life of millions of women around the world (1). The pathogenesis of endometriosis is still unknown, and the mechanisms whereby endometriotic lesions establish, progress, and migrate to extrapelvic sites are not well understood (2). Human studies are limited by ethical and practical considerations including: i) the need for repeated surgical procedures to monitor disease progression; ii) difficulties in controlling variables (individual genetic variation, environment, diet); and iii) difficulties in studying the early steps of disease development. Animal models of this disease, such as the rat autotransplantation model developed by Vernon and Wilson in 1985 (3), would therefore offer an invaluable tool to study the early steps of disease pathogenesis and to test novel therapeutics.
Despite the clear advantages of using animal models in biomedical research, whether results obtained using experimental models of disease could be extrapolated to the human scenario remains controversial (4–7). Therefore, animal models need to be carefully evaluated to ensure that they accurately represent the disease they are meant to mimic. Care must always be taken when making extrapolations from a rat model to the human, especially since the rat does not have spontaneously occurring endometriosis and it does not menstruate. However, rats with surgically induced endometriosis exhibit similar pathophysiological symptoms as humans with spontaneous endometriosis. The rat ectopic and eutopic endometrium have the same differential secretory pattern of protein synthesis and are histologically similar to what has been observed in human endometriosis. Furthermore, rats with surgically induced endometriosis exhibit a decrease in their fecundity and NK cell activity that parallels the human disease (8–11). Moreover, we have recently shown that, similar to the human disease, this animal model is characterized by a dysregulation of the TNF system systemically and at the peritoneal level (12).
The aim of the present study was to further evaluate the validity of this animal model by identifying functional biological categories in common with human disease. We propose that gene expression profiling and functional characterization of transcripts that are differentially regulated in the experimental condition could serve as the basis for validating physiological mechanisms at play in the natural disease. Such transcripts may, in turn, represent possible therapeutic targets for this incurable condition. There have been some reports of gene expression and protein production patterns that are shared by experimental and human disease, but these are mostly single gene/protein studies. cDNA microarrays are increasingly being used to identify gene expression profiles associated with complex genetic diseases of unknown etiology (13). This powerful technology reveals disease-specific patterns in gene expression, thereby accelerating the identification of candidate genes (14).
In order to expedite the identification of relevant genes that may play a role in the establishment, survival and growth of ectopic endometrium, we applied cDNA microarray technology to compare gene expression patterns of ectopic and normal endometrium in a well-known rat model of endometriosis. To validate the cDNA microarray results, selected genes were further evaluated by RT-PCR, and gene expression profiles were analyzed using GoMiner™, a systems biology data mining tool (15). GoMiner™ identified significantly enriched Gene Ontology (GO) categories, and the results were compared with what has already been reported in the literature for the human disease, including previous cDNA microarray studies on endometriosis (16–22). In this study we report the identification of common pathway-specific patterns of gene expression that support the use of this model for pre-clinical drug testing.
MATERIALS AND METHODS
The experiments reported herein were performed in accordance with the principles described in the “Guide for the Care and Use of Laboratory Animals”, Publication No. DHMS (NIH) 86-23.
Animal Model and Collection of Tissues
Studies were performed with 15 female Sprague-Dawley rats weighing 275–300g (Southern Veterinary Service, PSM, PR). All animals were maintained in restricted-access rooms with a controlled temperature (23°C) and a 12 hour light-dark cycle. Standard laboratory chow and drinking water were provided ad libitum. All experimental procedures involving animals were approved by the Animal Care and Use Committee at Ponce School of Medicine.
Intestinal endometriosis was induced surgically in mature female rats under pentobarbital anesthesia, based on the method by Vernon and Wilson (1985) (3). Briefly, the distal 1 cm of the right uterine horn was removed and immersed in warm (37°C) sterile culture medium. The endometrium was exposed by opening lengthwise with a pair of sterile scissors and four pieces of uterine horn measuring 2mm × 2mm were cut. Four implants of uterine tissue were sutured next to the mesenteric vessels of the small intestine in the experimental group (n = 9). In the control group (n = 6), four silk sutures were attached to the mesentery of the intestine without implants, and the uterine horn was massaged with fingertips for two minutes. All animals were allowed to recover for 60 days before sacrificing with an overdose of sodium pentobarbital.
Vaginal cytologic smears were carried out for all rats before and after surgical intervention, as well as at the time of sacrifice, in order to monitor their reproductive cyclicity. A laparotomy was performed and the peritoneal cavity was opened and systematically examined for the presence of implants and the original sutures. Peritoneal fluid was aseptically aspirated using a sterile micropipette taking care not to contaminate with blood, a smear with Wright’s stain was prepared for quantification of white blood cells (wbc), and the remainder stored at −80°C for RNA analyses. The number of wbc per high power field was determined in 5 randomly selected fields as previously described (12). The classification of implants in terms of grades of growth was carried out following the criteria described by Ingelmo et al. (1999) (23). Tissue samples (e.g., uterine horn, endometrial implants), were immediately immersed in RNAlater reagent (Ambion, Austin, TX) and stored in tightly closed containers at −80°C.
Total RNA Extraction and Linear Amplification
Total RNA was isolated by standard methods using a commercially available RNA isolation kit (Qiagen, Valencia, CA). The integrity of the extracted RNA was evaluated with capillary electrophoresis using a Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA). RNA quantity was determined by spectrophotometry at OD260 in a GeneQuant™ DNA/RNA calculator (Pharmacia, Piscataway, NJ). Linear amplification of RNA was done following a modified protocol of a previously described method (24). The quality and quantity of amplified RNA (aRNA) were determined by capillary electrophoresis and spectrophotometry as described above.
cDNA Microarrays
Rat-specific PIQOR™ Toxicology arrays (Miltenyi Biotec, Cologne, Germany) consisting of 1,252 selected cDNA fragments were used to generate gene expression profiles of ectopic (array #1: a pooled sample of four endometriotic vesicles from three rats, 1–2 vesicles from each) versus eutopic endometrium (array #2: uterine rat tissue from a control rat). Two micrograms (μg) of aRNA from eutopic and ectopic endometrium were reverse transcribed in a reaction containing 8 μl of 5x First Strand Buffer (Invitrogen, Carlsbad, CA), 2μl Primer Mix (oligo dT and randomeres, Miltenyi Biotech GmbH, Cologne, Germany), 2 μl low C-dNTPs (10mM dATP, 10 mM dGTP, 10 dTTP, 4 mM dCTP), 2 μl FluoroLink Cy3/5-dCTP (Amersham Pharmacia Biotech, Freiburg, Germany), respectively, 4 μl 0.1 M DTT and 1 μl RNasin (20–40 U) (Promega, Madison, WI). Reverse transcription was conducted by incubation with SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA) at 42°C for 30 min.
After RNAase H treatment, Cy3- and Cy5-labeled samples were combined and purified using Qiaquick columns (Qiagen, Valencia, CA). Samples diluted 1:1 in pre-warmed 2x hybridization solution were then subjected to a predefined PIQOR rat toxicology microarray. Hybridization and post-hybridization washes were carried out as described before (25). Slides were scanned by Miltenyi on a ScanArray 4000 Lite scanner (Perkin-Elmer, Wellesley, MA). ImaGene software version 4.1 was used for signal quantification and analysis as described (26). Normalized ratios are shown as Cy5 signal intensity divided by Cy3 signal intensity of the respective gene. Microarray experiments were performed according to MIAME guidelines (27).
Validation of Microarray Data by Real-Time RT-PCR
To validate the gene expression data obtained with cDNA microarrays, real-time RT-PCR was performed on 9 selected genes, using total RNA from endometriotic vesicles from experimental rats (n = 6) and uterine horn tissues of control animals (n = 5). The six experimental rats used for RT-PCR did not overlap with those used for microarrays (n = 3). The five control rats used for the RT-PCR experiments did not include the control rat used for microarrays. Genes were selected based on level of expression and plausible role in disease etiology, and included PDGRB (2.1-fold), LOXL1 (2.1-fold), IL2RG (3.2-fold), BCL2 (9.1-fold), OPG (4.8-fold), MMP9 (24.3-fold), TGFB (2.4-fold), APRIL (3.1-fold), and PGH2 (2.2-fold). Primers were designed based on published sequence data using Primer3 software and synthesized by a commercial vendor (IDT DNA Technology, Inc., Coralville, IA).
In brief, total RNA was isolated from tissues using the Trizol LS reagent (Invitrogen, Carlsbad, CA). To remove contaminating DNA, samples were treated with DNAse I (DNA-free, Ambion, Austin, TX). Reverse transcription was performed on the PTC-200 thermalcycler (MJ Research, Waltham, MA) using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) following the manufacturer’s protocol. After cDNA synthesis, PCR reactions were performed in triplicate with specific oligo-primer pairs using the iQ SYBR Green Super Mix kit according to the manufacturer’s recommendations (Bio-Rad, Hercules, CA). The PCR amplification profile was as follows: 94°C for 4 min followed by 50 cycles of denaturation at 94°C/30 sec, gene-specific annealing temperature/30 sec, and extension at 72°C/40 sec. Annealing temperatures per primer set were determined empirically. A melting curve was generated after each run to verify the specificity of the primers, shown by the presence of a single band and no primer-dimer artifacts. Real-time analysis of PCR amplification was performed with an iCycler iQ Optical System software, version 3.0a (Bio-Rad, Hercules, CA).
Relative expression levels were calculated for each sample after normalization against the housekeeping gene HPRT, using the ΔΔCt method for comparing relative fold-expression differences (28). Statistical analysis was performed using unpaired two-tailed t tests to compare relative mRNA expression levels in experimental and control samples (GraphPad InStat 3). Statistical significance was defined as a p value of <0.05.
GO Category Analysis of Gene Expression Data
In order to better understand the biological meaning of the cDNA microarray results obtained, gene expression data was analyzed using GoMiner™, a freely available computer-based data mining tool that automates the functional categorization of differentially expressed genes according to their biological, cellular and molecular functions. GoMiner™, which uses the hierarchical structure of the Gene Ontology program, calculates enrichment or depletion of functional categories (Biological Process, Cellular Component, and Molecular Function). Potentially important categories are easily identified by sorting quantitative and statistical results by either enrichment factor or p-value. GoMiner™ allows for bioinformatic integration by providing links to external databases such as LocusLink, PubMed, GeneCards, NCBI’s Structure Database, and pathway maps (BioCarta and KEGG).
RESULTS
Animal Model
All rats were sacrificed at the same time, exactly 60 days after surgery. Vaginal cytologic smears were carried out for all rats before and after the surgical intervention in order to monitor their reproductive cyclicity. The estrous cycle stage (i.e., estrus, anestrus, proestrus, metestrus) at the time of sacrifice was also recorded. While rats are surgically induced when in estrous (or proestrus in some cases), there was a spread among the various cycle stages within both control and experimental rats at the time of sacrifice; however, there was no statistically significant difference in the cycle stage between the groups. Also, there was no difference in the body weight between experimental and control rats at the time of sacrifice.
After sacrifice, classification of the implants was performed as described by Ingelmo (1999) (23). Briefly, vesicles at the suture sites (four sutures per rat) were classified in grades 1 to 4 (1 = no vesicle, 2 = vesicle is < 2 mm in diameter, 3 = vesicle is ≥ 2 mm but < 4.5 mm in diameter, 4 = vesicle is ≥ 4.5 mm in diameter). The experimental (implanted) rats (n = 9) developed a vesicle in 67% of their sutures. None of the six control rats developed a vesicle at the site of the suture. The peritoneal fluid of experimental rats had a significantly higher wbc number (48.85 ± 12.38 cells per high-power field) compared to controls (8.96 ± 1.92; p<0.05).
cDNA Microarray Analysis
From the 1,252 genes printed on the microarrays, 123 were found to be more than twofold overexpressed and 45 were underexpressed (≥0.5-fold) in ectopic endometrium as compared to eutopic tissue (Table 1). From this gene list, genes that have been previously associated to human endometriosis were identified by conducting a comprehensive search of the literature using PubMed. Novel genes which have not been previously associated to human disease were also identified; further characterization of such transcripts is expected to result in the generation of new hypotheses that would need to be tested.
Table 1.
Genes differentially regulated in ectopic and eutopic endometrium of a rat model of endometriosis
| NAME (by fold-expression) | Fold-expression | % SDa | Previously shown to be associated to human endometriosis |
|---|---|---|---|
| MMP12: (MMP12 OR HME) MACROPHAGE METALLOELASTASE PRECURSOR | 70.13 | 7 % | |
| MMP9: (MMP9 OR CLG4B) 92 KDA TYPE IV COLLAGENASE PRECURSOR | 24.33 | 22 % | 46–50 |
| OPN: (SPP1 OR OPN) OSTEOPONTIN PRECURSOR (BONE SIALOPROTEIN 1 | 24.14 | 3 % | 51 |
| MMP13: (MMP13) COLLAGENASE 3 PRECURSOR | 22.30 | 74 % | |
| FABE: (FABP5 OR MAL1 OR KLBP OR FABPE) FATTY ACID-BINDING PROTEIN, EPIDERMAL (E-FABP) | 14.55 | 7 % | |
| CCL3_RAT: (CCL3 OR SCYA3 OR G0S19-1 OR MIP1A) SMALL INDUCIBLE CYTOKINE A3 PRECURSOR (CCL3) (MACROPHAGE INFLAMMATORY PROTEIN 1-ALPHA) | 13.78 | 30 % | |
| ITGB2: (ITGB2 OR CD18) INTEGRIN BETA-2 PRECURSOR (CELL SURFACE ADHESION GLYCOPROTEINS LFA-1/CR3/P150,95 BETA-SUBUNIT | 13.67 | 11 % | 52 |
| CCL9_RAT: (SCYA9 OR SCYA10 OR MRP2) SMALL INDUCIBLE CYTOKINE A9 PRECURSOR (MACROPHAGE INFLAMMATORY PROTEIN 1-GAMMA) (MIP-1-GAMMA) | 12.35 | 20 % | |
| FABI: (FABP2) FATTY ACID-BINDING PROTEIN, INTESTINAL (I-FABP) (FABPI). | 10.26 | 57 % | |
| LBP: (LBP) LIPOPOLYSACCHARIDE-BINDING PROTEIN PRECURSOR (LBP). | 9.99 | 21 % | |
| DMBT1: (DMBT1) DMBT1 PROTEIN. EBNERIN. (CRPD OR CRP OR CRP-DUCTIN) CRP-DUCTIN PRECURSOR (CRP). | 9.36 | 5 % | |
| BCL2A1: (BCL2A1 OR BFL1 OR GRS OR BCL2L5) BCL2-RELATED PROTEIN A1 (BFL-1 PROTEIN) (HEMOPOIETIC-SPECIFIC EARLY RESPONSE PROTEIN) (GRS PROTEIN). | 9.14 | 16 % | 53–55 |
| S100A9: (S100A9 OR CAGB) CALGRANULIN B (MIGRATION INHIBITORY FACTOR-RELATED PROTEIN 14) (MRP-14) (P14) (LEUKOCYTE L1 COMPLEX HEAVY CHAIN) | 8.99 | 21 % | 56 |
| C3: (C3) COMPLEMENT C3 PRECURSOR. | 7.81 | 35 % | 18,57 |
| ITGAM: (ITGAM OR CR3A OR CD11B) INTEGRIN ALPHA-M PRECURSOR (CELL SURFACE GLYCOPROTEIN MAC-1 ALPHA SUBUNIT) (CR-3 ALPHA CHAIN) (CD11B) | 6.88 | 44 % | |
| CD36: (CD36 OR GP4 OR GP3B OR FAT) PLATELET GLYCOPROTEIN IV (GPIV) (GPIIIB) (CD36 ANTIGEN) (PAS-4 PROTEIN) | 6.70 | 14 % | |
| CTGF: (CTGF OR HCS24) CONNECTIVE TISSUE GROWTH FACTOR PRECURSOR (HYPERTROPHIC CHONDROCYTE-SPECIFIC PROTEIN 24). | 6.57 | 12 % | 58 |
| ANPEP: (ANPEP OR PEPN OR APN OR CD13 OR LAP1 OR LAP-1) AMINOPEPTIDASE N (MICROSOMAL AMINOPEPTIDASE) (GP150) | 6.48 | 5 % | |
| DUSP1: (DUSP1 OR PTPN10 OR MKP1 OR CL100 OR HVH1) DUAL SPECIFICITY PROTEIN PHOSPHATASE 1 (MAP KINASE PHOSPHATASE-1) (MKP-1) | 6.38 | 9 % | 59 |
| ATF3: (ATF3) CYCLIC-AMP-DEPENDENT TRANSCRIPTION FACTOR ATF-3 (ACTIVATING TRANSCRIPTION FACTOR 3). | 6.33 | 18 % | |
| FGR: (FGR OR SRC2) PROTO-ONCOGENE TYROSINE-PROTEIN KINASE FGR (P55-FGR) (C-FGR). | 5.94 | 44 % | |
| LIPL: (LPL OR LIPD) LIPOPROTEIN LIPASE PRECURSOR (LPL). | 5.94 | 6 % | |
| TNC: (TNC OR HXB OR TN-C) TENASCIN PRECURSOR (TN) (CYTOTACTIN) (NEURONECTIN) (GLIOMA-ASSOCIATED-EXTRACELLULAR MATRIX ANTIGEN) | 5.90 | 14 % | 60 |
| INTEGRINB6: (ITGB6) INTEGRIN BETA-6 PRECURSOR. | 5.61 | 22 % | 19,61,62 |
| CXCR4: (CXCR4 OR LESTR OR CMKAR4 OR SDF1R) C-X-C CHEMOKINE RECEPTOR TYPE 4 (CXC-R4) (CXCR-4) (STROMAL CELL-DERIVED FACTOR 1 RECEPTOR) | 5.48 | 5 % | |
| SCYB1_RAT: (SCYB1 OR GRO1 OR GRO OR MGSA) GROWTH REGULATED PROTEIN PRECURSOR (CXCL1) (CYTOKINE-INDUCED NEUTROPHIL CHEMOATTRACTANT) (CINC-1) (PLATELET-DERIVED GROWTH FACTOR- INDUCIBLE PROTEIN KC) | 5.43 | 38 % | 63 |
| CXCL4_RAT: (SCYB4 OR PF4) PLATELET FACTOR 4 PRECURSOR (PF-4) (ONCOSTATIN A) | 4.92 | 8 % | |
| MUC1: (MUC1) MUCIN 1 PRECURSOR (MUC-1) (POLYMORPHIC EPITHELIAL MUCIN) (PEM) (PEMT) (TUMOR-ASSOCIATED MUCIN) (CARCINOMA-ASSOCIATED MUCIN) (TUMOR-ASSOCIATED EPITHELIAL MEMBRANE ANTIGEN) (EMA) | 4.92 | 22 % | 64,65 |
| CD53: (CD53 OR MOX44 OR OX-44) LEUKOCYTE SURFACE ANTIGEN CD53 (CELL SURFACE GLYCOPROTEIN CD53) (LEUKOCYTE ANTIGEN MRC OX-44). | 4.87 | 6 % | |
| MMP7: (MMP7 OR MPSL1 OR PUMP1) MATRILYSIN PRECURSOR (UTERINE METALLOPROTEINASE) (MATRIX METALLOPROTEINASE-7) (MMP-7) | 4.85 | 12 % | |
| S100A8: (S100A8 OR CAGA) CALGRANULIN A (MIGRATION INHIBITORY FACTOR-RELATED PROTEIN 8) (MRP-8) (CYSTIC FIBROSIS ANTIGEN) (CFAG) (P8) | 4.81 | 18 % | 56 |
| TNFRSF11B: (TNFRSF11B OR OPG OR OCIF) OSTEOPROTEGERIN PRECURSOR (OSTEOCLASTOGENESIS INHIBITORY FACTOR) (OCIF) (TUMOR NECROSIS FACTOR RECEPTOR SUPERFAMILY MEMBER 11B). | 4.81 | 23 % | 66 |
| CD3Z-CD3H: (CD3Z OR T3Z OR TCRZ) T-CELL SURFACE GLYCOPROTEIN CD3 ZETA CHAIN PRECURSOR (T-CELL RECEPTOR T3 ZETA CHAIN) (CD3Z OR CD3H) | 4.74 | - % | |
| UPA: (PLAU) UROKINASE-TYPE PLASMINOGEN ACTIVATOR PRECURSOR (UPA) (U-PLASMINOGEN ACTIVATOR). | 4.71 | 8 % | 67,68 |
| CCL4: (SCYA4 OR MIP1B OR LAG1) SMALL INDUCIBLE CYTOKINE A4 PRECURSOR (MACROPHAGE INFLAMMATORY PROTEIN 1-BETA) (MIP-1-BETA) (T-CELL ACTIVATION PROTEIN 2) (ACT-2) | 4.54 | - % | |
| MOA-TF: (MITF OR MI) MICROPHTHALMIA-ASSOCIATED TRANSCRIPTION FACTOR (PUTATIVE TRANSCRIPTION FACTOR MI) | 4.42 | 40 % | |
| RAC2: (RAC2) RAS-RELATED C3 BOTULINUM TOXIN SUBSTRATE 2 (P21-RAC2) (SMALL G PROTEIN) (GX) | 4.31 | 7 % | |
| LUMICAN: (LDC) LUMICAN PRECURSOR (LUM) (KERATAN SULFATE PROTEOGLYCAN). | 4.27 | 19 % | |
| CD72: (LY-32 OR LYB-2 OR CD72) B-CELL DIFFERENTIATION ANTIGEN CD72 (LYB-2). | 4.23 | - % | |
| IL18: (IL18 OR IGIF) INTERLEUKIN-18 PRECURSOR (IL-18) (INTERFERON-GAMMA INDUCING FACTOR) (INTERLEUKIN-1 GAMMA) (IL-1 GAMMA). | 4.15 | 20 % | 69 |
| IL1B: (IL1B) INTERLEUKIN-1 BETA PRECURSOR (IL-1 BETA) (CATABOLIN). | 3.96 | 20 % | 70 |
| SELPLG: (SELPLG) P-SELECTIN GLYCOPROTEIN LIGAND 1 PRECURSOR (PSGL-1) (SELECTIN P LIGAND) (CD162 ANTIGEN). | 3.93 | 31 % | |
| THAS: (TBXAS1 OR CYP5) THROMBOXANE-A SYNTHASE (TXA SYNTHASE) (TXS). | 3.91 | 20 % | |
| THROMBOSPONDIN1: (THBS1 OR TSP1 OR TSP) THROMBOSPONDIN 1 PRECURSOR. | 3.88 | 16 % | 71 |
| CYP7B1: (CYP7B1) CYTOCHROME P450 7B1 (OXYSTEROL 7-ALPHA-HYDROXYLASE) | 3.83 | 27 % | |
| LYL1: (LYL1) LYL-1 PROTEIN. | 3.73 | 63 % | |
| TEF_BAD: (TEF) THYROTROPH EMBRYONIC FACTOR. | 3.67 | 89 % | |
| MMP11: (MMP11 OR STMY3) STROMELYSIN-3 PRECURSOR (MATRIX METALLOPROTEINASE-11) (MMP-11) | 3.66 | 14 % | |
| NDRG1: (NDRG1 OR NRD1 OR RTP OR DRG1 OR CAP43 OR NDRL OR TDD5) NDRG1 PROTEIN (DIFFERENTIATION-RELATED GENE 1 PROTEIN) (DRG1) | 3.60 | 9 % | |
| CD74: (CD74 OR DHLAG OR II) HLA CLASS II HISTOCOMPATIBILITY ANTIGEN, GAMMA CHAIN (HLA-DR ANTIGENS ASSOCIATED INVARIANT CHAIN) | 3.56 | 4 % | 16,18 |
| ALCAM: (ALCAM) CD166 ANTIGEN PRECURSOR (ACTIVATED LEUKOCYTE-CELL ADHESION MOLECULE) (ALCAM) | 3.50 | 5 % | |
| CD79A: (CD79A OR IGA OR MB1 OR MB-1) B-CELL ANTIGEN RECEPTOR COMPLEX ASSOCIATED PROTEIN ALPHA-CHAIN PRECURSOR (IG-ALPHA) | 3.49 | 12 % | |
| JUNB: (JUNB) TRANSCRIPTION FACTOR JUN-B (G0S3). | 3.48 | 4 % | |
| ASC: (ASC OR TMS1 OR PYCARD OR CARD5) APOPTOSIS-ASSOCIATED SPECK-LIKE PROTEIN (TARGET OF METHYLATION-INDUCED SILENCING 1) (CASPASE RECRUITMENT DOMAIN PROTEIN 5). | 3.46 | 8 % | |
| VAV: (VAV) VAV PROTO-ONCOGENE | 3.46 | 15 % | |
| ITGA4: (ITGA4 OR VLA-4) INTEGRIN ALPHA-4 PRECURSOR (INTEGRIN ALPHA-IV) (VLA-4) (CD49D) (LYMPHOCYTE-PEYER’S PATCH ADHESION MOLECULES ALPHA SUBUNIT) (LPAM ALPHA SUBUNIT). | 3.43 | 13 % | |
| FBLN2: (FBLN2) FIBULIN-2 PRECURSOR. | 3.31 | 13 % | |
| COL11A1: (COL11A1) COLLAGEN ALPHA 1(XI) CHAIN PRECURSOR. | 3.28 | 26 % | 19,72,73 |
| IL2RG: (IL2RG) CYTOKINE RECEPTOR COMMON GAMMA CHAIN PRECURSOR (GAMMA-C) (INTERLEUKIN- 2 RECEPTOR GAMMA CHAIN) (IL-2R GAMMA CHAIN) (P64) (CD132 ANTIGEN). | 3.18 | 14 % | 22 |
| ZF9: (COPEB OR KLF6 OR BCD1 OR CPBP) CORE PROMOTER ELEMENT-BINDING PROTEIN (B-CELL DERIVED PROTEIN 1) (PROTO-ONCOGENE BCD1) | 3.16 | 7 % | |
| CCR5-CCR2: (CCR5 OR CMKBR5) C-C CHEMOKINE RECEPTOR TYPE 5 (C-C CKR-5) (CC-CKR-5) (CCR-5) (CCR5) (HIV-1 FUSION CO-RECEPTOR) (CHEMR13) | 3.09 | 29 % | |
| FIP2: (FIP2 OR NEMO2) TUMOR NECROSIS FACTOR ALPHA-INDUCIBLE CELLULAR PROTEIN CONTAINING LEUCINE ZIPPER DOMAINS (HUNTINGTIN INTERACTING PROTEIN L/HYPL) | 3.09 | 17 % | |
| APRIL: (TNFSF13 OR APRIL OR TALL2 OR ZTNF2) TUMOR NECROSIS FACTOR LIGAND SUPERFAMILY MEMBER 13 (A PROLIFERATION-INDUCING LIGAND) (APRIL) | 3.08 | 12 % | |
| CXCL13: (BLC OR BCA1) B LYMPHOCYTE CHEMOATTRACTANT PRECURSOR (CXC CHEMOKINE BLC) (B CELL-ATTRACTING CHEMOKINE 1) (BCA-1) (ANGIE). | 3.07 | 8 % | 63,74 |
| HMOX1: (HMOX1 OR HO1 OR HO) HEME OXYGENASE 1 (HO-1). | 3.07 | 19 % | |
| LCP2: (LCP2) LYMPHOCYTE CYTOSOLIC PROTEIN 2 (SH2 DOMAIN-CONTAINING LEUCOCYTE PROTEIN OF 76 KDA) (SLP-76 TYROSINE PHOSPHOPROTEIN) (SLP76). | 3.03 | 24 % | |
| CD37: (CD37) LEUKOCYTE ANTIGEN CD37. | 3.01 | 10 % | |
| FN1: (FN1 OR FN) FIBRONECTIN PRECURSOR (FN) (COLD-INSOLUBLE GLOBULIN) (CIG). | 2.93 | 7 % | |
| RBP2: (RBP2 OR CRBP2) RETINOL-BINDING PROTEIN II, CELLULAR (CRBP-II). | 2.93 | 43 % | 75 |
| SFA2: (BATF) ATF-LIKE BASIC LEUCINE ZIPPER TRANSCRIPTIONAL FACTOR B-ATF (SF-HT-ACIVATED GENE-2) (SFA-2). | 2.88 | 15 % | |
| GJA1_1: (GJA1) GAP JUNCTION ALPHA-1 PROTEIN (CONNEXIN 43) (CX43) (GAP JUNCTION 43 KDA HEART PROTEIN). | 2.87 | 11 % | 76 |
| GADD45: (GADD45A OR DDIT1 OR GADD45) GROWTH ARREST AND DNA-DAMAGE-INDUCIBLE PROTEIN GADD45 ALPHA (DNA-DAMAGE INDUCIBLE TRANSCRIPT 1) | 2.79 | 3 % | 18 |
| ALDH6: (ALDH1A3 OR ALDH6) ALDEHYDE DEHYDROGENASE 6 (RALDH3) RETINALDEHYDE DEHYDROGENASE 3 | 2.76 | 8 % | |
| SOD2: (SOD2 OR SOD-2) SUPEROXIDE DISMUTASE [MN], MITOCHONDRIAL PRECURSOR | 2.75 | 12 % | 77 |
| KAI1: (KAI1 OR CD82 OR SAR2) CD82 ANTIGEN (INDUCIBLE MEMBRANE PROTEIN R2) (C33 ANTIGEN) (IA4) (METASTASIS SUPPRESSOR KANGAI 1) (SUPPRESSOR OF TUMORIGENICITY-6). | 2.73 | 9 % | |
| CCL2_RAT: (SCYA2 OR MCP1) SMALL INDUCIBLE CYTOKINE A2 PRECURSOR (MONOCYTE CHEMOTACTIC PROTEIN 1) (MCP-1) (MONOCYTE CHEMOATTRACTANT PROTEIN-1) | 2.67 | 17 % | 78,79 |
| LYSYLOXIDASE: (LOX) PROTEIN-LYSINE 6-OXIDASE PRECURSOR (LYSYL OXIDASE). | 2.67 | 22 % | |
| CD83: (CD83) ANTIGEN PRECURSOR (CELL SURFACE PROTEIN HB15) (B-CELL ACTIVATION PROTEIN). | 2.63 | 7 % | |
| CP: (CP) CERULOPLASMIN PRECURSOR (FERROXIDASE). | 2.62 | 18 % | |
| WNT2: (WNT2 OR IRP OR WNT-2) WNT-2 PROTEIN PRECURSOR (IRP PROTEIN) (INT-1 RELATED PROTEIN). | 2.57 | 11 % | |
| TIMP1: (TIMP1 OR TIMP OR CLGI) METALLOPROTEINASE INHIBITOR 1 PRECURSOR (TIMP-1) (ERYTHROID POTENTIATING ACTIVITY) (EPA) (TISSUE INHIBITOR OF METALLOPROTEINASES | 2.55 | 4 % | 68,80 |
| WNT4: (WNT4 OR WNT-4) WNT-4 PROTEIN PRECURSOR (UNQ426/PRO864). | 2.50 | 29 % | |
| BST1: (BST1 OR BP3 OR BP-3 OR LY65) ADP-RIBOSYL CYCLASE 2 PRECURSOR (CYCLIC ADP-RIBOSE HYDROLASE 2) | 2.48 | 10 % | |
| AHR: (AHR) AH RECEPTOR (ARYL HYDROCARBON RECEPTOR). | 2.45 | 17 % | 81 |
| CYP2D1-CYP2D5_RAT: (CYP2D1 OR CYP2D-1) (CYP2D5 OR CYP2D-5) CYTOCHROME P450 2D1 (CYPIID1) | 2.45 | 23 % | |
| ICAM1: (ICAM1 OR ICAM-1) INTERCELLULAR ADHESION MOLECULE 1 PRECURSOR (ICAM-1) (MAJOR GROUP RHINOVIRUS RECEPTOR) (CD54) (MALA-2). | 2.44 | 15 % | 12,82,83 |
| TGFB1_1: (TGFB1 OR TGFB) TRANSFORMING GROWTH FACTOR BETA 1 PRECURSOR (TGF-BETA 1). | 2.44 | 6 % | 16,74,84-86 |
| COL15A1: (COL15A1) COLLAGEN ALPHA 1(XV) CHAIN PRECURSOR. | 2.42 | 7 % | |
| TF: (TF) SEROTRANSFERRIN PRECURSOR (TRANSFERRIN) (SIDEROPHILIN) (BETA-1-METAL BINDING GLOBULIN) (PRO1400). | 2.40 | 8 % | 87,88 |
| PAP3_RAT: (PAP3 OR REG3G) PANCREATITIS-ASSOCIATED PROTEIN 3 PRECURSOR (REG III-GAMMA). | 2.39 | - % | |
| THY1: (THY1) THY-1 MEMBRANE GLYCOPROTEIN PRECURSOR (THY-1 ANTIGEN) (CDW90) (CD90 ANTIGEN). | 2.39 | 8 % | |
| OSF: (OSTF1 OR SH3D3 OR SH3P2) OSTEOCLAST STIMULATING FACTOR 1 (SH3 DOMAIN PROTEIN 3). | 2.38 | 13 % | |
| CSF1R: (CSF1R OR CSFMR OR FMS) MACROPHAGE COLONY STIMULATING FACTOR I RECEPTOR PRECURSOR (CSF-1-R) | 2.36 | 12 % | |
| BTK: (BTK OR ATK OR AGMX1 OR BPK) TYROSINE-PROTEIN KINASE BTK (BRUTON’S TYROSINE KINASE) | 2.35 | 29 % | |
| CDKN1A: (CDKN1A OR CIP1 OR MDA6 OR CYCLIN-DEPENDENT KINASE INHIBITOR 1 (MELANOMA DIFFERENTIATION ASSOCIATED PROTEIN 6) (MDA-6) | 2.30 | 20 % | |
| PTAFR: (PTAFR OR PAFR) PLATELET ACTIVATING FACTOR RECEPTOR (PAF-R). | 2.29 | 20 % | |
| FN1_EIIIA: (FN1 OR FN) FIBRONECTIN PRECURSOR (FIBRONECTIN EIIIA DOMAIN). | 2.28 | 11 % | |
| BACH1: (BACH1) TRANSCRIPTION REGULATOR PROTEIN BACH1 (BTB AND CNC HOMOLOG 1) (HA2303). | 2.26 | 29 % | |
| ERO1L: (ERO1L) ERO1-LIKE PROTEIN ALPHA PRECURSOR (OXIDOREDUCTIN 1-LALPHA) (ENDOPLASMIC OXIDOREDUCTIN 1-LIKE PROTEIN) | 2.25 | 17 % | |
| IL1R2: (IL1R2 OR IL1RB) INTERLEUKIN-1 RECEPTOR, TYPE II PRECURSOR (IL-1R-2) (IL-1R-BETA). | 2.25 | 19 % | 74,89 |
| CD2: (CD2) T-CELL SURFACE ANTIGEN CD2 PRECURSOR (LEU-5) (LFA-2) (LFA-3 RECEPTOR) (ERYTHROCYTE RECEPTOR) | 2.24 | 16 % | |
| DEC1: (BHLHB2 OR SHARP-2 OR STRA14) STIMULATED BY RETINOIC ACID 14 (BASIC-HELIX-LOOP-HELIX PROTEIN | 2.23 | 15 % | |
| SOX9: (SOX9) TRANSCRIPTION FACTOR SOX-9. | 2.22 | 50 % | |
| TNFSF12: (TNFSF12 OR APO3L OR DR3LG) TUMOR NECROSIS FACTOR LIGAND SUPERFAMILY MEMBER 12 (TNF-RELATED WEAK INDUCER OF APOPTOSIS) (TWEAK) | 2.19 | 225 % | |
| PGH2: (PTGS2 OR COX2) PROSTAGLANDIN G/H SYNTHASE 2 PRECURSOR (CYCLOOXYGENASE-2) (COX-2) (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASE 2) | 2.18 | 26 % | 20,21,90 |
| SOCS3: (SOCS3) STAT INDUCED STAT INHIBITOR-3 (PROTEIN EF-10). (CISH3 OR SOCS-3 OR SOCS3) CYTOKINE INDUCIBLE SH2-CONTAINING PROTEIN 3 | 2.16 | - % | |
| ABME: (APOBEC1) APOLIPOPROTEIN B MRNA EDITING PROTEIN (HEPR) (APOBEC-1). | 2.15 | 16 % | |
| INTEGRINB7: (ITGB7) INTEGRIN BETA-7 PRECURSOR. | 2.15 | 23 % | |
| TLR4: (TLR4) TOLL-LIKE RECEPTOR 4 PRECURSOR (HTOLL). | 2.15 | 19 % | |
| SRC: (SRC OR SRC1) PROTO-ONCOGENE TYROSINE-PROTEIN KINASE SRC (C-SRC). | 2.12 | 13 % | |
| LRP: (MVP OR LRP) MAJOR VAULT PROTEIN (MVP) (LUNG RESISTANCE-RELATED PROTEIN). | 2.09 | 16 % | |
| LAMB3: (LAMB3) LAMININ BETA-3 CHAIN PRECURSOR (LAMININ B1K CHAIN) (KALININ B1 CHAIN). | 2.08 | 14 % | |
| C1S: (C1S) COMPLEMENT C1S COMPONENT PRECURSOR (C1 ESTERASE). | 2.07 | 4 % | 16,18 |
| FMO5: (FMO5) DIMETHYLANILINE MONOOXYGENASE 5 (HEPATIC FLAVIN-CONTAINING MONOOXYGENASE 5) (FMO 5) (DIMETHYLANILINE OXIDASE 5). | 2.06 | 42 % | |
| PDGFRB: (PDGFRB OR PDGFR) BETA PLATELET-DERIVED GROWTH FACTOR RECEPTOR PRECURSOR (PDGF-R-BETA) (CD140B ANTIGEN). | 2.06 | 10 % | 20,21 |
| LAMP2: (LAMP2 OR LAMP-2) LYSOSOME-ASSOCIATED MEMBRANE GLYCOPROTEIN 2 PRECURSOR (LAMP-2) (LYSOSOMAL MEMBRANE GLYCOPROTEIN-TYPE B) (LGP-B) | 2.05 | 10 % | |
| LOXL1: (LOX OR RRG) PROTEIN-LYSINE OXIDASE HOMOLOG PRECURSOR (LYSYL OXIDASE HOMOLOG) (LYSYL OXIDASE-LIKE PROTEIN) (RAS EXCISION PROTEIN). | 2.05 | 28 % | 22 |
| POLD4: (POLD4 OR POLDS) DNA POLYMERASE DELTA SUBUNIT 4 (DNA POLYMERASE DELTA SUBUNIT P12). | 2.05 | 15 % | |
| ENO2: (ENO2) GAMMA ENOLASE (2-PHOSPHO-D-GLYCERATE HYDRO-LYASE) | 2.04 | 3 % | |
| DCP1: (DCP1 OR DCP OR ACE) ANGIOTENSIN-CONVERTING ENZYME, SOMATIC ISOFORM PRECURSOR (ACE) (DIPEPTIDYL CARBOXYPEPTIDASE I) | 2.00 | 17 % | 91 |
| GLUL: (GLUL OR GLNS) GLUTAMINE SYNTHETASE (GLUTAMATE--AMMONIA LIGASE). | 2.00 | 10 % | |
| PRKCE: (PRKCE OR PKCE) PROTEIN KINASE C, EPSILON TYPE (NPKC-EPSILON). | 2.00 | 19 % | 20,21 |
| ERP5: (CABP1 OR PDIA6 OR 1700015E05RIK) PROTEIN DISULFIDE ISOMERASE A6 PRECURSOR (PROTEIN DISULFIDE ISOMERASE P5). | 0.50 | 6 % | |
| ILF: (ILF1 OR ILF) INTERLEUKIN ENHANCER-BINDING FACTOR 1 (CELLULAR TRANSCRIPTION FACTOR ILF-1). | 0.50 | 16 % | |
| PLC: (PLC-L) PHOSPHOLIPASE C (130KDA-INS (1,4,5)P3 BINDING PROTEIN). | 0.50 | 30 % | |
| POD1: (TCF21 OR POD1 OR COR1) MESODERM-SPECIFIC BASIC-HELIX-LOOP-HELIX PROTEIN. | 0.50 | 13 % | |
| SMAD5: (MADH5 OR SMAD5) MOTHERS AGAINST DECAPENTAPLEGIC HOMOLOG 5 (SMAD 5) (MOTHERS AGAINST DPP HOMOLOG 5) (SMAD5) (HSMAD5) | 0.50 | 20 % | 92 |
| IL12A: (IL12A OR NKSF1) INTERLEUKIN-12 ALPHA CHAIN PRECURSOR (IL-12A) (CYTOTOXIC LYMPHOCYTE MATURATION FACTOR 35 KDA SUBUNIT) (NK CELL STIMULATORY FACTOR CHAIN 1) (NKSF1). | 0.49 | 13 % | 93 |
| RFC5: (RFC5) ACTIVATOR 1 36 KDA SUBUNIT (REPLICATION FACTOR C 36 KDA SUBUNIT) (A1 36 KDA SUBUNIT) (RF-C 36 KDA SUBUNIT) (RFC36). | 0.49 | 9 % | |
| CPT2: (CPT2) CARNITINE O-PALMITOYLTRANSFERASE II, MITOCHONDRIAL PRECURSOR (CPT II). | 0.48 | 12 % | |
| HJ2: (HJ2 JAGGED2) NOTCH LIGAND JAGGED 2. | 0.48 | - % | |
| MMP21-22-23: (MMP-23 OR MMP21/22 OR MIFR-1 OR MIFR OR DJ283E3.2) MMP-23 (MIFR/FEMALYSIN) (DJ283E3.2.1) (MATRIX METALLOPROTEINASE MMP21/22A (MIFR1)) (MATRIX METALLOPROTEINASE 23B) | 0.48 | 9 % | |
| NOP40: (EBNA1BP2 OR EBP2) PROBABLE RRNA PROCESSING PROTEIN EBP2 (EBNA1 BINDING PROTEIN 2) (NUCLEOLAR PROTEIN P40). | 0.48 | 3 % | |
| TM4SF3: (TM4SF3) TUMOR-ASSOCIATED ANTIGEN CO-029 | 0.48 | 7 % | |
| ESR1: (ESR1 OR NR3A1 OR ESR) ESTROGEN RECEPTOR (ER) (ESTRADIOL RECEPTOR). | 0.47 | 1 % | 94,95 |
| HSP40-3: (DNAJB5 OR HSC40) DNAJ HOMOLOG SUBFAMILY B MEMBER 5 (HEAT SHOCK PROTEIN HSP40-3) (HEAT SHOCK PROTEIN COGNATE 40) (HSC40). | 0.47 | 26 % | 96,97 |
| FKBP5: (FKBP5) (54 KDA PROGESTERONE RECEPTOR-ASSOCIATED IMMUNOPHILIN) (FKBP54) (P54) (FF1 ANTIGEN) (HSP90 BINDING IMMUNOPHILIN). | 0.46 | - % | |
| LRRN3: (LRRN3) LEUCINE-RICH REPEAT PROTEIN PRECURSOR (FRAGMENT). | 0.46 | 14 % | |
| ACS-2: (FACL3 OR ACS3 OR LACS3) LONG-CHAIN-FATTY-ACID-COA LIGASE 3 (LONG-CHAIN ACYL-COA SYNTHETASE 3) (LACS 3) | 0.45 | 5 % | |
| IL4R: (IL4R OR IL4RA OR 582J2.1) INTERLEUKIN-4 RECEPTOR ALPHA CHAIN PRECURSOR (IL-4R-ALPHA) (CD124 ANTIGEN) | 0.45 | 7 % | |
| TFRC_MIDDLE: (TFRC) TRANSFERRIN RECEPTOR PROTEIN (TFR1) (CD71 ANTIGEN) | 0.45 | 26 % | |
| THBD: (THBD OR THRM) THROMBOMODULIN PRECURSOR (FETOMODULIN) (TM) | 0.45 | 11 % | |
| VGR2: (KDR OR FLK1) VASCULAR ENDOTHELIAL GROWTH FACTOR RECEPTOR 2 PRECURSOR (VEGFR-2) (KINASE INSERT DOMAIN RECEPTOR) (FLK-1). | 0.45 | - % | 12 |
| FMO1: (FMO1) DIMETHYLANILINE MONOOXYGENASE 1 (FETAL HEPATIC FLAVIN-CONTAINING MONOOXYGENASE 1) (FMO 1) | 0.44 | 13 % | |
| POLD2: (POLD2) DNA POLYMERASE DELTA SMALL SUBUNIT | 0.43 | 11 % | |
| SULT1A1_RAT: (SULT1A1 OR ST1A1) ARYL SULFOTRANSFERASE (PHENOL SULFOTRANSFERASE) (PST-1) (SULFOKINASE) (ARYL SULFOTRANSFERASE IV) (ASTIV) | 0.43 | 3 % | |
| SYK: (SYK) TYROSINE-PROTEIN KINASE SYK (SPLEEN TYROSINE KINASE). | 0.43 | 8 % | |
| ID4: (ID4) DNA-BINDING PROTEIN INHIBITOR ID-4. | 0.41 | 8 % | |
| BCG1: (BCG1 OR MAGED2) BREAST CANCER ASSOCIATED GENE 1 PROTEIN JCL-1 (DJ14O9.2 OR BCG1) DJ14O9.2 (MELANOMA-ASSOCIATED ANTIGEN MAGE LIKE) (JCL-1) | 0.40 | 12 % | |
| IGF1: (IGF-I OR IGF1) INSULIN-LIKE GROWTH FACTOR I PRECURSOR (SOMATOMEDIN). (IGF1 OR IBP1) INSULIN-LIKE GROWTH FACTOR IA PRECURSOR (IGF-IA) | 0.40 | 4 % | |
| FKBP14: (FKBP14 OR FKBP22) FK506 BINDING PROTEIN 14 PRECURSOR (PEPTIDYL-PROLYL CIS-TRANS ISOMERASE) (PPIASE) (ROTAMASE) | 0.39 | 27 % | |
| FKBP11: (FKBP11 OR FKBP19) FK506 BINDING PROTEIN 11 PRECURSOR (PEPTIDYL-PROLYL CIS-TRANS ISOMERASE) (PPIASE) (ROTAMASE) | 0.37 | 8 % | |
| FKBP4: (FKBP4) P59 PROTEIN (HSP BINDING IMMUNOPHILIN) (HBI) (ROTAMASE) (52 KDA FK506 BINDING PROTEIN) (P52) (FKBP59) (HSP56). | 0.36 | 6 % | |
| MT1_RAT: (MT1) METALLOTHIONEIN-I (MT-I). | 0.32 | 4 % | |
| TRK-B: (NTRK2 OR TRKB) BDNF/NT-3 GROWTH FACTORS RECEPTOR PRECURSOR (TRKB TYROSINE KINASE) | 0.31 | 23 % | 20,21,98 |
| UMAT: (ABCB6 OR MTABC OR MTABC3 OR ABCB7 OR ABC7) ABC TRANSPORTER UMAT (MT-ABC TRANSPORTER) (MITOCHONDRIAL ABC TRANSPORTER 3) ATP-BINDING CASSETTE | 0.28 | 34 % | |
| WNT5A: (WNT5A OR WNT-5A) WNT-5A PROTEIN PRECURSOR. | 0.26 | 24 % | 59 |
| EGFR-LONG: (EGFR OR ERBB1) EPIDERMAL GROWTH FACTOR RECEPTOR PRECURSOR (RECEPTOR PROTEIN-TYROSINE KINASE ERBB-1). | 0.24 | 38 % | 99,100 |
| MT2_RAT: (MT2) METALLOTHIONEIN-II (MT-II). | 0.24 | 6 % | |
| FKHL16: (FOXM1 OR FKHL16 OR HFH11 OR WIN OR MPP2) FORKHEAD PROTEIN M1 (FORKHEAD-RELATED PROTEIN FKHL16) (HEPATOCYTE NUCLEAR FACTOR 3 FORKHEAD HOMOLOG 11) | 0.23 | - % | |
| GSTM1_RAT: (GSTM1 OR GST1) GLUTATHIONE S-TRANSFERASE MU 1 (GSTM1-1) (HB SUBUNIT 4) (GTH4) (GSTM1A-1A) (GSTM1B-1B) (GST CLASS-MU). | 0.23 | 7 % | 101 |
| NGFR: (NGFR OR TNFRSF16) LOW-AFFINITY NERVE GROWTH FACTOR RECEPTOR PRECURSOR (NGF RECEPTOR) (LOW AFFINITY NEUROTROPHIN RECEPTOR P75NTR). | 0.19 | 34 % | |
| FUT2: (FUT2 OR SEC2) GALACTOSIDE 2-ALPHA-L-FUCOSYLTRANSFERASE 2 (GDP-L-FUCOSE:BETA-D-GALACTOSIDE 2-ALPHA-L-FUCOSYLTRANSFERASE 2) | 0.15 | 8 % | |
| GLI1: (GLI OR GLI1) ZINC FINGER PROTEIN GLI1 (GLIOMA-ASSOCIATED ONCOGENE) (ONCOGENE GLI). | 0.14 | 20 % | |
| LEFTY1-LEFTY2_RAT: (EBAF OR TGFB4 OR LEFTA OR LEFTYA) TRANSFORMING GROWTH FACTOR BETA 4 PRECURSOR (TGF-BETA 4) (ENDOMETRIAL BLEEDING-ASSOCIATED FACTOR) | 0.14 | 51 % | 102 |
| HSD11B2: (HSD11B2 OR HSD11K) CORTICOSTEROID 11-BETA-DEHYDROGENASE, ISOZYME 2 (11-DH2) | 0.05 | 10 % | 103 |
| ORM1: (ORM1 OR AGP1) ALPHA-1-ACID GLYCOPROTEIN 1 PRECURSOR (AGP 1) (OROSOMUCOID 1) (OMD 1). | 0.03 | 23 % |
Percent standard deviation (otherwise known as the relative standard deviation RSD) is obtained by multiplying the standard deviation by 100 and dividing this product by the average
Real-time RT-PCR Validation of Microarray Data
To validate the microarray data, we conducted real-time RT-PCR quantification of the expression of 9 selected genes in a second set of samples (not included in the microarray experiments). RNA was extracted from vesicles obtained from 6 experimental rats, and from uterine horn samples from 5 control animals. Due to the limited amount of RNA obtained from each vesicle, RNA obtained from two to three vesicles per animal was pooled. Relative expression levels of the genes of interest was compared to those of a reference gene. We tested four different reference genes (HPRT, CYP, β-actin, and GAPDH), and found no significant differences in the expression between groups, i.e., none of the tested reference genes were differentially regulated during the disease process and therefore all were valid to normalize the data (29). Arbitrarily, we selected HPRT as our reference gene for analysis. Relative expression levels were calculated for each sample after normalization against HPRT, using the ΔΔCt method for comparing relative fold-expression differences. All PCR reactions had efficiencies of over 90%. From the 9 selected genes, RT-PCR analysis validated the results for seven: PDGFA, LOXL1, IL2RG, BCL2, OPG, MMP9, and TGFB. In Figure 1, scatter plots for each gene show the mean and individual variation in gene expression levels in experimental vs. control rats. For LOXL1, IL2RG, BCL2, MMP9 and TGFB, the differences in means between experimental and control groups reached statistical significance. A comparison between the cDNA microarray and RT-PCR expression data is shown in Figure 2.
Figure 1.
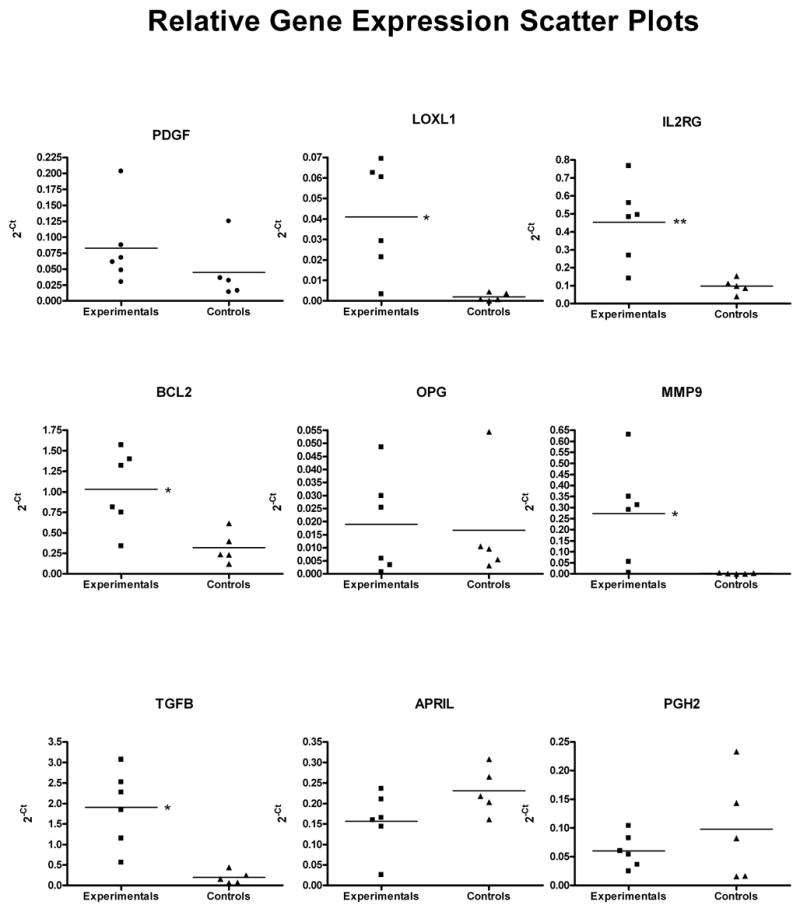
Scatter plots of gene expression levels in experimental (n=6) and control (n=5) rats analyzed by RT-PCR. Unpaired t-tests were conducted to determine significant differences in the mean level of gene expression between the groups. Significance was set at p<0.05; * indicates a p value <0.05, ** a p value <0.01.
Figure 2.
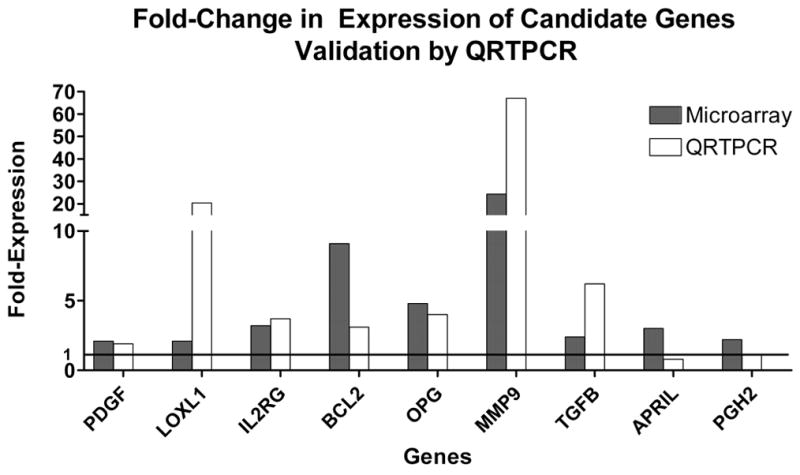
Comparison of fold-expression of candidate genes in ectopic versus eutopic endometrium analyzed by either microarrays or real-time QRT-PCR.
Functional Categorization of Microarray Data
Data mining using GoMiner identified 58 potentially important functional categories based on both a high enrichment factor and statistical significance (p-value <0.5). A total of 41 Biological Process (121 genes)-, eight Cellular Component (113 genes)-, and nine Molecular Function (112 genes)-categories were significantly enriched (Table 2). The largest group of the functional categories was, as expected, those related to immune modulation/inflammation. In particular, IL-6 biosynthesis and action was one of the categories with the largest enrichment factor value (6.32), together with positive regulation of T cell proliferation (6.32), and neutrophil chemotaxis (4.74). Other interesting categories— which strongly relate to the human condition— were integrin binding (6.32), extracellular matrix (2.56), response to wounding (2.25), angiogenesis (2.28), and metallopeptidase activity (2.95) (Table 3).
Table 2.
Significantly enriched or depleted Go Categories in ectopic versus eutopic endometrium
| Term | GoMiner | Fisher |
|---|---|---|
| Biological process: | E | P |
| organismal physiological process | 1.29 | p=0.05 |
| regulation of organismal physiological process | 2.19 | p=0.01 |
| positive regulation of organismal physiological process | 3.16 | p=0.01 |
| angiogenesis | 2.28 | p=0.05 |
| histogenesis | 1.99 | p=0.04 |
| cartilage development | 4.74 | p=0.01 |
| tube development | 5.44 | p=0.05 |
| regulation of ossification | 10.89 | p=0.01 |
| negative regulation of bone remodeling | 6.32 | p=0.02 |
| cell adhesion | 1.84 | p=0.01 |
| cell cycle arrest | 3.16 | p=0.05 |
| G1/S transition of mitotic cell cycle | 5.44 | p=0.05 |
| cell homeostasis | 2.81 | p=0.04 |
| ion homeostasis | 2.6 | p=0.04 |
| transition metal ion homeostasis | 3.42 | p=0.03 |
| response to wounding | 2.25 | p=0.00 |
| response to external stimulus | 1.6 | p=0.01 |
| response to abiotic stimulus | 2 | p=0.01 |
| response to chemical substance | 2.71 | p=0.00 |
| response to biotic stimulus | 1.53 | p=0.01 |
| response to external biotic stimulus | 2.2 | p=0.00 |
| response to pest, pathogen or parasite | 2.11 | p=0.00 |
| defense response | 1.69 | p=0.00 |
| cellular defense response (sensu Vertebrata) | 2.81 | p=0.04 |
| immune response | 1.64 | p=0.01 |
| regulation of immune response | 2.33 | p=0.02 |
| positive regulation of immune response | 3.45 | p=0.00 |
| positive regulation of lymphocyte proliferation | 3.79 | p=0.03 |
| positive regulation of T-cell proliferation | 6.32 | p=0.02 |
| inflammatory response | 2.34 | p=0.01 |
| chemotaxis | 3.35 | p=0.00 |
| immune cell chemotaxis | 4.22 | p=0.01 |
| immune cell migration | 3.61 | p=0.01 |
| neutrophil chemotaxis | 4.74 | p=0.01 |
| cytokine production | 3.16 | p=0.01 |
| cytokine biosynthesis | 2.81 | p=0.04 |
| positive regulation of cytokine biosynthesis | 6.32 | p=0.02 |
| interleukin-6 biosynthesis | 6.32 | p=0.02 |
| regulation of interleukin-6 biosynthesis | 6.32 | p=0.02 |
| collagen catabolism | 3.95 | p=0.00 |
| response to reactive oxygen species | 6.32 | p=0.02 |
| Cellular Component: | ||
| cell surface | 2.81 | p=0.04 |
| extracellular region | 1.57 | p=0.00 |
| extracellular matrix | 2.56 | p=0.00 |
| extracellular space | 1.49 | p=0.00 |
| plasma membrane | 1.51 | p=0.03 |
| intrinsic to membrane | 1.33 | p=0.03 |
| vacuole | 2.6 | p=0.04 |
| lytic vacuole | 2.6 | p=0.04 |
| Molecular Function: | ||
| integrin binding | 6.32 | p=0.00 |
| ion binding | 1.51 | p=0.05 |
| lipid binding | 4.22 | p=0.01 |
| metal ion binding | 1.51 | p=0.05 |
| cation binding | 1.58 | p=0.04 |
| metallopeptidase activity | 2.95 | p=0.00 |
| metalloendopeptidase activity | 2.71 | p=0.01 |
| transition metal ion binding | 2.17 | p=0.01 |
| transition metal ion transport | 6.32 | p=0.02 |
Table 3.
Differentially expressed genes within relevant Go categories
| Go Category | Gene |
|---|---|
| Defense response: | SPP1, CD2, CD4, IL18, CXCR4, CDKN1A, CEBPB, IL1B, CD4, IL18, PTAFR, SYK, B2M, LBP, CCL2, S100A8, C3, TLR4, ITGAM, ICAM1, CXCL1, IL12A, BST1, CCL4, CD74, SOD2, IL4R, IL2RG, FN1 |
| Inflammatory response | ITGAM, PTAFR, CCL4, SPP1, CCL2, S100A8, C3, CXCL1, TLR4, IL1B, TGFB1 |
| Immune cell migration (Neutrophils) | IL1B, SPP1, ITGAM, CXCL1 |
| Angiogenesis | ANPEP, HAND2, IL18, CTGF, ID1 |
| Bone remodeling | PTGER4, OPG, SPP1, SMAD5 |
| Response to reactive oxygen species | SOD2, GPX1 |
| Cell adhesion | CD2, ICAM1, SPP1, CTGF, ITGAM CD4, FN1, CD36, ALCAM, COL11A1, IL18, TNC, CXCR4, ITGA4 |
| Extracellular matrix | SPP1, LAMA3, TNC, FN1, CTGF, HSPG2, COL11A1, OPG, TGFB1, MMP7, MMP9, MMP11, MMP12, MMP13, MMP14, TIMP1, TIMP2 |
| Programmed cell death | OPN, HMOX1, NGFR, IGF1, GPX1, CEBPB, OPG, BCL2, FAIM, DDIT3, IL18, CDKN1A, CD2, AHR, TGFB1 |
| Response to wounding | THBD, C3, FN1, DMBT1, TLR4, SOD2, CCL4, B2M, S100A8, TM4SF3, IL1B, CXCL1, IL18, CCL2, ITGAM, SPP1, CD4, PTAFR |
| Histogenesis | SOX9, PTGER4, CTGF, SMAD5, DMBT1, COL11A1, SPP1 |
| Cell ion homeostasis | MT1A, EDN1, TF, CP |
| Metallopeptidase function | MMP7, MMP9, MMP11, MMP12, MMP13, MMP14, TIMP1, TIMP2 |
DISCUSSION
At the present time the cause of endometriosis and its natural history are still unknown, treatment options are limited, and there is no cure for this debilitating disease. Animal models represent an invaluable tool to study the initiating events leading to survival and establishment of endometriotic implants at ectopic sites, as well as for the development of novel therapeutic strategies. Drug discovery and development require that model organisms used in preclinical assays can accurately predict the clinical efficacy and safety of new drugs. One of the major obstacles to drug discovery is the lack of— or inadequacy of— animal models available to conduct preclinical studies of safety and efficacy, target identification and validation, and drug screening. Therefore, determining the validity of animal models of disease has great implications for the pharmaceutical and biotechnology fields.
Animal model validation has been limited to pathological, histological, and pharmacological considerations. Single-gene studies have been conducted in an attempt to genetically validate animal models, but this approach cannot provide a broader picture (30). For instance, we have identified common gene expression phenomena between experimental and human disease, such as the significant decrease in expression of the TNF receptor 2 (Tnfrsf1b) (12). However, for a complex disease such as endometriosis, with no clear understanding of its etiology and progression, and for which no specific biomarker has been identified despite decades of research, there are very few obvious candidate genes to study.
In this study we proposed that gene expression profiling is the most appropriate tool to validate a much needed accurate, inexpensive, and accessible preclinical model for endometriosis. Therefore, we applied a comprehensive, high-throughput technology such as cDNA microarrays to solve the complex question at hand: Is the autotransplantation rat model of endometriosis a valid model of this disease? To answer this question we conducted cDNA microarray analysis to compare gene expression profiles of ectopic and eutopic endometrium in this animal model.
To validate the cDNA microarray results, real-time RT-PCR analysis of the level of expression of selected genes was conducted. This technology constitutes an independent way of validating the cDNA microarray results, while providing a cost-effective way of quantifying gene expression levels in additional sample sets. Of the nine genes initially selected for microarray data validation (PDGFA, LOXL1, IL2RG, BCL2, OPG, MMP9, TGFB, APRIL, PGH2) we confirmed the results for the first seven (Figure 2). Discrepancies between microarray and real-time PCR results in both direction and level of expression have been reported before, providing further support for the use of real-time RT-PCR to confirm cDNA microarray data (31,32).
A difficulty related to cDNA microarray studies relates to the fact that large gene sets are identified in a single experiment, making these large-scale studies problematic for data interpretation. While gene annotations (National Center for Biotechnology Information/Entrez/Online Mendelian Inheritance in Man Database, and others) are helpful, searching for patterns or for interesting gene functions that can be ascribed to disease is labor intensive, subjective, and therefore impractical. Data mining tools such as GoMiner™ make selecting significant trends from such large data sets a more user-friendly process. GoMiner™ compares the expression of genes from an experiment against the distribution of all of the genes of the genome on the GO hierarchy of gene functions. Those branches that are not enriched by chance alone are flagged, and the investigator can easily select those interesting categories for further experimentation (15).
GO category analysis was conducted to provide biological meaning and functional coherence to the results. Using GoMiner™, we analyzed the biological classifications of the 168 genes that were differentially-expressed (123 upregulated, 45 downregulated) in ectopically growing endometrium. We observed that, in particular, defense-related cellular pathways were significantly enriched, including inflammatory response (10 genes), immune response (23 genes), and chemotaxis (9 genes). In addition, angiogenesis (5 genes), response to wounding (18 genes), programmed cell death (14 genes), metallopeptidase function (8 genes), cell adhesion (14), extracellular matrix (16 genes) and collagen metabolism (6 genes) were also significantly enriched. Of the downregulated genes, only tube development (2 genes), regulation of ossification (3 genes), and G1/S transition of mitotic cell cycle (3 genes) reached statistical significance (Table 3).
It is now widely accepted that angiogenesis plays a major role in the pathogenesis of endometriosis (34–36). In fact, angiogenic inhibitors have been proposed as a novel therapy for this disease, since it has been shown that they suppress the growth of endometriotic lesions in an animal model (37,38). Likewise, both integrin and metalloproteinase expression have been associated with the metastatic potential of endometriosis lesions. The fact that the immune system and inflammatory mechanisms are activated in endometriosis is also well known. This study also served to identify new gene expression profiles that may characterize endometriosis. Interesting novel functional categories that deserve further exploration include: metal ion binding and transport, regulation of bone ossification and remodeling, cell cycle arrest and response to reactive oxygen species. These findings are expected to promote the development of novel hypotheses that will likely guide future research directions.
The present study has uncovered many interesting biological themes shared between the natural and experimental disease. In agreement with observations made in patients, we observed gene- and pathway-specific patterns of expression that may explain the observed survival and growth of ectopic endometrium in rats. Endometriosis is most likely the result of the stepwise activation of a complex series of molecular events that begin when endometrial cells present in the menstrual fluid reach the peritoneal cavity by retrograde menstruation (according to Sampson’s theory). Our observations support the current knowledge from both the natural and experimental disease, which can be summarized as follows (Figure 3): the initial attachment of cells that reach the peritoneum to the extracellular matrix [adhesion molecule, integrin expression], is followed by tissue invasion [metalloprotease (MMPs) and tissue inhibitor of metalloprotease activity (TIMP)], cell growth to form lesions [cell division, growth factors, steroid hormones, k-ras mutations], survival at the ectopic site [angiogenesis, resistance to apoptosis, induction of local immunosuppression], and finally activation of wound healing mechanisms ensues which may result in the fribrosis, scarring and adhesion formation that characterize severe endometriosis. Concomitantly, inflammatory [neutrophil chemotaxis, complement] and immune mechanisms [T cell activation, cytokines, chemokines] are activated in response to the growth of cells at the abnormal location, and these probably feed into the proposed pathway.
Figure 3.
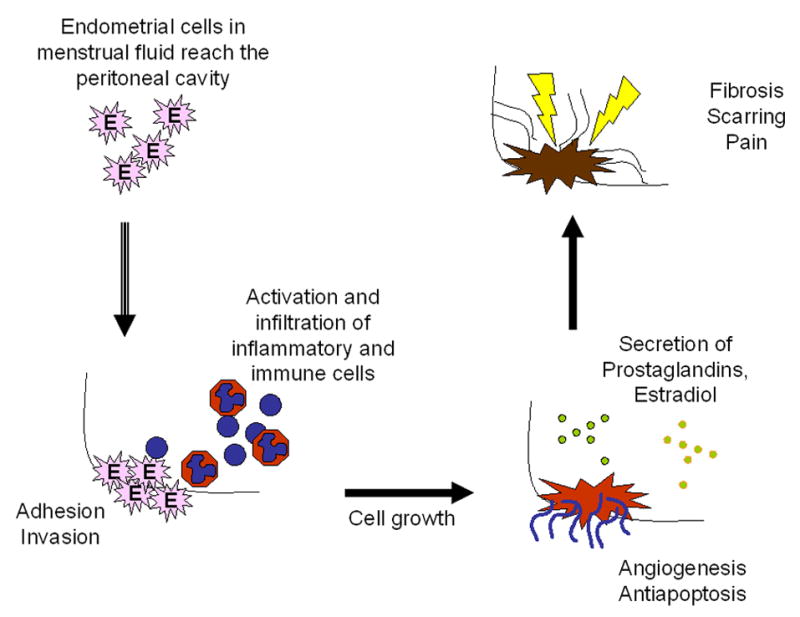
A model of disease for endometriosis based on data from both animal and human studies
It should be noted that there are limitations to the use of cDNA microarrays for the study of endometriosis. Endometriotic tissue is heterologous, i.e., it is a mixture of endometrium, myometrium, serosa, connective tissue and immune cells. Therefore, there is no guarantee that the gene expression profiles that we have identified derive from tissue that is significantly contributing to the pathophysiology of this disease. The recent application of laser capture microdissection to molecular studies will undoubtedly facilitate follow up studies of gene expression in the different cell lineages that comprise heterogeneous tissues such as the endometrial lesions (20). Also, since cDNA microarrays are assaying the message for protein synthesis but not the actual protein levels, it is important that follow up studies are conducted to determine the extent to which relevant proteins are produced. Similarly, functional studies in vitro and in vivo should ask whether differences in protein production are indeed related to disease.
In summary, while it is generally accepted that non-human primate models of endometriosis most closely resemble the disease (39), this study provides evidence in support of the autotransplantation rat model as a simple, inexpensive and useful alternative to study certain aspects of this condition. This model of endometriosis originally developed by Vernon and Wilson in 1985, has already been used for testing possible new drugs, including studies showing for the first time the potential of anti-TNF agents for the treatment of endometriosis (40–45). The common themes of gene expression between the experimental and the natural disease described herein should be considered evidence in support of this surgically-induced rat model as an appropriate and invaluable tool to study the natural history of endometriosis and test novel therapeutics for this incurable disease.
Acknowledgments
The authors wish to acknowledge the data analysis support provided by Dr. Sükrü Tuzmen at TGen Corporation, Tuczon, AZ. Thanks also go to the RCMI Publications Office and the Molecular Biology Core, supported by RCMI Grant # 2 G12 RR03050-18.
Footnotes
Where the work was done: Ponce School of Medicine, Ponce, Puerto Rico
Presented at: Society for the Study of Reproduction Annual Meeting, Quebec, Canada, July 24-27, 2005
Financial support: These studies were supported by S06-GM08239 (C.B.A. and I.F.) from the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mahmood TA, Templeton A. Prevalence and genesis of endometriosis. Hum Reprod. 1991;6:544–549. doi: 10.1093/oxfordjournals.humrep.a137377. [DOI] [PubMed] [Google Scholar]
- 2.Witz CA, Allsup KT, Montoya-Rodríguez IA, Vaughan SL, Centonze VE, Schenken RS. Pathogenesis of endometriosis— current research. Hum Fertil. 2003;6:34–40. doi: 10.1080/1464770312331368973. [DOI] [PubMed] [Google Scholar]
- 3.Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–694. [PubMed] [Google Scholar]
- 4.Story L, Kennedy S. Animal studies in endometriosis: a review. ILAR J. 2004;45:132–138. doi: 10.1093/ilar.45.2.132. [DOI] [PubMed] [Google Scholar]
- 5.Sharpe-Timms KL. Using rats as a research model for the study of endometriosis. Ann NY Acad Sci. 2002;955:318–327. doi: 10.1111/j.1749-6632.2002.tb02792.x. [DOI] [PubMed] [Google Scholar]
- 6.Vernon MW. Experimental endometriosis in laboratory animals as a research model. Prog Clin Biol Res. 1990;323:49–60. [PubMed] [Google Scholar]
- 7.D’Hooghe TM, Mwenda JM, Hill JA. A critical review of the use and application of the baboon as a model for research in women’s reproductive health. Gynecol Obstet Invest. 2004;57(1):1–60. Epub 2003 Dec 29. [Google Scholar]
- 8.Sharpe KL, Vernon MW. Polypeptides synthesized and released by rat ectopic uterine implants differ from those of the uterus in culture. Biol Reprod. 1993;48:1334–1340. doi: 10.1095/biolreprod48.6.1334. [DOI] [PubMed] [Google Scholar]
- 9.Sharpe KL, Bertero MC, Vernon MW. Detection of a progesterone-induced secretory protein synthesized by the uteri but not the endometriotic implants of rats with induced endometriosis. Fertil Steril. 1991;55:403–410. [PubMed] [Google Scholar]
- 10.Uchiide I, Ihara T, Sugamata M. Pathological evaluation of the rat endometriosis model. Fertil Steril. 2002;78:782–786. doi: 10.1016/s0015-0282(02)03327-7. [DOI] [PubMed] [Google Scholar]
- 11.Mizumoto Y, Hirata J, Tokuoka S, Furuya K, Kikuchi Y, Nagata I. Effect of culture supernatants of endometriotic lesions, uterine endometrium and peritoneum from rats with experimental endometriosis on the natural killer activity of spleen cells. Gynecol Obstet Invest. 1996;41:122–127. doi: 10.1159/000292056. [DOI] [PubMed] [Google Scholar]
- 12.Rojas-Cartagena C, Appleyard CB, Santiago OI, Flores I. Experimental Intestinal Endometriosis is Characterized by Increased Levels of Soluble TNFRSFa and Downregulation of Tnfrsfa and Tnfrsfb Gene Expression. Biol Reprod. 2005;73:1211–1218. doi: 10.1095/biolreprod.105.044131. Epub Aug 10. [DOI] [PubMed] [Google Scholar]
- 13.Hughes TR, Shoemaker DD. cDNA microarrays for expression profiling. Curr Opin Chem Biol. 2005:21–25. doi: 10.1016/s1367-5931(00)00163-0. [DOI] [PubMed] [Google Scholar]
- 14.Albertson DG, Pinkel D. Genomic microarrays in human genetic disease and cancer. Hum Mol Genet. 2003;12:R145–52. doi: 10.1093/hmg/ddg261. [DOI] [PubMed] [Google Scholar]
- 15.Zeeberg BR, Feng W, Wang G, Wang MD, Fojo AT, Sunshine M, Narasimhan S, Kane DW, Reinhold WC, Lababidi S, Bussey KJ, Riss J, Barrett JC, Weinstein JN. GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biol. 2003;4:R28. doi: 10.1186/gb-2003-4-4-r28. Epub 2003 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eyster KM, Boles AL, Brannian JD, Hansen KA. DNA microarray analysis of gene expression markers of endometriosis. Fertil Steril. 2002;77:38–42. doi: 10.1016/s0015-0282(01)02955-7. [DOI] [PubMed] [Google Scholar]
- 17.Lebovic DI, Baldocchi RA, Mueller MD, Taylor RN. Altered expression of a cell-cycle suppressor gene, Tob-1, in endometriotic cells by cDNA array analyses. Fertil Steril. 2002;78:849–854. doi: 10.1016/s0015-0282(02)03319-8. [DOI] [PubMed] [Google Scholar]
- 18.Arimoto T, Katagiri T, Oda K, Tsunoda T, Yasugi T, Osuga Y, Yoshikawa H, Nishii O, Yano T, Taketani Y, Nakamura Y. Genome-wide cDNA microarray analysis of gene-expression profiles involved in ovarian endometriosis. Int J Oncol. 2003;22:551–560. [PubMed] [Google Scholar]
- 19.Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaki S, Canis M, Vaurs-Barriere C, Pouly JL, Boespflug-Tanguy O, Penault Llorca F, Dechelotte P, Dastugue P, Okamura B, Mage G. DNA microarray analysis of gene expression profiles in deep endometriosis using laser capture microdissection. Mol Hum Reprod. 2004;10:719–728. doi: 10.1093/molehr/gah097. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzaki S, Canis M, Vaurs-Barriere C, Boespflug-Tanguy O, Dastugue B, Mage G. DNA microarray analysis of gene expression in eutopic endometrium from patients with deep endometriosis using laser capture microdissection. Fertil Steril. 2005;84 (Suppl 2):1180–1190. doi: 10.1016/j.fertnstert.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 22.Flores I, Rivera E, Mousses S, Chen Y, Rozenblum E. Identification of molecular markers for endometriosis in blood lymphocytes using DNA microarrays. Fertil Steril. 2006;85:1676–1683. doi: 10.1016/j.fertnstert.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 23.Ingelmo MR, Quereda F, Acién P. Intraperitoneal and subcutaneus treatment of experimental endometriosis with recombinant human interferon-α-2b in a murine model. Fertil Steril. 1999;71:907–911. doi: 10.1016/s0015-0282(99)00087-4. [DOI] [PubMed] [Google Scholar]
- 24.Eberwine J. Amplification of mRNA populations using aRNA generated from immobilized oligo(dT)-T7 primed cDNA. Biotechniques. 1996;20:584–591. doi: 10.2144/19962004584. [DOI] [PubMed] [Google Scholar]
- 25.Diegman J, Junker K, Gerstmayer B, Bosio A, Hindermann W, Rosenhahn J, von Eggeling F. Identification of CD70 as a diagnostic marker for clear cell renal cell carcinoma by gene expression profiling, real-time RT-PCR and immunohistochemistry. Eur J Cancer. 2005;41:1794–1801. doi: 10.1016/j.ejca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Hedenfalk I, Duggan D, Chen Y, Radmacher M, Bittner M, Simon R, et al. Gene-expression profiles in hereditary breast cancer. N Engl J Med. 2001;344:539–548. doi: 10.1056/NEJM200102223440801. [DOI] [PubMed] [Google Scholar]
- 27.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, et al. Minimum information about a microarray experiment (MIAME)- toward standards for microarray data. Nat Genet. 2001;29:365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta Ct) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- 30.Fang Z, Yang S, Gurates B, Tamura M, Simpson E, Evans E, Bulun SE. Genetic or enzymatic disruption of aromatase inhibits the growth of ectopic uterine tissue. J Clin Endocrinol Metab. 2002;87:3460–3466. doi: 10.1210/jcem.87.7.8683. [DOI] [PubMed] [Google Scholar]
- 31.Orr WE, Song BK, Geisert EE., Jr Comparing the use of Affymetrix to spotted oligonucleotide microarrays using two retinal pigment epithelium cell lines. Mol Vis. 2003;9:482–496. [PMC free article] [PubMed] [Google Scholar]
- 32.Jenson SD, Robetorye RS, Bohling SD, Schumacher JA, Morgan JW, Lim MS, Elenitoba-Johnson KS. Validation of cDNA microarray gene expression data obtained from linearly amplified RNA. Mol Pathol. 2003;56:307–312. doi: 10.1136/mp.56.6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JSM, Katari G, Sachidanandam R. GObar: A Gene Ontology based analysis and visualization tool for gene sets. BMC Bioinformatics. 2005;6:189. doi: 10.1186/1471-2105-6-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLaren J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update. 2000;6:45–55. doi: 10.1093/humupd/6.1.45. [DOI] [PubMed] [Google Scholar]
- 35.Groothuis PG, Nap AW, Winterhager E, Grummer R. Vascular development in endometriosis. Angiogenesis. 2005;8:147–156. doi: 10.1007/s10456-005-9005-x. Epub 2005 Oct 7. [DOI] [PubMed] [Google Scholar]
- 36.Print C, Valtola R, Evans A, Lessan K, Malik S, Smith S. Soluble factors from human endometrium promote angiogenesis and regulate the endothelial cell transcriptome. Hum Reprod. 2004;19:2356–66. doi: 10.1093/humrep/deh411. Epub 2004 Jul 8. [DOI] [PubMed] [Google Scholar]
- 37.Becker CM, Sampson DA, Rupnick MA, Rohan RM, Efstathiou JA, Short SM, Taylor GA, Folkman J, D’Amato RJ. Endostatin inhibits the growth of endometriotic lesions but does not affect fertility. Fertil Steril. 2005;84 (Suppl 2):1144–1155. doi: 10.1016/j.fertnstert.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 38.Laschke MW, Elitzsch A, Vollmar B, Vajkoczy P, Menger MD. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet-derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Human Reproduction. 2006;21:262–268. doi: 10.1093/humrep/dei308. [DOI] [PubMed] [Google Scholar]
- 39.D’Hooghe TM, Mwenda JM, Hill JA. A critical review of the use and application of the baboon as a model for research in women’s reproductive health. Gynecol Obstet Invest. 2004;57(1):1–60. Epub 2003 Dec 29. [Google Scholar]
- 40.Tjaden B, Galetto D, Woodruff JD, Rock JA. Time-related effects of RU486 treatment in experimentally induced endometriosis in the rat. Fertil Steril. 1993;59:437–440. doi: 10.1016/s0015-0282(16)55705-7. [DOI] [PubMed] [Google Scholar]
- 41.Keenan JA, Williams-Boyce PK, Massey PJ, Chen TT, Caudle MR, Bukovsky A. Regression of endometrial explants in a rat model of endometriosis treated with the immune modulators loxoribine and levamisole. Fertil Steril. 1999;72:135–141. doi: 10.1016/s0015-0282(99)00157-0. [DOI] [PubMed] [Google Scholar]
- 42.Dogan E, Saygili U, Posaci C, Tuna B, Caliskan S, Altunyurt S, Saatli B. Regression of endometrial explants in rats treated with the cyclooxygenase-2 inhibitor rofecoxib. Fertil Steril. 2004;82 (Suppl 3):1115–1120. doi: 10.1016/j.fertnstert.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 43.Lebovic DI, Kir M, Casey CL. Peroxisome proliferator-activated receptor-gamma induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril. 2004;82 (Suppl 3):1008–1013. doi: 10.1016/j.fertnstert.2004.02.148. [DOI] [PubMed] [Google Scholar]
- 44.Matsuzaki S, Canis M, Darcha C, Dallel R, Okamura K, Mage G. Cyclooxygenase-2 selective inhibitor prevents implantation of eutopic endometrium to ectopic sites in rats. Fertil Steril. 2004;82:1609–1615. doi: 10.1016/j.fertnstert.2004.07.946. [DOI] [PubMed] [Google Scholar]
- 45.D’Antonio M, Martelli F, Peano S, Papoian R, Borrelli F. Ability of recombinant human TNF binding protein-1 (r-hTBP-1) to inhibit the development of experimentally-induced endometriosis in rats. J Reprod Immunol. 2000;48:81–98. doi: 10.1016/s0165-0378(00)00073-5. [DOI] [PubMed] [Google Scholar]
- 46.Mulayim N, Savlu A, Guzeloglu-Kayisli O, Kayisli UA, Arici A. Regulation of endometrial stromal cell matrix metalloproteinase activity and invasiveness by interleukin-8. Fertil Steril. 2004;81 (Suppl 1):904–911. doi: 10.1016/j.fertnstert.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Chung HW, Wen Y, Chun SH, Nezhat C, Woo BH, Lake Polan M. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: a rationale for endometriotic invasiveness. Fertil Steril. 2001;75:152–159. doi: 10.1016/s0015-0282(00)01670-8. [DOI] [PubMed] [Google Scholar]
- 48.Ueda M, Yamashita Y, Takehara M, Terai Y, Kumagai K, Ueki K, Kanda K, Hung YC, Ueki M. Gene expression of adhesion molecules and matrix metalloproteinases in endometriosis. Gynecol Endocrinol. 2002;16:391–402. [PubMed] [Google Scholar]
- 49.Collette T, Bellehumeur C, Kats R, Maheux R, Mailloux J, Villeneuve M, Akoum A. Evidence for an increased release of proteolytic activity by the eutopic endometrial tissue in women with endometriosis and for involvement of matrix metalloproteinase-9. Hum Reprod. 2004;19:1257–1264. doi: 10.1093/humrep/deh290. Epub 2004 Apr 22. [DOI] [PubMed] [Google Scholar]
- 50.Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87:4782–4791. doi: 10.1210/jc.2002-020418. [DOI] [PubMed] [Google Scholar]
- 51.Lessey BA. Implantation defects in infertile women with endometriosis. Ann N Y Acad Sci. 2002;955:265–80. doi: 10.1111/j.1749-6632.2002.tb02787.x. discussion 293–5, 396–406. [DOI] [PubMed] [Google Scholar]
- 52.García-Velasco JA, Seli E, Arici A. Regulation of monocyte chemotactic protein-1 expression in human endometrial stromal cells by integrin-dependent cell adhesion. Biol Reprod. 1999;61:548–552. doi: 10.1095/biolreprod61.2.548. [DOI] [PubMed] [Google Scholar]
- 53.Jones RK, Searle RF, Bulmer JN. Apoptosis and bcl-2 expression in normal human endometrium, endometriosis and adenomyosis. Hum Reprod Update. 1998;4:702–709. doi: 10.1093/humrep/13.12.3496. [DOI] [PubMed] [Google Scholar]
- 54.Cameron RI, Maxwell P, Jenkins D, McCluggage WG. Immunohistochemical staining with MIB1, bcl2 and p16 assists in the distinction of cervical glandular intraepithelial neoplasia from tubo-endometrial metaplasia, endometriosis and microglandular hyperplasia. Histopathology. 2002;41:313–321. doi: 10.1046/j.1365-2559.2002.01465.x. [DOI] [PubMed] [Google Scholar]
- 55.Nishida M, Nasu K, Ueda T, Fukuda J, Takai N, Miyakawa I. Endometriotic cells are resistant to interferon-gamma-induced cell growth inhibition and apoptosis: a possible mechanism involved in the pathogenesis of endometriosis. Mol Hum Reprod. 2005;11:29–34. doi: 10.1093/molehr/gah133. Epub 2004 Dec 3. [DOI] [PubMed] [Google Scholar]
- 56.Hayrabedyan S, Kyurkchiev S, Kehayov I. FGF-1 and S100A13 possibly contribute to angiogenesis in endometriosis. J Reprod Immunol. 2005;67:87–101. doi: 10.1016/j.jri.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Tao XJ, Sayegh RA, Isaacson KB. Increased expression of complement component 3 in human ectopic endometrium compared with the matched eutopic endometrium. Fertil Steril. 1997;68:460–467. doi: 10.1016/s0015-0282(97)00254-9. [DOI] [PubMed] [Google Scholar]
- 58.Rageh MA, Moussad EE, Wilson AK, Brigstock DR. Steroidal regulation of connective tissue growth factor (CCN2; CTGF) synthesis in the mouse uterus. Mol Pathol. 2001;54:338–346. doi: 10.1136/mp.54.5.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Y, Kajdacsy-Balla A, Strawn E, Basir Z, Halverson G, Jailwala P, Wang Y, Wang X, Ghosh S, Guo SW. Transcriptional characterizations of differences between eutopic and ectopic endometrium. Endocrinology. 2006;147:232–246. doi: 10.1210/en.2005-0426. Epub 2005 Sep 29. [DOI] [PubMed] [Google Scholar]
- 60.Harrington DJ, Lessey BA, Rai V, Bergqvist A, Kennedy S, Manek S, Barlow DH, Mardon HJ. Tenascin is differentially expressed in endometrium and endometriosis. J Pathol. 1999;187:242–248. doi: 10.1002/(SICI)1096-9896(199901)187:2<242::AID-PATH221>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 61.Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643–649. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 62.Chrobak A, Gmyrek GB, Sozanski R, Sieradzka U, Paprocka M, Gabrys M, Jerzak M. The influence of extracellular matrix proteins on T-cell proliferation and apoptosis in women with endometriosis or uterine leiomyoma. Am J Reprod Immunol. 2004;51:123–129. doi: 10.1046/j.8755-8920.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 63.Nishida M, Nasu K, Fukuda J, Kawano Y, Narahara H, Miyakawa I. Down-regulation of interleukin-1 receptor type 1 expression causes the dysregulated expression of CXC chemokines in endometriotic stromal cells: a possible mechanism for the altered immunological functions in endometriosis. J Clin Endocrinol Metab. 2004;89:5094–5100. doi: 10.1210/jc.2004-0354. [DOI] [PubMed] [Google Scholar]
- 64.Matalliotakis IM, Goumenou AG, Mulayim N, Karkavitsas N, Koumantakis EE. High concentrations of the CA-125, CA 19-9 and CA 15-3 in the peritoneal fluid between patients with and without endometriosis. Arch Gynecol Obstet. 2005;271:40–45. doi: 10.1007/s00404-004-0645-7. Epub 2004 Jul 9. [DOI] [PubMed] [Google Scholar]
- 65.Hombach-Klonisch S, Kehlen A, Fowler PA, Huppertz B, Jugert JF, Bischoff G, Schluter E, Buchmann J, Klonisch T. Regulation of functional steroid receptors and ligand-induced responses in telomerase-immortalized human endometrial epithelial cells. J Mol Endocrinol. 2005;34:517–534. doi: 10.1677/jme.1.01550. [DOI] [PubMed] [Google Scholar]
- 66.Harada M, Osuga Y, Hirata T, Hirota Y, Koga K, Yoshino O, Morimoto C, Fujiwara T, Momoeda M, Yano T, Tsutsumi O, Taketani Y. Concentration of osteoprotegerin (OPG) in peritoneal fluid is increased in women with endometriosis. Hum Reprod. 2004;19:2188–2191. doi: 10.1093/humrep/deh412. Epub 2004 Jul 8. [DOI] [PubMed] [Google Scholar]
- 67.Bulletti C, Flamigni C, de Ziegler D. Implantation markers and endometriosis. Reprod Biomed Online. 2005;11:464–468. doi: 10.1016/s1472-6483(10)61142-x. [DOI] [PubMed] [Google Scholar]
- 68.Ramon L, Gilabert-Estelles J, Castello R, Gilabert J, Espana F, Romeu A, Chirivella M, Aznar J, Estelles A. mRNA analysis of several components of the plasminogen activator and matrix metalloproteinase systems in endometriosis using a real-time quantitative RT-PCR assay. Hum Reprod. 2005;20:272–8. doi: 10.1093/humrep/deh571. Epub 2004 Dec 3. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X, Lin J, Qian Y, Deng L. Decreased levels of interleukin-18 in peritoneal fluid but not in serum of patients with endometriosis. Fertil Steril. 2004;81:1229–1234. doi: 10.1016/j.fertnstert.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 70.Akoum A, Lemay A, Maheux R. Estradiol and interleukin-1beta exert a synergistic stimulatory effect on the expression of the chemokine regulated upon activation, normal T cell expressed, and secreted in endometriotic cells. J Clin Endocrinol Metab. 2002;87:5785–5792. doi: 10.1210/jc.2002-020106. [DOI] [PubMed] [Google Scholar]
- 71.Tan XJ, Lang JH, Liu DY, Shen K, Leng JH, Zhu L. Expression of vascular endothelial growth factor and thrombospondin-1 mRNA in patients with endometriosis. Fertil Steril. 2002;78:148–153. doi: 10.1016/s0015-0282(02)03187-4. [DOI] [PubMed] [Google Scholar]
- 72.Koks CA, Groothuis PG, Dunselman GA, de Goeij AF, Evers JL. Adhesion of menstrual endometrium to extracellular matrix: the possible role of integrin alpha(6)beta(1) and laminin interaction. Mol Hum Reprod. 2000;6:170–177. doi: 10.1093/molehr/6.2.170. [DOI] [PubMed] [Google Scholar]
- 73.Matsuzaki S, Canis M, Darcha C, Dechelotte P, Pouly JL, Bruhat MA. Fibrogenesis in peritoneal endometriosis. A semi-quantitative analysis of type-I collagen. Gynecol Obstet Invest. 1999;47:197–199. doi: 10.1159/000010094. [DOI] [PubMed] [Google Scholar]
- 74.Dimitriadis E, Stoikos C, Stafford-Bell M, Clark I, Paiva P, Kovacs G, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2006;69:53–64. doi: 10.1016/j.jri.2005.07.004. Epub 2005 Nov 28. [DOI] [PubMed] [Google Scholar]
- 75.Orlandi A, Ferlosio A, Ciucci A, Sesti F, Lifschitz-Mercer B, Gabbiani G, Spagnoli LG, Czernobilsky B. Cellular retinol-binding protein-1 expression in endometrial stromal cells: physiopathological and diagnostic implications. Histopathology. 2004;45:511–517. doi: 10.1111/j.1365-2559.2004.01963.x. [DOI] [PubMed] [Google Scholar]
- 76.Regidor PA, Regidor M, Schindler AE, Winterhager E. Aberrant expression pattern of gap junction connexins in endometriotic tissues. Mol Hum Reprod. 1997;3:375–381. doi: 10.1093/molehr/3.5.375. [DOI] [PubMed] [Google Scholar]
- 77.Ota H, Igarashi S, Hatazawa J, Tanaka T. Immunohistochemical assessment of superoxide dismutase expression in the endometrium in endometriosis and adenomyosis. Fertil Steril. 1999;72:129–134. doi: 10.1016/s0015-0282(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 78.Gmyrek GB, Sozanski R, Jerzak M, Chrobak A, Wickiewicz D, Skupnik A, Sieradzka U, Fortuna W, Gabrys M, Chelmonska-Soyta A. Evaluation of monocyte chemotactic protein-1 levels in peripheral blood of infertile women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;122:199–205. doi: 10.1016/j.ejogrb.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 79.Konno R, Yamada-Okabe H, Fujiwara H, Uchiide I, Shibahara H, Ohwada M, et al. Role of immunoreactions and mast cells in pathogenesis of human endometriosis-morphologic study and gene expression analysis. Hum Cell. 2003;16:141–149. doi: 10.1111/j.1749-0774.2003.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 80.Chung HW, Lee JY, Moon HS, Hur SE, Park MH, Wen Y, Polan ML. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil Steril. 2002;78:787–795. doi: 10.1016/s0015-0282(02)03322-8. [DOI] [PubMed] [Google Scholar]
- 81.Tsuchiya M, Katoh T, Motoyama H, Sasaki H, Tsugane S, Ikenoue T. Analysis of the AhR, ARNT, and AhRR gene polymorphisms: genetic contribution to endometriosis susceptibility and severity. Fertil Steril. 2005;84:454–458. doi: 10.1016/j.fertnstert.2005.01.130. [DOI] [PubMed] [Google Scholar]
- 82.Kusume T, Maeda N, Izumiya C, Yamamoto Y, Hayashi K, Oguri H, Nishimori Y, Fukaya T. Human leukocyte antigen expression by peritoneal macrophages from women with pelvic endometriosis is depressed but coordinated with costimulatory molecule expression. Fertil Steril. 2005 Apr;83(Suppl 1):1232–40. doi: 10.1016/j.fertnstert.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 83.Wu MH, Yang BC, Lee YC, Wu PL, Hsu CC. The differential expression of intercellular adhesion molecule-1 (ICAM-1) and regulation by interferon-gamma during the pathogenesis of endometriosis. Am J Reprod Immunol. 2004;51:373–380. doi: 10.1111/j.1600-0897.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 84.Johnson MC, Torres M, Alves A, Bacallao K, Fuentes A, Vega M, Boric MA. Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-beta1 and bax genes. Reprod Biol Endocrinol. 2005;3:45. doi: 10.1186/1477-7827-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsieh YY, Chang CC, Tsai FJ, Peng CT, Yeh LS, Lin CC. Polymorphism for transforming growth factor beta 1-509 (TGF-B1-509): association with endometriosis. Biochem Genet. 2005;43:203–210. doi: 10.1007/s10528-005-5211-x. [DOI] [PubMed] [Google Scholar]
- 86.Roberts M, Luo X, Chegini N. Differential regulation of interleukins IL-13 and IL-15 by ovarian steroids, TNF-alpha and TGF-beta in human endometrial epithelial and stromal cells. Mol Hum Reprod. 2005 Oct;11(10):751–60. doi: 10.1093/molehr/gah233. Epub 2005 Oct 27. [DOI] [PubMed] [Google Scholar]
- 87.Lang GA, Yeaman GR. Autoantibodies in endometriosis sera recognize a Thomsen-Friedenreich-like carbohydrate antigen. J Autoimmun. 2001;16:151–161. doi: 10.1006/jaut.2000.0465. [DOI] [PubMed] [Google Scholar]
- 88.Mathur SP, Lee JH, Jiang H, Arnaud P, Rust PF. Levels of transferrin and alpha 2-HS glycoprotein in women with and without endometriosis. Autoimmunity. 1999;29:121–127. doi: 10.3109/08916939908995381. [DOI] [PubMed] [Google Scholar]
- 89.Bellehumeur C, Collette T, Maheux R, Mailloux J, Villeneuve M, Akoum A. Increased soluble interleukin-1 receptor type II proteolysis in the endometrium of women with endometriosis. Hum Reprod. 2005;20:1177–1184. doi: 10.1093/humrep/deh749. Epub 2005 Feb 10. [DOI] [PubMed] [Google Scholar]
- 90.Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–606. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 91.Hsieh YY, Chang CC, Tsai FJ, Hsu CM, Lin CC, Tsai CH. Angiotensin I-converting enzyme ACE 2350*G and ACE-240*T-related genotypes and alleles are associated with higher susceptibility to endometriosis. Mol Hum Reprod. 2005;11:11–14. doi: 10.1093/molehr/gah131. Epub 2004 Nov 5. [DOI] [PubMed] [Google Scholar]
- 92.Luo X, Xu J, Chegini N. The expression of Smads in human endometrium and regulation and induction in endometrial epithelial and stromal cells by transforming growth factor-beta. J Clin Endocrinol Metab. 2003;88:4967–4976. doi: 10.1210/jc.2003-030276. [DOI] [PubMed] [Google Scholar]
- 93.Gallinelli A, Chiossi G, Giannella L, Marsella T, Genazzani AD, Volpe A. Different concentrations of interleukins in the peritoneal fluid of women with endometriosis: relationships with lymphocyte subsets. Gynecol Endocrinol. 2004;18:144–151. doi: 10.1080/09513590310001653044. [DOI] [PubMed] [Google Scholar]
- 94.Harris HA, Bruner-Tran KL, Zhang X, Osteen KG, Lyttle CR. A selective estrogen receptor-beta agonist causes lesion regression in an experimentally induced model of endometriosis. Hum Reprod. 2005;20:936–941. doi: 10.1093/humrep/deh711. Epub 2004 Dec 23. [DOI] [PubMed] [Google Scholar]
- 95.Matsuzaki S, Murakami T, Uehara S, Canis M, Sasano H, Okamura K. Expression of estrogen receptor alpha and beta in peritoneal and ovarian endometriosis. Fertil Steril. 2001;75:1198–1205. doi: 10.1016/s0015-0282(01)01783-6. [DOI] [PubMed] [Google Scholar]
- 96.Nip MM, Miller D, Taylor PV, Gannon MJ, Hancock KW. Expression of heat shock protein 70 kDa in human endometrium of normal and infertile women. Hum Reprod. 1994;9:1253–1256. doi: 10.1093/oxfordjournals.humrep.a138689. [DOI] [PubMed] [Google Scholar]
- 97.Noda T, Murakami T, Terada Y, Yaegashi N, Okamura K. Increased production of tumor necrosis factor-alpha by peritoneal fluid mononuclear cells induced by 60-kDa heat shock protein in women with minimal to mild endometriosis. Am J Reprod Immunol. 2003;50:427–432. doi: 10.1034/j.1600-0897.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 98.Kligman I, Grifo JA, Witkin SS. Expression of the 60 kDa heat shock protein in peritoneal fluids from women with endometriosis: implications for endometriosis-associated infertility. Hum Reprod. 1996;11:2736–2738. doi: 10.1093/oxfordjournals.humrep.a019200. [DOI] [PubMed] [Google Scholar]
- 99.Anaf V, Simon P, El Nakadi I, Fayt I, Simonart T, Buxant F, Noel JC. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002;17:1895–1900. doi: 10.1093/humrep/17.7.1895. [DOI] [PubMed] [Google Scholar]
- 100.Hsieh YY, Chang CC, Tsai FJ, Lin CC, Tsai CH. T homozygote and allele of epidermal growth factor receptor 2073 gene polymorphism are associated with higher susceptibility to endometriosis and leiomyomas. Fertil Steril. 2005;83:796–799. doi: 10.1016/j.fertnstert.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 101.Huang JC, Yeh J. Quantitative analysis of epidermal growth factor receptor gene expression in endometriosis. J Clin Endocrinol Metab. 1994;79:1097–1101. doi: 10.1210/jcem.79.4.7962280. [DOI] [PubMed] [Google Scholar]
- 102.Guo SW. Glutathione S-transferases M1/T1 gene polymorphisms and endometriosis: a meta-analysis of genetic association studies. Mol Hum Reprod. 2005;11:729–743. doi: 10.1093/molehr/gah206. Epub 2005 Nov 16. [DOI] [PubMed] [Google Scholar]
- 103.Tabibzadeh S, Mason JM, Shea W, Cai Y, Murray MJ, Lessey B. Dysregulated expression of ebaf, a novel molecular defect in the endometria of patients with infertility. J Clin Endocrinol Metab. 2000;85:2526–2536. doi: 10.1210/jcem.85.7.6674. [DOI] [PubMed] [Google Scholar]
- 104.Darnel AD, Archer TK, Yang K. Regulation of 11beta-hydroxysteroid dehydrogenase type 2 by steroid hormones and epidermal growth factor in the Ishikawa human endometrial cell line. J Steroid Biochem Mol Biol. 1999;70:203–210. doi: 10.1016/s0960-0760(99)00116-8. [DOI] [PubMed] [Google Scholar]


