Abstract
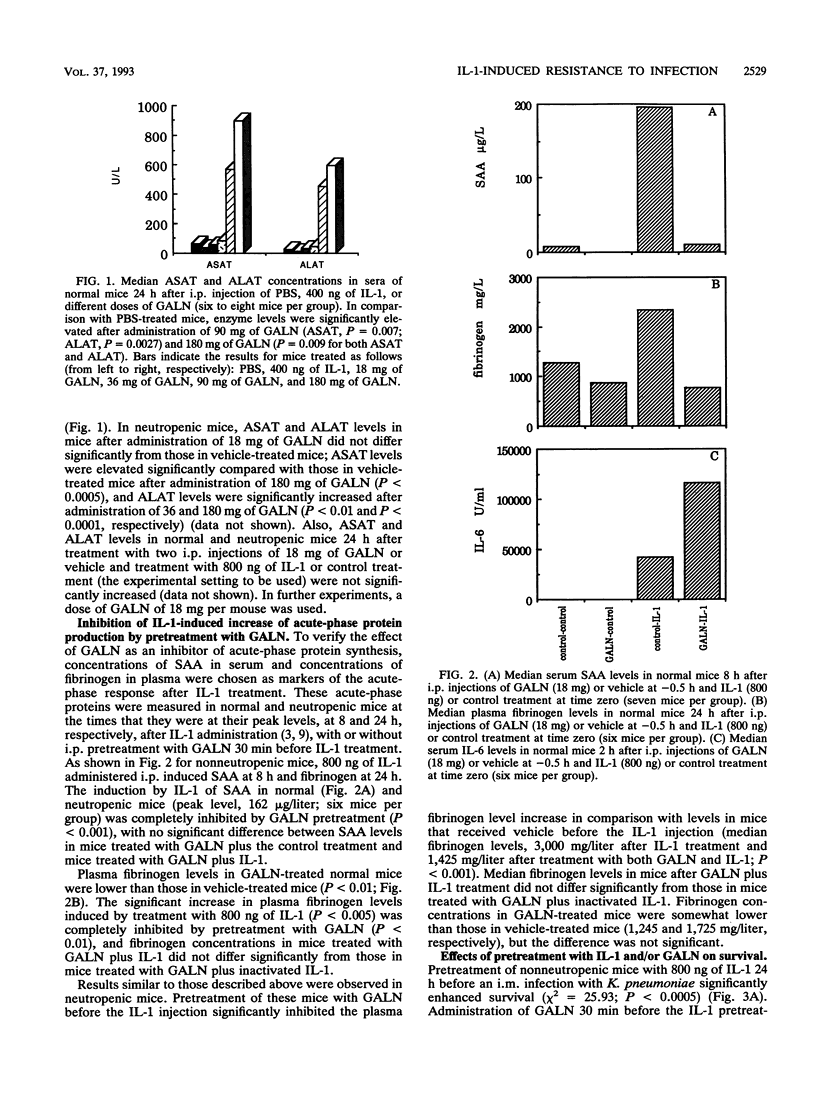
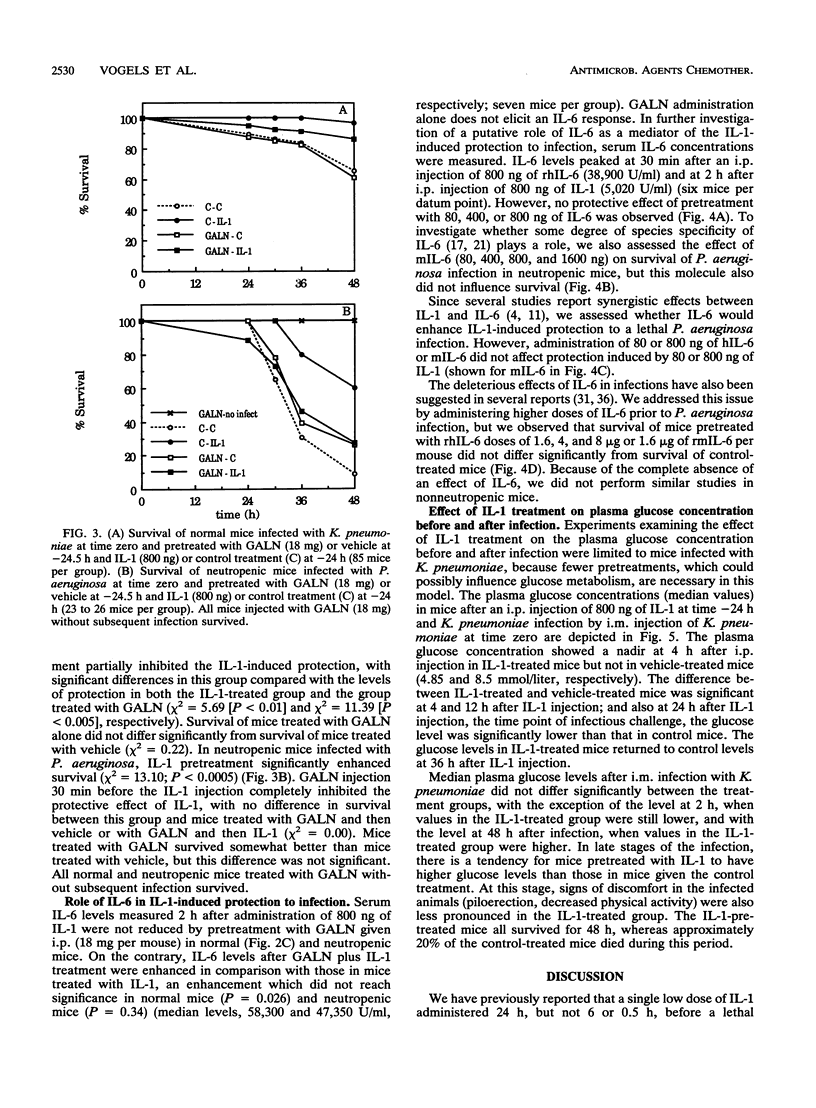
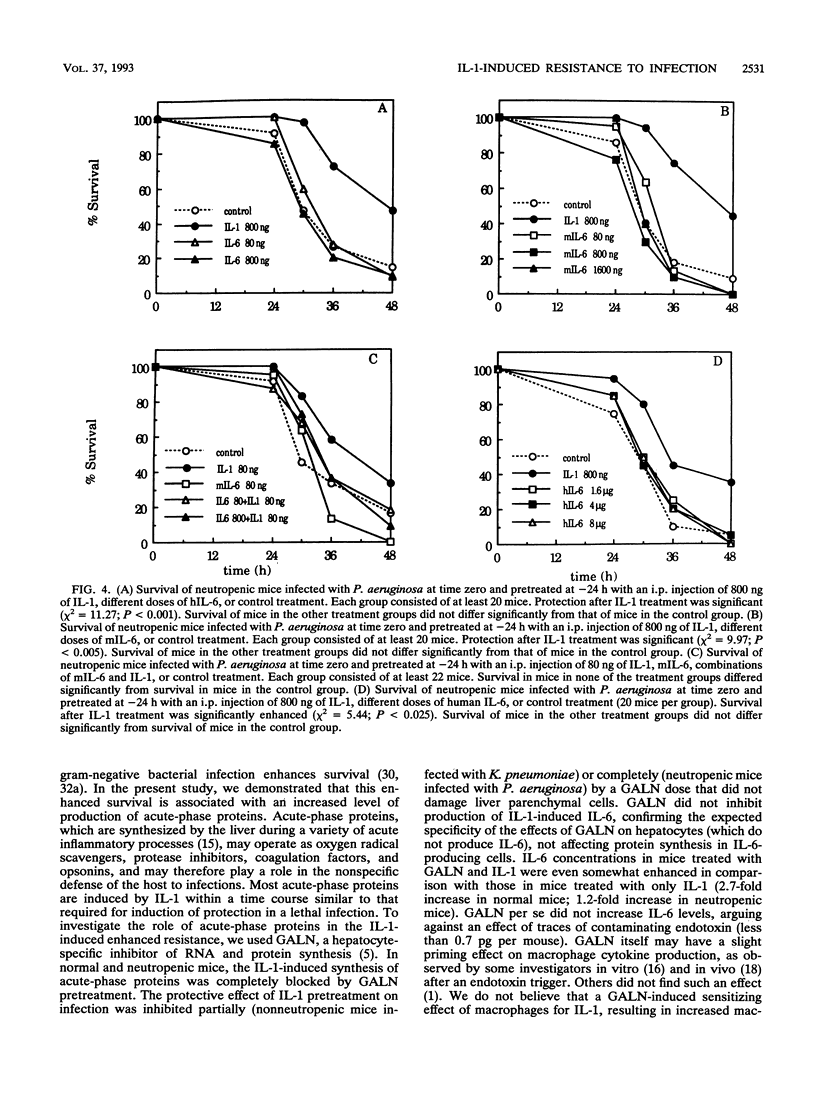
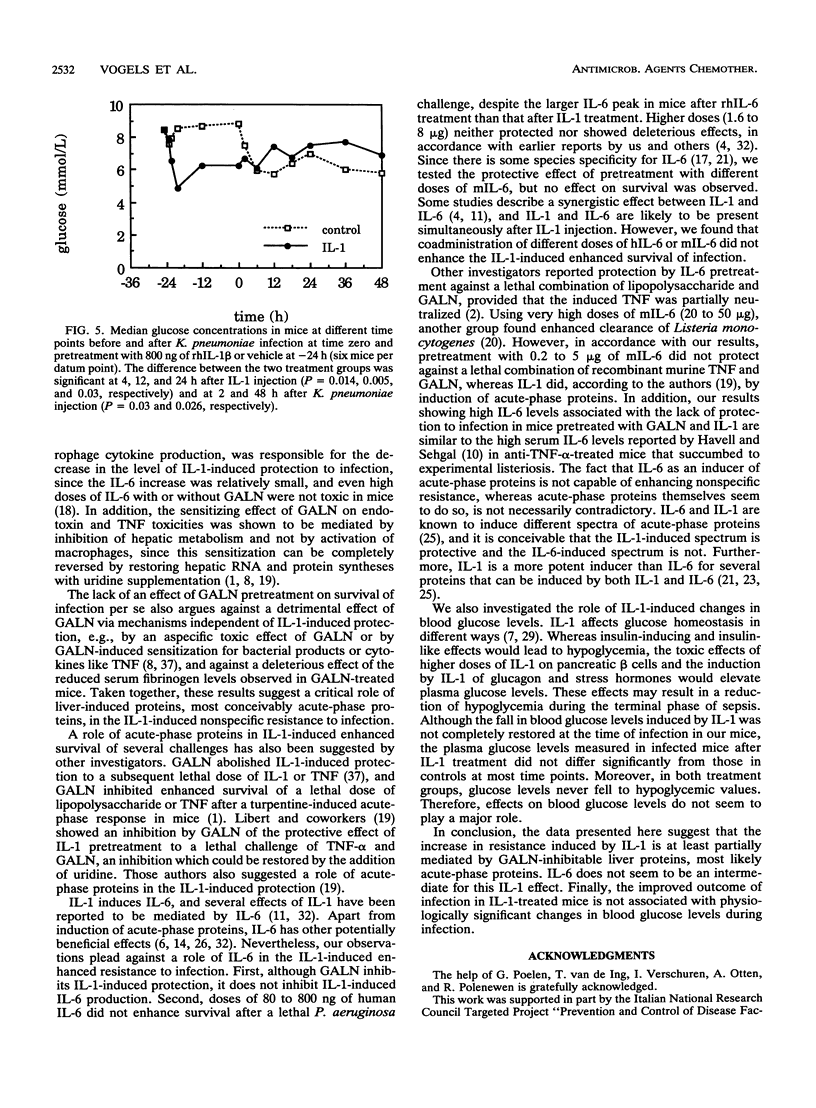
Treatment with a single low dose (80 to 800 ng) of interleukin-1 (IL-1) 24 h before a lethal bacterial challenge of granulocytopenic and normal mice enhances nonspecific resistance. Since IL-1 induces secretion of acute-phase proteins, liver proteins which possess several detoxifying effects, we investigated the role of these proteins in the IL-1-induced protection. Inhibition of liver protein synthesis with D-galactosamine (GALN) completely inhibited the IL-1-induced synthesis of acute-phase proteins. GALN pretreatment abolished the protective effect of IL-1 on survival completely (neutropenic mice infected with Pseudomonas aeruginosa) or partially (nonneutropenic mice infected with Klebsiella pneumoniae). Pretreatment with IL-6, a cytokine induced by IL-1, did not reproduce the protection offered after IL-1 pretreatment, nor did it enhance or deteriorate the IL-1-enhanced resistance to infection. A protective effect of IL-1 via effects on glucose homeostasis during the acute-phase response was investigated by comparing plasma glucose levels in IL-1-treated mice and control mice before and during infection. Although glucose levels in IL-1-pretreated mice were somewhat higher in the later stages of infection, no significant differences from levels in control mice were present, and the glucose levels in control-treated animals never fell to hypoglycemic values. We conclude that the IL-1-induced nonspecific resistance is mediated neither by the induction of IL-6 nor by the effects of IL-1 on glucose homeostasis. Acute-phase proteins generated after IL-1 pretreatment, however, seem to play a critical role in the IL-1-induced protection to infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcorn J. M., Fierer J., Chojkier M. The acute-phase response protects mice from D-galactosamine sensitization to endotoxin and tumor necrosis factor-alpha. Hepatology. 1992 Jan;15(1):122–129. doi: 10.1002/hep.1840150121. [DOI] [PubMed] [Google Scholar]
- Barton B. E., Jackson J. V. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993 Apr;61(4):1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Erroi A., Villa P., Ghezzi P. Dexamethasone modulation of in vivo effects of endotoxin, tumor necrosis factor, and interleukin-1 on liver cytochrome P-450, plasma fibrinogen, and serum iron. J Leukoc Biol. 1989 Sep;46(3):254–262. doi: 10.1002/jlb.46.3.254. [DOI] [PubMed] [Google Scholar]
- Czuprynski C. J., Haak-Frendscho M., Maroushek N., Brown J. F. Effects of recombinant human interleukin-6 alone and in combination with recombinant interleukin-1 alpha and tumor necrosis factor alpha on antibacterial resistance in mice. Antimicrob Agents Chemother. 1992 Jan;36(1):68–70. doi: 10.1128/aac.36.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K., Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;(71):77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Stimulation of antibacterial macrophage activities by B-cell stimulatory factor 2 (interleukin-6). Infect Immun. 1990 Jan;58(1):269–271. doi: 10.1128/iai.58.1.269-271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn K. N. Hormonal control of metabolism in trauma and sepsis. Clin Endocrinol (Oxf) 1986 May;24(5):577–599. doi: 10.1111/j.1365-2265.1986.tb03288.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P., Sipe J. D. Dexamethasone modulation of LPS, IL-1, and TNF stimulated serum amyloid A synthesis in mice. Lymphokine Res. 1988 Summer;7(2):157–166. [PubMed] [Google Scholar]
- Havell E. A., Sehgal P. B. Tumor necrosis factor-independent IL-6 production during murine listeriosis. J Immunol. 1991 Jan 15;146(2):756–761. [PubMed] [Google Scholar]
- Helle M., Brakenhoff J. P., De Groot E. R., Aarden L. A. Interleukin 6 is involved in interleukin 1-induced activities. Eur J Immunol. 1988 Jun;18(6):957–959. doi: 10.1002/eji.1830180619. [DOI] [PubMed] [Google Scholar]
- Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol. 1992 Jan;62(1 Pt 2):S60–S65. doi: 10.1016/0090-1229(92)90042-m. [DOI] [PubMed] [Google Scholar]
- Kharazmi A., Nielsen H., Rechnitzer C., Bendtzen K. Interleukin 6 primes human neutrophil and monocyte oxidative burst response. Immunol Lett. 1989 May;21(2):177–184. doi: 10.1016/0165-2478(89)90056-4. [DOI] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Lasfargues A., Chaby R. Endotoxin-induced tumor necrosis factor (TNF): selective triggering of TNF and interleukin-1 production by distinct glucosamine-derived lipids. Cell Immunol. 1988 Aug;115(1):165–178. doi: 10.1016/0008-8749(88)90171-2. [DOI] [PubMed] [Google Scholar]
- Leebeek F. W., Fowlkes D. M. Construction and functional analysis of hybrid interleukin-6 variants. Characterization of the role of the C-terminus for species specificity. FEBS Lett. 1992 Jul 20;306(2-3):262–264. doi: 10.1016/0014-5793(92)81013-c. [DOI] [PubMed] [Google Scholar]
- Libert C., Van Bladel S., Brouckaert P., Fiers W. The influence of modulating substances on tumor necrosis factor and interleukin-6 levels after injection of murine tumor necrosis factor or lipopolysaccharide in mice. J Immunother (1991) 1991 Aug;10(4):227–235. doi: 10.1097/00002371-199108000-00001. [DOI] [PubMed] [Google Scholar]
- Libert C., Van Bladel S., Brouckaert P., Shaw A., Fiers W. Involvement of the liver, but not of IL-6, in IL-1-induced desensitization to the lethal effects of tumor necrosis factor. J Immunol. 1991 Apr 15;146(8):2625–2632. [PubMed] [Google Scholar]
- Liu Z., Simpson R. J., Cheers C. Recombinant interleukin-6 protects mice against experimental bacterial infection. Infect Immun. 1992 Oct;60(10):4402–4406. doi: 10.1128/iai.60.10.4402-4406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikage T., Mizushima Y., Sakamoto K., Yano S. Prevention of fatal infections by recombinant human interleukin 1 alpha in normal and anticancer drug-treated mice. Cancer Res. 1990 Apr 1;50(7):2099–2104. [PubMed] [Google Scholar]
- Neta R., Perlstein R., Vogel S. N., Ledney G. D., Abrams J. Role of interleukin 6 (IL-6) in protection from lethal irradiation and in endocrine responses to IL-1 and tumor necrosis factor. J Exp Med. 1992 Mar 1;175(3):689–694. doi: 10.1084/jem.175.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Van Damme J., Rieder H., Meyer zum Büschenfelde K. H. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol. 1988 Aug;18(8):1259–1264. doi: 10.1002/eji.1830180817. [DOI] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Sipe J. D., Gonnerman W. A., Loose L. D., Knapschaefer G., Xie W. J., Franzblau C. Direct binding enzyme-linked immunosorbent assay (ELISA) for serum amyloid A (SAA). J Immunol Methods. 1989 Dec 20;125(1-2):125–135. doi: 10.1016/0022-1759(89)90085-9. [DOI] [PubMed] [Google Scholar]
- Sironi M., Breviario F., Proserpio P., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989 Jan 15;142(2):549–553. [PubMed] [Google Scholar]
- Thibault V., Terlain B., Ekindjian O. G. Interleukine-1 et régulation de l'homéostasie du glucose. Pathol Biol (Paris) 1993 Feb;41(2):178–186. [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Van der Meer J. W., Helle M., Aarden L. Comparison of the effects of recombinant interleukin 6 and recombinant interleukin 1 on nonspecific resistance to infection. Eur J Immunol. 1989 Feb;19(2):413–416. doi: 10.1002/eji.1830190229. [DOI] [PubMed] [Google Scholar]
- Vogels M. T., Lindley I. J., Curfs J. H., Eling W. M., van der Meer J. W. Effects of interleukin-8 on nonspecific resistance to infection in neutropenic and normal mice. Antimicrob Agents Chemother. 1993 Feb;37(2):276–280. doi: 10.1128/aac.37.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels M. T., Sweep C. G., Hermus A. R., van der Meer J. W. Interleukin-1-induced nonspecific resistance to bacterial infection in mice is not mediated by glucocorticosteroids. Antimicrob Agents Chemother. 1992 Dec;36(12):2785–2789. doi: 10.1128/aac.36.12.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels M. T., van der Meer J. W. Use of immune modulators in nonspecific therapy of bacterial infections. Antimicrob Agents Chemother. 1992 Jan;36(1):1–5. doi: 10.1128/aac.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Holtmann H., Engelmann H., Nophar Y. Sensitization and desensitization to lethal effects of tumor necrosis factor and IL-1. J Immunol. 1988 May 1;140(9):2994–2999. [PubMed] [Google Scholar]
- van der Meer J. W., Barza M., Wolff S. M., Dinarello C. A. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]


