Abstract
COPI-coated vesicles mediate protein transport within the early secretory pathway. Their coat consists of ADP ribosylation factor (ARF1, a small guanosine nucleotide binding protein), and coatomer, a cytosolic complex composed of seven subunits, α- to ζ-coat proteins (COPs). For coat formation that initiates budding of a vesicle, ARF1 is recruited to the Golgi membrane from the cytosol in its GTP-bound form, and subsequently, coatomer can bind to the membrane. To identify a minimal structure of coatomer capable to bind to Golgi membranes in an ARF1-dependent manner, we have established a procedure to dissociate coatomer under conditions that allow reassociation of the subunits to a complete and functional complex. After dissociation, subunits or subcomplexes can be isolated and may be expected to be functional. Herein we describe isolation of a subcomplex of coatomer consisting of β- and δ-COPs that is able to bind to Golgi membranes in an ARF1- and GTP-dependent manner.
COPI vesicles mediate protein transport within the early secretory pathway (1). In this study we focus on the structure and function of mammalian coatomer (2), a cytosolic complex of seven subunits [α- to ζ-coat proteins (COPs)] (3–13), that with ADP ribosylation factor (ARF1) (14, 15) cover the surface of COPI-coated vesicles (16).
The molecular mechanisms underlying the budding of a COPI vesicle from the Golgi are known in part: ARF1 in its GDP-bound state is a soluble cytosolic protein. After contact with the Golgi membrane GDP is exchanged with GTP (14), a reaction catalyzed by a Golgi-attached nucleotide exchange protein (17–19). ARF1–GTP is tightly bound to the membrane, and this binding is saturable (20). Coatomer can be recruited from the cytosol to the Golgi membrane only after binding ARF1–GTP. The role of ARF1 in this mechanism is viewed controversially. In contrast to a direct interaction of coatomer with ARF1–GTP on the Golgi membrane, an indirect role of ARF1–GTP has been suggested. According to this concept, ARF1–GTP activates a membrane-bound phospholipase D (21, 22), resulting in increased amounts of phosphatidic acid (PA) in the Golgi membrane, and this rise in PA would stimulate the formation of phosphatidylinositol 4,5-bisphosphate (PIP2) (23). PIP2 and PA are thought to represent the binding site(s) for coatomer (24). However, ARF1 has recently been shown to interact directly with coatomer, dependent on the presence of GTP (25). This interaction seems to persist during budding because it is found also in isolated COPI vesicles.
To study the minimal requirements for the interaction of Golgi membranes with coatomer subunits, we have established a method that reversibly dissociates coatomer into subunits and subcomplexes so that such subcomplexes can be isolated in a native state that will allow functional studies of the isolated proteins. Herein, we describe the dissociation of isolated coatomer into substructures under conditions that allow their reassociation to a structurally and functionally complete complex. After dissociation of the complex, we have characterized various subcomplexes and isolated a β/δ-COP dimer that binds to Golgi membranes in an ARF1- and GTP-dependent manner.
MATERIALS AND METHODS
Materials.
Dimethyl maleic anhydride (DMMA) was purchased from Sigma. ARF1 was prepared as described (20). The isolation of rabbit liver Golgi membranes was performed as described (26).
Antibodies.
For Western blot analysis, the following antibodies were used: anti-peptide antibodies to α-COP (antibody 883), β′-COP (C1PL), and δ-COP (antibody 877) were produced and purified as described (3, 7, 10). A mouse mAb, M3A5, to β-COP (27) was produced in hybridoma cells donated by T. Kreis (University of Geneva, Switzerland). For immunoprecipitation experiments the following antibodies were used: a mouse mAb, CM1A10 (28), was produced in mice from hybridoma cells donated by James E. Rothman (Memorial Sloan Kettering Cancer Center, New York). Anti-peptide antibodies that recognize the C termini of α-COP (antibody 1409) and β′-COP (antibody 891) were produced as described (10, 29). A anti-peptide antibody to β-COP was raised in rabbits against the internal peptide sequence EAGELKPEEEITVGPVQK coupled to keyhole limpet hemocyanin as described (30). Polyclonal anti-γ-COP and anti-δ-COP antibodies were raised in rabbits against the recombinant His-tagged proteins. Polyclonal anti-ɛ-COP (12) and anti-ζ-COP antibodies (13) were provided by James E. Rothman and used for both Western blot analysis and immunoprecipitation.
Isolation of Coatomer.
Coatomer was purified in its native state from rabbit liver cytosol (starting with 15–20 g of cytosolic protein) by using a modified version of the protocol as described (2). Protein was precipitated with ammonium sulfate at a final concentration of 35%. The precipitate was resuspended in 200 mM KCl/25 mM Tris⋅HCl, pH 7.4/1 mM DTT/1 mM phenylmethylsulfonyl fluoride/0.5 mM 1,10-phenanthroline/pepstatin A (1 μg/ml)/aprotinin (2 μg/ml)/leupeptin (0.5 μg/ml). [Buffer abbreviations are as follows: x mM K, x mM KCl; T, 25 mM Tris⋅HCl; D, 1 mM DTT; G, 10% (wt/vol) glycerol.] The suspension was dialyzed against 200 mM KTD (pH 7.4). Insoluble material was removed by centrifugation for 1 h at 100,000 × g. The supernatants were filtrated through 0.45-μm (pore size) membrane filters and loaded onto a 300-ml DEAE-Sepharose FF column (Pharmacia) equilibrated with 200 mM KTDG (pH 7.4). Protein was eluted with a linear 2-liter gradient from 200 mM to 1 M KTDG (pH 7.4). Fractions containing β′-COP immunoreactivity (elution at 0.28–0.4M KCl) were pooled, and the conductivity was adjusted to the equivalent of 200 mM KTDG (pH 7.4). The pool was loaded onto a 6-ml ResourceQ column (Pharmacia) equilibrated with the same buffer. Proteins were eluted with a linear 200-ml gradient from 200 mM to 1 M KTDG (pH 7.4). Fractions containing β′-COP immunoreactivity (0.36–0.4 M KCl) were pooled, adjusted to a final conductivity to the equivalent of 200 mM KTDG (pH 7.4), and applied to a 1-ml ResourceQ column. Coatomer was eluted with 0.5 M KTDG (pH 7.4). This protocol yields 50–70% pure coatomer at a protein concentration of about 5 mg/ml.
Reversible Modification of Coatomer with DMMA.
Isolated coatomer was dialyzed against 100 mM KCl/50 mM Hepes⋅KOH, pH 8.5/1 mM DTT (100 mM KHD, pH 8.5; where H is 50 mM Hepes⋅KOH) and adjusted to a protein concentration of 1 mg/ml. DMMA treatment was performed for 1 h at 4°C with final concentrations of 0.5 mM, 1.5 mM, or 3.5 mM DMMA (stock solution was 100 mg/ml in dioxane, stored in a dry atmosphere). To regenerate Lys ɛ-amino-groups of DMMA-treated protein, samples were dialyzed against 100 mM KHD (pH 6.7) for 3 h at room temperature or overnight in the cold room.
Sucrose Density Centrifugation.
Samples of 700 μl were applied onto a 11-ml sucrose step gradient (32.5–7.5% sucrose, six 1.75-ml steps) with a 1-ml 42.5% sucrose cushion in 25 mM Hepes⋅KOH, pH 8.5/100 mM KCl. The gradients were centrifuged for 24 h at 180,000 × g in a SW 41 rotor (Beckman) at 4°C and fractionated into 20 fractions.
Immunoprecipitation, SDS/PAGE, and Immunoblot Analysis.
Antibodies against the various COPs were incubated with protein A-Sepharose (CL-4B, Pharmacia) on a rotator in 25 mM Tris⋅HCl, pH 7.4/100 mM NaCl/1 mM EDTA/0.5% Nonidet P-40 (IP buffer) for 2 h at room temperature. For immunoprecipitation with CM1A10, the antibody was covalently coupled to protein G-agarose (28). After washing with IP buffer, regenerated coatomer or putative subcomplexes were added and incubation was continued for 2 h at room temperature. Beads were collected by centrifugation and washed five times with IP buffer and once with buffer without detergent before dissociation in reducing or nonreducing SDS/PAGE sample buffer at 95°C. SDS/PAGE was performed with the following gel systems: 7.5% gels under reducing conditions were used for the detection of β-, α-, β′-, γ-, and δ-COPs; 15% gels under nonreducing conditions were used for the detection of ɛ- and ζ-COPs; and 7.5–16.5% gradient gels under reducing conditions were used when all COPs were to be separated on one gel. In separating gels, the ratio of acrylamide to bisacrylamide was 100:1 (wt/wt). For immunoblot analysis, peroxidase-conjugated secondary antibodies (goat anti-mouse for M3A5 antibody and goat anti-rabbit for all other antibodies) and enhanced chemiluminescence (ECL, Amersham) were used as a detection system.
Isolation of a β/δ-COP Dimer.
Isolated coatomer modified with 1.5 mM DMMA in 100 mM KHD (pH 8.5) was loaded onto a Mono Q PC 1.6/5 column (SMART-System, Pharmacia) equilibrated with the same buffer. Proteins were eluted with a linear 4-ml gradient of 100 mM to 1 M KHD (pH 8.5). Fractions were analyzed by SDS/PAGE and Western blotting with antibodies against the various COPs.
Golgi Binding Assay.
Golgi binding assays were carried out as described (28) with 7 μg of rabbit liver Golgi membranes, 2.2 μg of myristylated ARF1, and 25 μM guanosine 5′-[γ-thio]triphosphate (GTP[γS]) or guanosine 5′-[β-thio]diphosphate (GDP[βS]) in a 50-μl reaction mixture.
RESULTS
Reversible Dissociation of Coatomer.
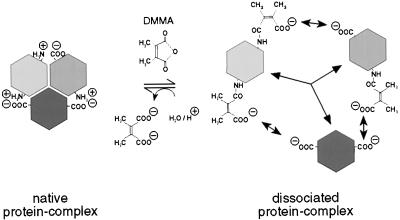
A method for the reversible chemical modification of Lys side chains was adopted to dissociate coatomer. This method has originally been used for the reversible dissociation of another quarternary structure, the multienzyme complex of yeast fatty acid synthase (31), and subsequently for a variety of other protein aggregates (32). Easily accessible ɛ-amino groups of Lys residues are acylated by DMMA, as depicted in Fig. 1. As a result the net charge of every Lys residue modified is changed by 2 charge units, because the positively charged amino group is converted into an uncharged amido group and, in addition, a free carboxylic function is introduced (Fig. 1). The number of Lys residues modified can easily be controlled by varying the concentrations of the reagent. In the resulting protein, ɛ-amides are highly sensitive to proton-assisted hydrolysis, because the two methyl groups of the reagent favor a planar intermediate state, resulting in intramolecular catalysis of the hydrolysis step (33). Thus, the modification can be reversed under extremely mild conditions: although it is quite stable at pH >8.5, it is efficiently hydrolyzed at pH <7, conditions that favor protein secondary and tertiary structures to be maintained. Disruption of the quarternary structure is believed to be because of charge repulsions caused by changing the net charge state of the derivatized proteins.
Figure 1.
Reaction mechanism of DMMA. Reaction of DMMA with ɛ-amino groups of Lys residues that are exposed on the surface of an oligomeric protein complex leads to dissociation of the complex. Reassociation may occur by removing the modification at slightly acidic pH (below 7).
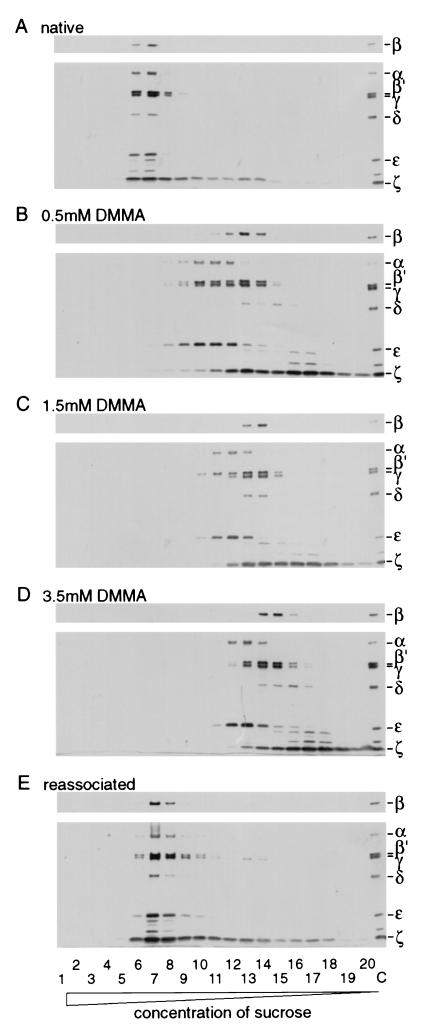
For dissociation, coatomer isolated from rabbit liver cytosol was incubated with various concentrations of DMMA, and dissociation of the complex was analyzed by sucrose density gradient centrifugation as depicted in Fig. 2. After fractionation of the gradients, the individual fractions were analyzed by Western blotting with antibodies against all seven COPs, α–ζ. Under the conditions used, underivatized coatomer is found in fractions 6–8 (with small amounts of ζ-COP “bleeding” into fractions with lower sucrose density; Fig. 2A). This pattern is strikingly changed by the addition of DMMA: increasing the amounts of reagent leads to a shift of the COPs to fractions of lower sucrose density, correlating to smaller sedimentation constants and thus to smaller molecular masses (Fig. 2 A–C). An aliquot of the sample modified with 1.5 mM DMMA was dialyzed against buffer at pH 6.7 and then reanalyzed on a sucrose density gradient. As shown in Fig. 2E, appreciable amounts of COPs (≈40%) are found in fractions of high sucrose concentration (fraction 6–8), indicative for the molecular mass of the native complex. The relative intensities of the individual COP immuno signals resemble those of the native complex, suggesting reassociation of the subunits in a stoichiometric manner. To analyze the structural integrity of the reassociated protein complex, we made use of mAb CM1A10 (28), known to exclusively precipitate intact coatomer. Immunoprecipitation with CM1A10 was performed with native coatomer and with the reassociated fractions. As shown in Fig. 3A, the antibody is able to precipitate the reassociated material, and the immunoreactivities of the individual COPs are very similar to those of the native complex. Thus, most of the reassociated material of fractions 6–8 in Fig. 2E resembles structurally intact coatomer. For a functional analysis of the reassociated complex, its capability to bind to isolated Golgi membranes in an ARF1- and GTP[γS]-dependent manner was probed. The result is depicted in Fig. 3B: a significant increase is observed in binding of the reassociated material, dependent on the presence of ARF1 and GTP[γS], similar to the binding of native coatomer as a control. The presence of coatomer in these binding studies was detected by β′-COP immunoreactivity.
Figure 2.
Sucrose gradient centrifugation of native, dissociated, and reassociated coatomer. Native coatomer was treated with the concentrations of DMMA indicated. To reassociate dissociated coatomer, the modified Lys ɛ-amino groups were regenerated by dialysis against 100 mM KCl/50 mM Hepes⋅KOH, pH 6.7/1 mM DTT (reassociation buffer). Samples of native, dissociated, and reassociated coatomer were separated on sucrose gradients and analyzed by SDS/PAGE on 7.5–16.5% gels, Western blotting with antibodies against all COPs. (A) Sedimentation of native coatomer. (B–D) Sedimentation of coatomer treated with the indicated concentrations of DMMA. (E) Sedimentation of DMMA-treated coatomer (1.5 mM) after dialysis against reassociation buffer. Lanes: 1–20, fractions from sucrose gradients collected from bottom to top; C, isolated coatomer as a standard.
Figure 3.
Structural and functional characterization of reassociated coatomer. Coatomer, native or reassociated, was separated by sucrose density centrifugation (see Fig. 2A, fraction 7, for native and Fig. 2E, fraction 7, for reassociated coatomer) and subjected to immunoprecipitation with an antibody directed against the native structure of the complex or to a Golgi binding assay. (A) Native and reassociated coatomer was immunoprecipitated with CM1A10 mouse mAb. The precipitates were analyzed by SDS/PAGE on 7.5–16.5% gels and Western blotting with antibodies against α-, β′-, γ-, δ-, ɛ-, and ζ-COPs. Lanes: i, 20% of the input; IP, immunoprecipitate. (B) Native or reassociated coatomer was incubated with ARF1 without membranes in the presence of GTP[γS] or with ARF1 and Golgi membranes in the presence of GDP[βS] or GTP[γS]. After incubation the samples were layered on a 15% sucrose cushion and centrifuged for 30 min at 15,000 × g. The supernatant was removed and the pellet was analyzed by SDS/PAGE and Western blotting. Immunostaining against β′-COP was used for the detection of coatomer.
Thus, after dissociation with DMMA, significant amounts of COPs are able to reassociate to yield a structurally and functionally intact coatomer complex.
Characterization of Subcomplexes.
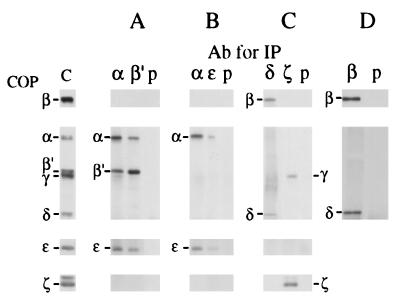
The relative sedimentation of the various COPs, depending on the concentration of DMMA used (Fig. 2 B–D), suggested that the individual fractions may contain coatomer subcomplexes in addition to individual COPs. Such specific interactions of COPs within subcomplexes may reveal additional information about the architecture of the complete complex. Therefore, we have analyzed such interactions by using coimmunoprecipitation experiments. Freshly dissociated coatomer was subjected to immunoprecipitation with various antibodies against the individual COPs, and the precipitates were examined by Western blotting for the presence of additional COPs, as shown in Fig. 4. From these experiments, we deduce that the following subcomplexes are present in the dissociate: α/β′/ɛ-COPs (Fig. 4A), α/ɛ-COPs (Fig. 4B), and β/δ-COPs and γ/ζ-COPs (Fig. 4C).
Figure 4.
Characterization of coatomer subcomplexes. (A–C) Coatomer was modified with 1.5 mM or 3.5 mM DMMA, separated by sucrose density centrifugation, and fractionated as shown in Fig. 2. Fractions were dialyzed against 100 mM KTD (pH 6.7) and subjected to immunoprecipitation. (A) Fraction 11 (Fig. 2C) was immunoprecipitated with antibodies against α-COP or β′-COP or with preimmune serum. (B) Fractions 12 and 13 (Fig. 2D) were immunoprecipitated with antibodies against α-COP or ɛ-COP or with preimmune serum. (C) Fractions 13 and 14 (Fig. 2C) were immunoprecipitated with antibodies against δ-COP or ζ-COP or with preimmune serum. The immunoprecipitates of A–C were separated on 7.5% gels under reducing conditions for the detection of β-, α-, β′-, γ-, and δ-COPs and on 15% gels under nonreducing conditions for the detection of ɛ- and ζ-COPs. (D) Coatomer was modified with 1.5 mM DMMA and subjected to Mono Q anion-exchange chromatography as shown in Fig. 5. Fraction 14 was used for immunoprecipitation with anti-β-COP serum or preimmune serum. Immunoprecipitation of the COPs was analyzed by SDS/PAGE on 7.5% gels under reducing conditions Western blotting with antibodies against β-COP and δ-COP.
Isolation of a β/δ-COP Dimer.
Sucrose density gradient fractionation was used to analyze the state of dissociation of coatomer after DMMA treatment (Fig. 2). However, this method did not lead to a separation that would allow purification of individual subcomplexes. Therefore, freshly dissociated coatomer was subjected to ion-exchange chromatography, and the fractions obtained were analyzed by SDS/PAGE and Western blotting. The result of such an experiment is shown in Fig. 5. Clearly, part of ζ-COP is present in its monomeric state and can be obtained in a pure form. In addition, β- and δ-COPs were eluted together (fractions 13–15) and separated from additional COPs. In later fractions, a variety of COPs were eluted with only partial separation. Stoichiometric interaction of β- and δ-COPs in fractions 13–15 was reconfirmed by coimmunoprecipitation as depicted in Fig. 4D. Thus, after DMMA treatment, a dimer of β- and δ-COPs can be isolated in amounts sufficient for functional studies.
Figure 5.
Isolation of a β/δ-COP dimer. Isolated coatomer was modified with 1.5 mM DMMA and chromatographed on a Mono Q anion-exchange column. Proteins were eluted with a salt gradient. The resulting fractions were analyzed for COPs by SDS/PAGE on 7.5–16.5% gradient gels and Western blotting with antibodies for all COPs. Fractions 13–15 containing β/δ-COP dimer were used for Golgi binding assay. ∗, Cross-reactivity with anti-ɛ-COP serum.
ARF1- and GTP[γS]-Dependent Binding to Golgi Membranes of a β/δ-COP Dimer.
The material containing β/δ-COP dimer (fraction 14 in Fig. 5) was dialyzed against slightly acidic buffer to remove the dimethyl maleic acid residues, and then the sample was analyzed to determine its ability to bind to Golgi membranes. In these experiments, Golgi membranes were pretreated with a high salt buffer to remove residual amounts of ARF1 and coatomer attached to the membranes (28). As shown in Fig. 6, neither with native coatomer nor with β/δ-COPs alone is a significant binding observed to Golgi membranes in the presence of ARF1 and GDP[βS]. However, after addition of ARF1 and GTP[γS], both β- and δ-COPs are recovered with the membranes, as is the complete set of COPs when native coatomer (visualized by the presence of β-, α-, β′-, γ-, and δ-COPs) is used as a control. Thus the function for the observed GTP[γS]- and ARF1-dependent binding of coatomer to Golgi membranes can be attributed to a minimal subcomplex, a dimer of β- and δ-COPs.
Figure 6.
Binding of β/δ-COP dimer to Golgi membranes. Native coatomer or isolated β/δ-COP dimer (Fig. 5, fraction 14) after dialysis against buffer pH 6.7 was incubated with ARF1 without membranes in the presence of GTP[γS] or with ARF1 and Golgi membranes in the presence of GDP[βS] or GTP[γS]. After incubation the samples were layered on a sucrose cushion and centrifuged. The supernatant was removed and the precipitate was analyzed by SDS/PAGE on 7.5–16.5% gradient gels and Western blotting with antibodies against β-, α-, β′-, γ-, and δ-COPs. As a control Golgi membranes were incubated with ARF1 and GTP[γS]. Lane i contains 20% of the sample.
DISCUSSION
In this study we used chemical modification of coatomer to dissociate the complex. The degree of dissociation can be controlled by the concentration of reagent used, allowing us to produce subcomplexes of various compositions and monomeric COPs. Moreover, modification is mild enough and reversible to allow reassembly of the subcomplexes and subunits into a structurally and functionally intact complex.
By using coimmunoprecipitation, a variety of subcomplexes of coatomer were characterized, a trimer of α/β′/ɛ-COPs, and dimers of α/ɛ-COPs, γ/ζ-COPs, and β/δ-COPs. These findings confirm COP–COP interactions that have been identified with several different methods (10, 34, 35).
Isolation of purified subcomplexes has allowed us to identify the minimal set of COPs required for ARF1-dependent interaction with the Golgi membrane during recruitment of cytosolic coatomer in the process of COPI vesicle budding. We find that an isolated β/δ-COP dimer binds to Golgi membranes in an ARF1- and GTP[γS]-dependent manner. This is in accordance with a direct interaction of ARF1 with β-COP in intact coatomer (25) and shows that this key function of coatomer is preserved within a minimal subcomplex. It remains to be established whether the δ-COP subunit that seems tightly associated with β-COP is needed to confer functionality to the β subunit. Partially purified subcomplexes of α/β′/ɛ-COPs and γ/ζ-COPs (fractions 16 and 19 in Fig. 5, respectively) were also probed for their binding to Golgi membranes (data not shown). These proteins bind to Golgi membranes even in the absence of ARF1 or GTP[γS]. However, control experiments revealed that this binding is likely to be unspecific, because binding of similar efficiency was observed also with highly purified mitochondrial membranes that do not bind intact coatomer. Therefore the ARF1- and GTP-dependent binding of coatomer to Golgi membranes seems to be conserved exclusively in the β/δ-COP subcomplex.
In addition to an interaction with membrane-bound ARF1, coatomer has been shown to bind to cytoplasmic tails of type I membrane proteins of the p24 family, present in Golgi membranes and COPI-coated vesicles (36, 37). This interaction involves coatomer subunits distinct from β/δ-COPs (ref. 38 and C. H., unpublished observation). Thus, recruitment of cytosolic coat proteins for the budding of a COPI vesicle involves a bimodal interaction of coatomer with the Golgi membrane: (i) ARF1- and GTP-dependent binding via β-COP and (ii) binding to a member of the p24 family via coatomer subunits distinct from β-COP. No significant binding of coatomer to Golgi membranes is observed in the absence of ARF1–GTP, although p23/p24 are present in the membranes. This implies that either the cytoplasmic tail of p23/p24 or the corresponding binding site within coatomer is not available. According to this model, binding of coatomer to Golgi membranes is initiated by the β-COP–ARF1–GTP interaction. This might induce the second binding site within the complex for the interaction with cytoplasmic domains of p23/p24. Alternatively, initiation of coat recruitment by ARF1–GTP might make available for coatomer the otherwise masked cytoplasmic tails of p23/p24. This would also explain why no specific binding with the other subunits and subcomplexes lacking β-COP was detected. For a complete understanding of COPI vesicle budding, it will be crucial to reveal how the additional second binding site for coatomer is generated, either within the complex or at the Golgi membrane.
ARF1- and GTP-dependent binding of a β/δ-COP subcomplex is also of note in light of an ongoing discussion about to the role of phospholipase D in coatomer recruitment. An interaction of coatomer with ARF1–GTP (25) and our finding that even an isolated β/δ-COP dimer binds to Golgi membranes in an ARF- and GTP-dependent manner supports a direct role of ARF in recruitment of coatomer to membranes. It does not exclude, however, an additional role via phospholipase D activation of ARF1 in COPI vesicle formation.
Acknowledgments
We thank Drs. T. Kreis and J. E. Rothman for providing antibodies and Dr. B. Helms for providing recombinant ARF1 and for critically reading the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 352, C6) and the Human Frontier Science Program.
ABBREVIATIONS
- COPI
protein coat composed of coatomer and ARF1
- ARF1
ADP ribosylation factor
- COP
coat protein
- DMMA
dimethyl maleic anhydride
- GTP[γS]
guanosine 5′-[γ-thio]triphosphate
- GDP[βS]
guanosine 5′-[β-thio]diphosphate
References
- 1.Rothman J E, Wieland F T. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 2.Waters M G, Serafini T, Rothman J E. Nature (London) 1991;349:248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- 3.Gerich B, Orci L, Tschochner H, Lottspeich F, Ravazzola M, Amherdt M, Wieland F, Harter C. Proc Natl Acad Sci USA. 1995;92:3229–3233. doi: 10.1073/pnas.92.8.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letourneur F, Gaynor E C, Hennecke S, Demolliere C, Duden R, Emr S D, Riezman H, Cosson P. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 5.Duden R, Griffiths G, Frank R, Argos P, Kreis T E. Cell. 1991;64:649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- 6.Serafini T, Stenbeck G, Brecht A, Lottspeich F, Orci L, Rothman J E, Wieland F T. Nature (London) 1991;349:215–220. doi: 10.1038/349215a0. [DOI] [PubMed] [Google Scholar]
- 7.Stenbeck G, Harter C, Brecht A, Herrmann D, Lottspeich F, Orci L, Wieland F T. EMBO J. 1993;12:2841–2845. doi: 10.1002/j.1460-2075.1993.tb05945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison-Lavoie K J, Lewis V A, Hynes G M, Collison K S, Nutland E, Willison K R. EMBO J. 1993;12:2847–2853. doi: 10.1002/j.1460-2075.1993.tb05946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harter C, Pavel J, Coccia F, Draken E, Wegehingel S, Tschochner H, Wieland F. Proc Natl Acad Sci USA. 1996;93:1902–1906. doi: 10.1073/pnas.93.5.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulstich D, Auerbach S, Orci L, Ravazzola M, Wegehingel S, Lottspeich F, Stenbeck G, Harter C, Wieland F T, Tschochner H. J Cell Biol. 1996;135:53–61. doi: 10.1083/jcb.135.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosson P, Démollière C, Hennecke S, Duden R, Letourneur F. EMBO J. 1996;15:1792–1798. [PMC free article] [PubMed] [Google Scholar]
- 12.Hara-Kuge S, Kuge O, Orci L, Amherdt M, Ravazzola M, Wieland F T, Rothman J E. J Cell Biol. 1994;124:883–892. doi: 10.1083/jcb.124.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuge O, Hara K S, Orci L, Ravazzola M, Amherdt M, Tanigawa G, Wieland F T, Rothman J E. J Cell Biol. 1993;123:1727–1734. doi: 10.1083/jcb.123.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serafini T, Orci L, Amherdt M, Brunner M, Kahn R A, Rothman J E. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- 15.Taylor T C, Kahn R A, Melancon P. Cell. 1992;70:69–79. doi: 10.1016/0092-8674(92)90534-j. [DOI] [PubMed] [Google Scholar]
- 16.Orci L, Palmer D J, Amherdt M, Rothman J E. Nature (London) 1993;364:732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- 17.Cardin P, Paris S, Antonny B, Robineau S, Beraud-Dufur S, Jackson C L, Chabre M. Nature (London) 1996;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- 18.Peyroche A, Paris S, Jackson C L. Nature (London) 1996;384:479–481. doi: 10.1038/384479a0. [DOI] [PubMed] [Google Scholar]
- 19.Rosa J L, Casarolimarano R P, Buckler A J, Vilaro S, Barbacid M. EMBO J. 1996;15:4262–4273. [PMC free article] [PubMed] [Google Scholar]
- 20.Helms J B, Palmer D J, Rothman J E. J Cell Biol. 1993;121:751–760. doi: 10.1083/jcb.121.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown H A, Gutowski S, Moomaw C R, Slaughter C, Sternweis P C. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 22.Cockcroft S, Thomas G M, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty N F, Truong O, Hsuan J J. Science. 1994;263:523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- 23.Liscovitch M, Chalifa V, Pertile P, Chen C S, Cantley L C. J Biol Chem. 1994;269:21403–21406. [PubMed] [Google Scholar]
- 24.Ktistakis N T, Brown H A, Waters M G, Sternweis P C, Roth M G. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L, Helms J B, Brügger B, Harter C, Martoglio B, Graf R, Brunner J, Wieland F T. Proc Natl Acad Sci USA. 1997;94:4418–4423. doi: 10.1073/pnas.94.9.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhotra V, Serafini T, Orci L, Shepherd J C, Rothman J E. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- 27.Allan V J, Kreis T E. J Cell Biol. 1986;103:2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer D J, Helms J B, Beckers C J, Orci L, Rothman J E. J Biol Chem. 1993;268:12083–12089. [PubMed] [Google Scholar]
- 29.Harter C, Draken E, Lottspeich F, Wieland F T. FEBS Lett. 1993;332:71–73. doi: 10.1016/0014-5793(93)80487-f. [DOI] [PubMed] [Google Scholar]
- 30.Kreis T E. EMBO J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wieland F T, Renner L, Verfürth C, Lynen F. Eur J Biochem. 1979;94:189–197. doi: 10.1111/j.1432-1033.1979.tb12885.x. [DOI] [PubMed] [Google Scholar]
- 32.Palacian E, Gonzalez P J, Pineiro M, Hernandez F. Mol Cell Biochem. 1990;97:101–111. doi: 10.1007/BF00221051. [DOI] [PubMed] [Google Scholar]
- 33.Bruice T C, Bakovic S J. Bioorganic Mechanisms. New York: Benjamin; 1966. [Google Scholar]
- 34.Lowe M, Kreis T E. J Biol Chem. 1995;270:31364–31371. doi: 10.1074/jbc.270.52.31364. [DOI] [PubMed] [Google Scholar]
- 35.Cosson P, Letourneur F. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- 36.Stamnes M A, Craighead M W, Hoe M H, Lampen N, Geromanos S, Tempst P, Rothman J E. Proc Natl Acad Sci USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms J B, Wieland F T. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiedler K, Veit M, Stamnes M A, Rothman J E. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]