Abstract
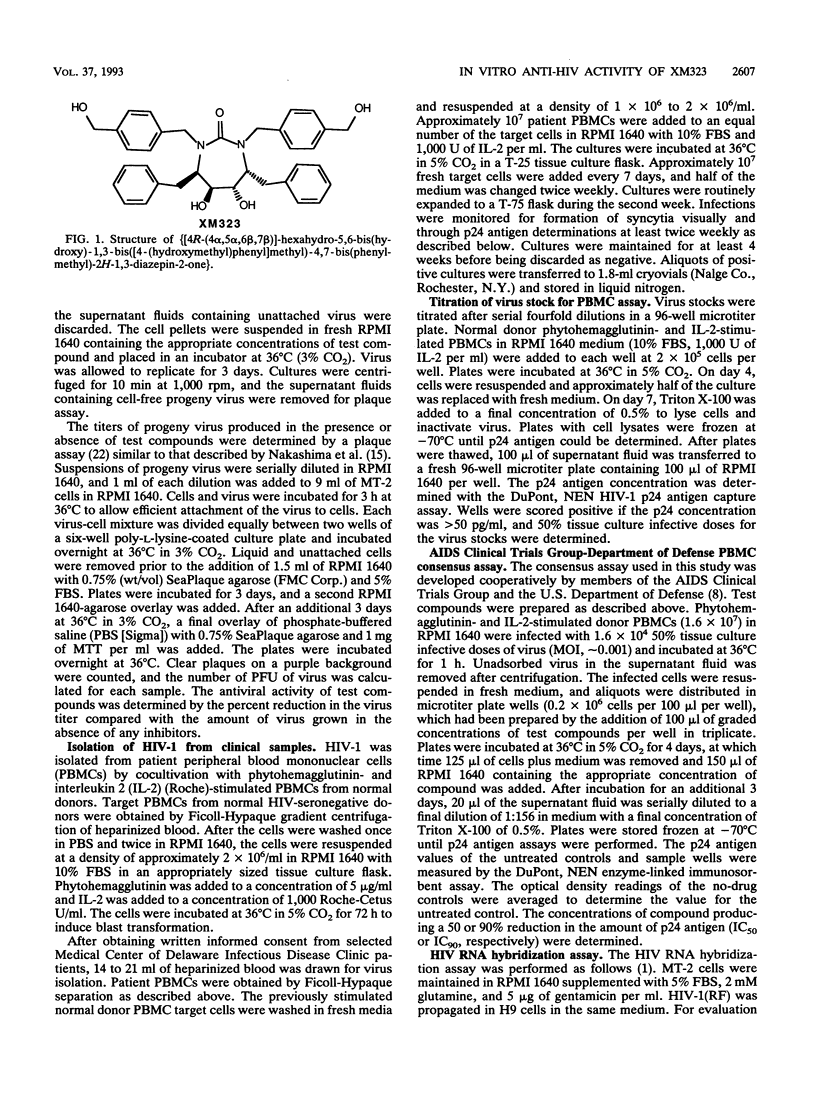
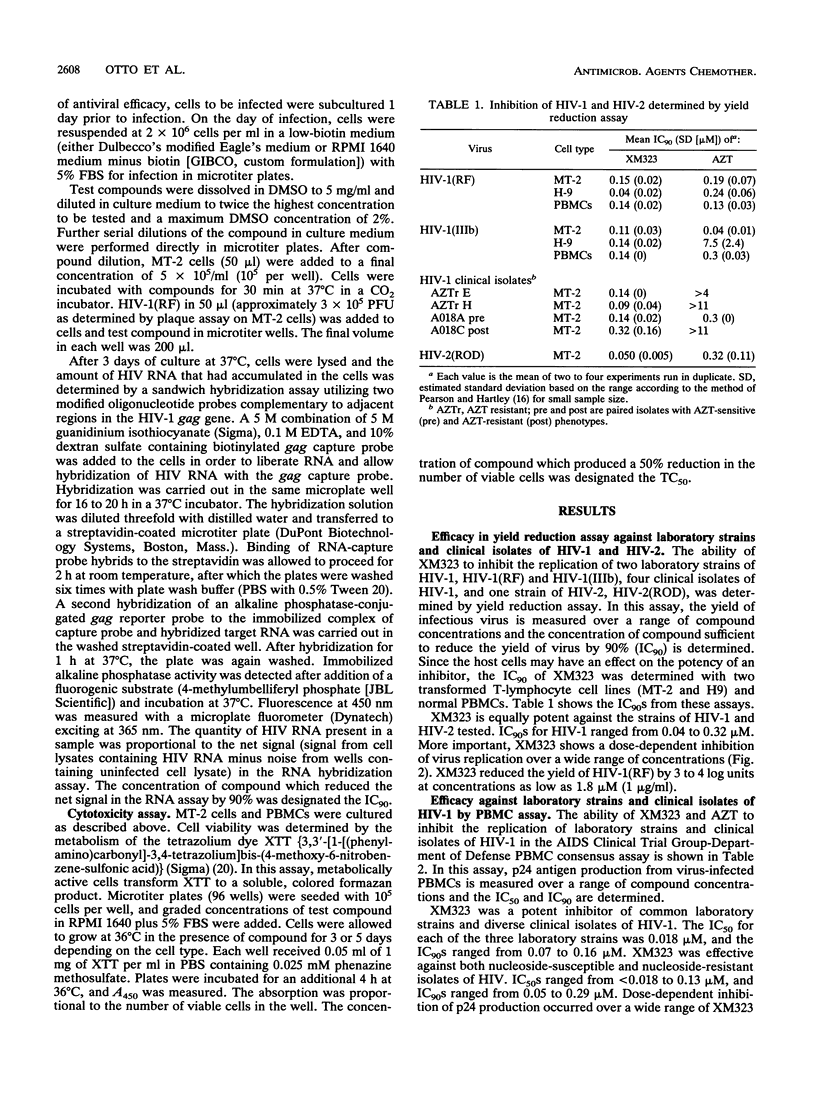
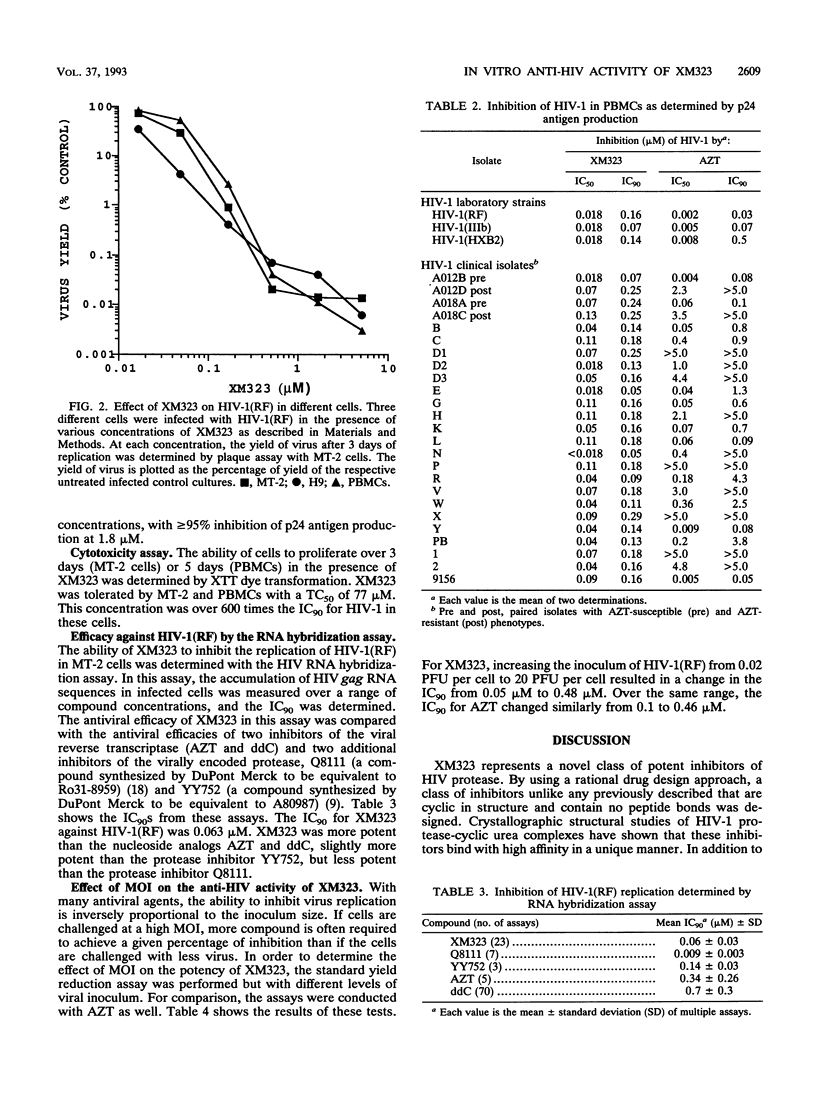
XM323 represents a novel class of potent inhibitors of human immunodeficiency virus (HIV) protease. In vitro studies have shown that inhibition of this enzyme translates into potent inhibition of replication of HIV type 1 (HIV-1) and HIV-2. The inhibition of virus replication was assessed with three assays designed to measure the production of infectious virus, viral RNA, or p24 antigen. The production of mature infectious virions was measured with a yield reduction assay. By this assay, several strains and isolates of HIV-1 and HIV-2 were shown to be susceptible to XM323 in two lymphoid cell lines (MT-2 and H9) and in normal peripheral blood mononuclear cells, with a concentration required for 90% inhibition (IC90) of 0.12 +/- 0.04 microM (mean +/- standard deviation). The production of HIV-1(RF) RNA was measured with an RNA hybridization-capture assay. With this assay, XM323 was shown to be a potent inhibitor of HIV-1(RF) replication, with an IC90 of 0.063 +/- 0.032 microM. A third measure of virus replication, the production of p24 viral antigen, an essential protein component of the virion, was determined with the AIDS Clinical Trial Group-Department of Defense peripheral blood mononuclear cell consensus assay. This assay was used for expanded testing of XM323 against 28 clinical isolates and laboratory strains of HIV-1. XM323 was shown to be equally effective against zidovudine-susceptible and zidovudine-resistant isolates of HIV-1, with an overall IC90 of 0.16 +/- 0.06 microM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Darke P. L., Nutt R. F., Brady S. F., Garsky V. M., Ciccarone T. M., Leu C. T., Lumma P. K., Freidinger R. M., Veber D. F., Sigal I. S. HIV-1 protease specificity of peptide cleavage is sufficient for processing of gag and pol polyproteins. Biochem Biophys Res Commun. 1988 Oct 14;156(1):297–303. doi: 10.1016/s0006-291x(88)80839-8. [DOI] [PubMed] [Google Scholar]
- Debouck C., Gorniak J. G., Strickler J. E., Meek T. D., Metcalf B. W., Rosenberg M. Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8903–8906. doi: 10.1073/pnas.84.24.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer G. B., Metcalf B. W., Tomaszek T. A., Jr, Carr T. J., Chandler A. C., 3rd, Hyland L., Fakhoury S. A., Magaard V. W., Moore M. L., Strickler J. E. Inhibition of human immunodeficiency virus 1 protease in vitro: rational design of substrate analogue inhibitors. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9752–9756. doi: 10.1073/pnas.86.24.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobelny D., Wondrak E. M., Galardy R. E., Oroszlan S. Selective phosphinate transition-state analogue inhibitors of the protease of human immunodeficiency virus. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1111–1116. doi: 10.1016/0006-291x(90)92010-w. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Japour A. J., Mayers D. L., Johnson V. A., Kuritzkes D. R., Beckett L. A., Arduino J. M., Lane J., Black R. J., Reichelderfer P. S., D'Aquila R. T. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. The RV-43 Study Group, the AIDS Clinical Trials Group Virology Committee Resistance Working Group. Antimicrob Agents Chemother. 1993 May;37(5):1095–1101. doi: 10.1128/aac.37.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf D. J., Norbeck D. W., Codacovi L., Wang X. C., Kohlbrenner W. E., Wideburg N. E., Paul D. A., Knigge M. F., Vasavanonda S., Craig-Kennard A. Structure-based, C2 symmetric inhibitors of HIV protease. J Med Chem. 1990 Oct;33(10):2687–2689. doi: 10.1021/jm00172a002. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade T. J., Tomasselli A. G., Liu L., Karacostas V., Moss B., Sawyer T. K., Heinrikson R. L., Tarpley W. G. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science. 1990 Jan 26;247(4941):454–456. doi: 10.1126/science.2405486. [DOI] [PubMed] [Google Scholar]
- Moore M. L., Bryan W. M., Fakhoury S. A., Magaard V. W., Huffman W. F., Dayton B. D., Meek T. D., Hyland L., Dreyer G. B., Metcalf B. W. Peptide substrates and inhibitors of the HIV-1 protease. Biochem Biophys Res Commun. 1989 Mar 15;159(2):420–425. doi: 10.1016/0006-291x(89)90008-9. [DOI] [PubMed] [Google Scholar]
- Roberts N. A., Martin J. A., Kinchington D., Broadhurst A. V., Craig J. C., Duncan I. B., Galpin S. A., Handa B. K., Kay J., Kröhn A. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990 Apr 20;248(4953):358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- Royer W. E., Jr, Love W. E., Fenderson F. F. Cooperative dimeric and tetrameric clam haemoglobins are novel assemblages of myoglobin folds. Nature. 1985 Jul 18;316(6025):277–280. doi: 10.1038/316277a0. [DOI] [PubMed] [Google Scholar]
- Schneider J., Kent S. B. Enzymatic activity of a synthetic 99 residue protein corresponding to the putative HIV-1 protease. Cell. 1988 Jul 29;54(3):363–368. doi: 10.1016/0092-8674(88)90199-7. [DOI] [PubMed] [Google Scholar]
- Scudiero D. A., Shoemaker R. H., Paull K. D., Monks A., Tierney S., Nofziger T. H., Currens M. J., Seniff D., Boyd M. R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988 Sep 1;48(17):4827–4833. [PubMed] [Google Scholar]
- Sham H. L., Betebenner D. A., Wideburg N. E., Saldivar A. C., Kohlbrenner W. E., Vasavanonda S., Kempf D. J., Norbeck D. W., Zhao C., Clement J. J. Potent HIV-1 protease inhibitors with antiviral activities in vitro. Biochem Biophys Res Commun. 1991 Mar 29;175(3):914–919. doi: 10.1016/0006-291x(91)91652-s. [DOI] [PubMed] [Google Scholar]
- Tomasselli A. G., Hui J. O., Sawyer T. K., Staples D. J., Bannow C., Reardon I. M., Howe W. J., DeCamp D. L., Craik C. S., Heinrikson R. L. Specificity and inhibition of proteases from human immunodeficiency viruses 1 and 2. J Biol Chem. 1990 Aug 25;265(24):14675–14683. [PubMed] [Google Scholar]
- Vacca J. P., Guare J. P., deSolms S. J., Sanders W. M., Giuliani E. A., Young S. D., Darke P. L., Zugay J., Sigal I. S., Schleif W. A. L-687,908, a potent hydroxyethylene-containing HIV protease inhibitor. J Med Chem. 1991 Mar;34(3):1225–1228. doi: 10.1021/jm00107a050. [DOI] [PubMed] [Google Scholar]


