Abstract
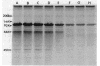
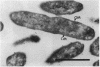
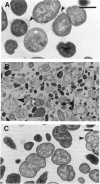
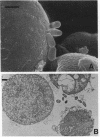
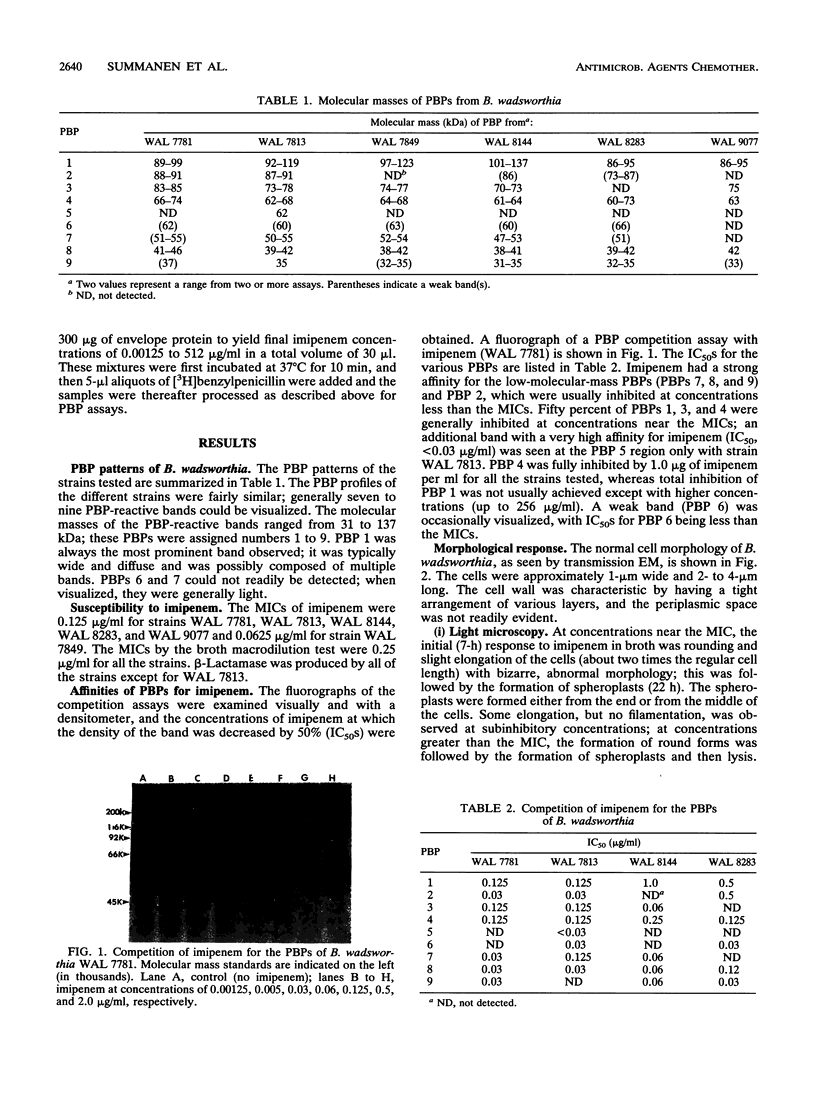
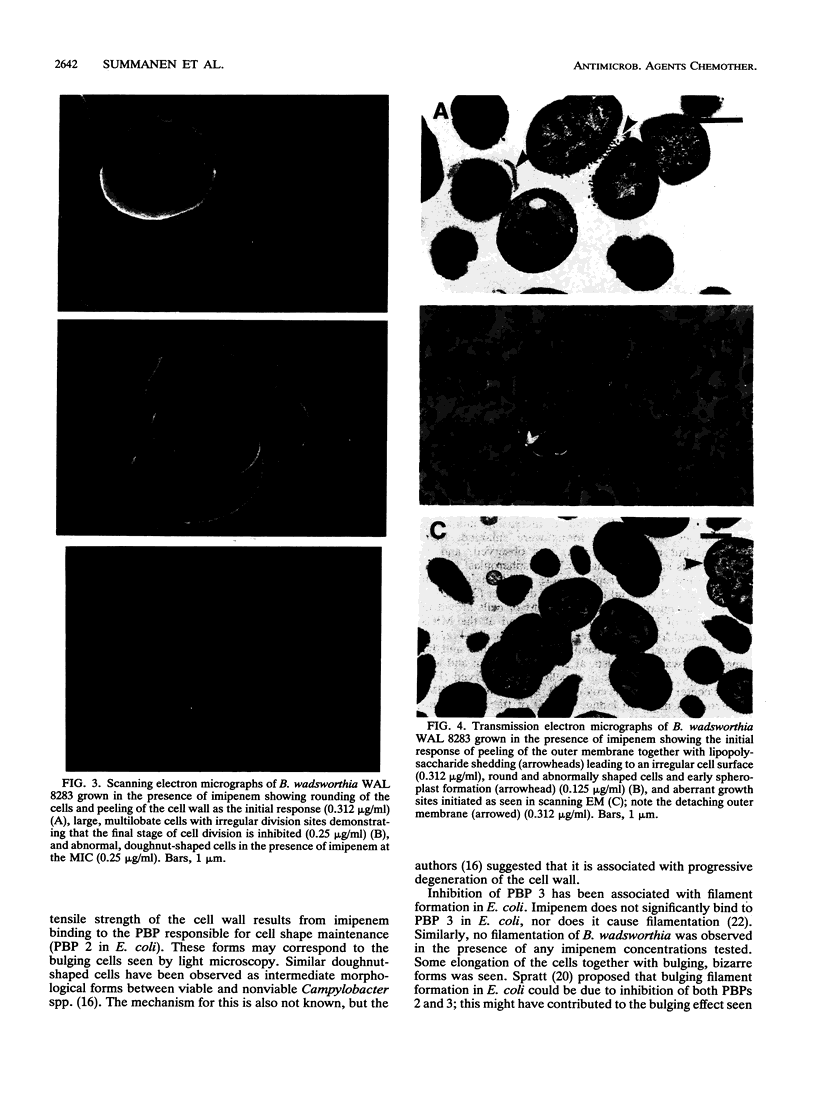
The penicillin-binding protein (PBP) patterns of six strains of Bilophila wadsworthia were investigated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis and subsequent fluorography of membrane preparations labelled with [3H]benzylpenicillin. The PBP profiles among the strains were similar; generally, seven to nine PBP-reactive bands could be visualized; their molecular weights ranged from 31 to 137 kDa. The relative affinities of the PBPs of four strains of B. wadsworthia for imipenem were examined and correlated with the morphological responses of the cells to imipenem. Morphological changes were examined by light and electron microscopies. Light microscopy revealed that at low concentrations (less than the MIC), imipenem induced the formation of rounded and bulging cells; rarely, elongation without filamentation was observed. In the presence of imipenem at the MIC, spheroplast formation was observed. Scanning and transmission electron microscopies revealed round forms together with larger, multilobate cells in the presence of subinhibitory concentrations of imipenem, suggesting that new growth sites were initiated while cell division was inhibited. Peeling of the outer membrane was also seen. Spheroplasts were very large (up to 30 microns in diameter) and stable in aqueous solution. Inhibition of the PBPs could be seen in the presence of low imipenem concentrations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron E. J., Bennion R., Thompson J., Strong C., Summanen P., McTeague M., Finegold S. M. A microbiological comparison between acute and complicated appendicitis. Clin Infect Dis. 1992 Jan;14(1):227–231. doi: 10.1093/clinids/14.1.227. [DOI] [PubMed] [Google Scholar]
- Baron E. J., Curren M., Henderson G., Jousimies-Somer H., Lee K., Lechowitz K., Strong C. A., Summanen P., Tunér K., Finegold S. M. Bilophila wadsworthia isolates from clinical specimens. J Clin Microbiol. 1992 Jul;30(7):1882–1884. doi: 10.1128/jcm.30.7.1882-1884.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron E. J., Summanen P., Downes J., Roberts M. C., Wexler H., Finegold S. M. Bilophila wadsworthia, gen. nov. and sp. nov., a unique gram-negative anaerobic rod recovered from appendicitis specimens and human faeces. J Gen Microbiol. 1989 Dec;135(12):3405–3411. doi: 10.1099/00221287-135-12-3405. [DOI] [PubMed] [Google Scholar]
- Bellido F., Veuthey C., Blaser J., Bauernfeind A., Pechère J. C. Novel resistance to imipenem associated with an altered PBP-4 in a Pseudomonas aeruginosa clinical isolate. J Antimicrob Chemother. 1990 Jan;25(1):57–68. doi: 10.1093/jac/25.1.57. [DOI] [PubMed] [Google Scholar]
- Bennion R. S., Baron E. J., Thompson J. E., Jr, Downes J., Summanen P., Talan D. A., Finegold S. M. The bacteriology of gangrenous and perforated appendicitis--revisited. Ann Surg. 1990 Feb;211(2):165–171. doi: 10.1097/00000658-199002000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta G. A., Park J. T. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J Bacteriol. 1981 Jan;145(1):333–340. doi: 10.1128/jb.145.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Competition of beta-lactam antibiotics for the penicillin-binding proteins of Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aerogenes, Proteus rettgeri, and Escherichia coli: comparison with antibacterial activity and effects upon bacterial morphology. Antimicrob Agents Chemother. 1979 Sep;16(3):325–328. doi: 10.1128/aac.16.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. S., Greenwood D. The morphological response of Pseudomonas aeruginosa to azthreonam, cefoperazone, ceftazidime and N-formimidoyl thienamycin. J Med Microbiol. 1984 Apr;17(2):159–169. doi: 10.1099/00222615-17-2-159. [DOI] [PubMed] [Google Scholar]
- Garcia M. M., Becker S. A., Brooks B. W., Berg J. N., Finegold S. M. Ultrastructure and molecular characterization of Fusobacterium necrophorum biovars. Can J Vet Res. 1992 Oct;56(4):318–325. [PMC free article] [PubMed] [Google Scholar]
- Handwerger S., Tomasz A. Alterations in kinetic properties of penicillin-binding proteins of penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1986 Jul;30(1):57–63. doi: 10.1128/aac.30.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume T., Ishino F., Nakagawa J., Tamaki S., Matsuhashi M. Studies on the mechanism of action of imipenem (N-formimidoylthienamycin) in vitro: binding to the penicillin-binding proteins (PBPs) in Escherichia coli and Pseudomonas aeruginosa, and inhibition of enzyme activities due to the PBPs in E. coli. J Antibiot (Tokyo) 1984 Apr;37(4):394–400. doi: 10.7164/antibiotics.37.394. [DOI] [PubMed] [Google Scholar]
- Johnson C. C., Wexler H. M., Becker S., Garcia M., Finegold S. M. Cell-wall-defective variants of Fusobacterium. Antimicrob Agents Chemother. 1989 Mar;33(3):369–372. doi: 10.1128/aac.33.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- NG L. K., Sherburne R., Taylor D. E., Stiles M. E. Morphological forms and viability of Campylobacter species studied by electron microscopy. J Bacteriol. 1985 Oct;164(1):338–343. doi: 10.1128/jb.164.1.338-343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoe T., Umemoto T., Sagawa H., Suginaka H. Filament formation of Fusobacterium nucleatum cells induced by mecillinam. Antimicrob Agents Chemother. 1981 Mar;19(3):487–489. doi: 10.1128/aac.19.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J., Wise R. Cefoxitin resistance in Bacteroides species: evidence indicating two mechanisms causing decreased susceptibility. J Antimicrob Chemother. 1987 Feb;19(2):161–170. doi: 10.1093/jac/19.2.161. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Wise R. Properties of the penicillin-binding proteins of four species of the genus Bacteroides. Antimicrob Agents Chemother. 1986 May;29(5):825–832. doi: 10.1128/aac.29.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V., Zimmermann W. Binding of thienamycin and clavulanic acid to the penicillin-binding proteins of Escherichia coli K-12. Antimicrob Agents Chemother. 1977 Sep;12(3):406–409. doi: 10.1128/aac.12.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Summanen P., Wexler H. M., Finegold S. M. Antimicrobial susceptibility testing of Bilophila wadsworthia by using triphenyltetrazolium chloride to facilitate endpoint determination. Antimicrob Agents Chemother. 1992 Aug;36(8):1658–1664. doi: 10.1128/aac.36.8.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Penicillin-binding proteins and the antibacterial effectiveness of beta-lactam antibiotics. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S260–S278. doi: 10.1093/clinids/8.supplement_3.s260. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Schwartz J. Penicillin-binding protein 7 and its relationship to lysis of nongrowing Escherichia coli. J Bacteriol. 1987 Nov;169(11):4912–4915. doi: 10.1128/jb.169.11.4912-4915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler H. M., Halebian S. Alterations to the penicillin-binding proteins in the Bacteroides fragilis group: a mechanism for non-beta-lactamase mediated cefoxitin resistance. J Antimicrob Chemother. 1990 Jul;26(1):7–20. doi: 10.1093/jac/26.1.7. [DOI] [PubMed] [Google Scholar]
- Wexler H. M. Susceptibility testing of anaerobic bacteria: myth, magic, or method? Clin Microbiol Rev. 1991 Oct;4(4):470–484. doi: 10.1128/cmr.4.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]