Abstract
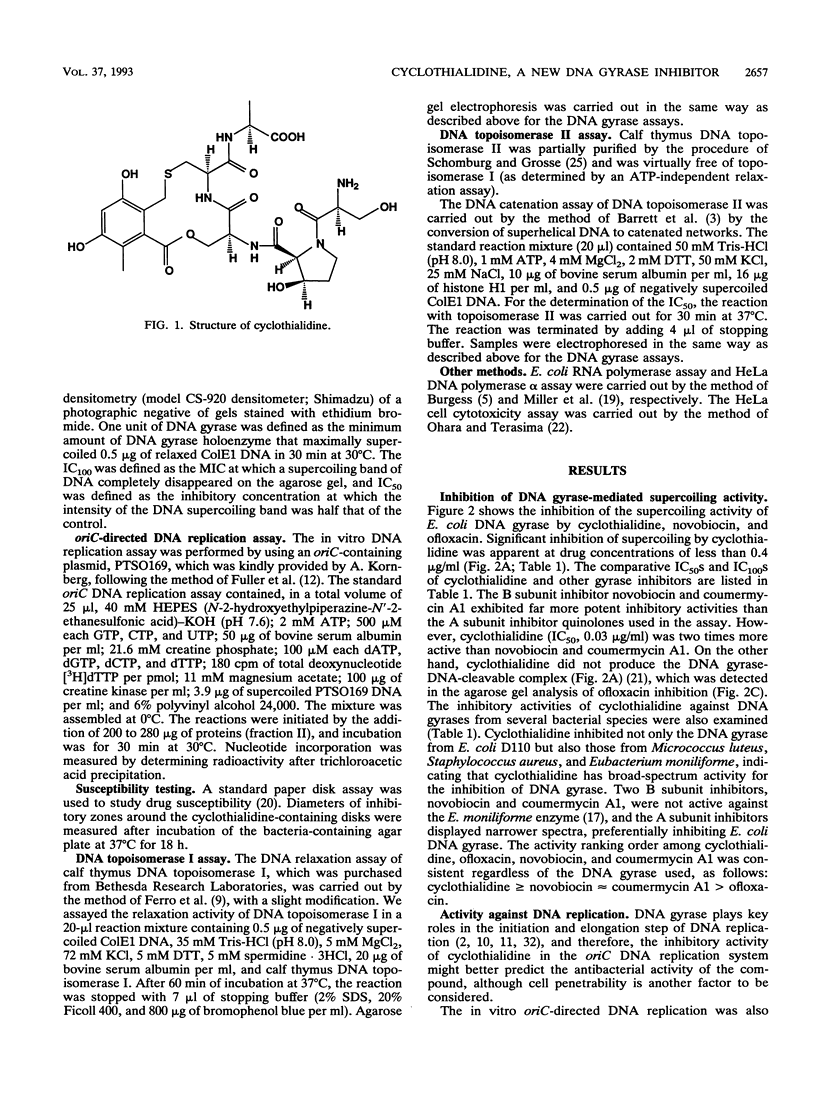
Cyclothialidine is a new DNA gyrase inhibitor isolated from Streptomyces filipinensis NR0484. Structurally, it belongs to a new class of natural products containing a unique 12-membered lactone ring that is partly integrated into a pentapeptide chain. Cyclothialidine was found to be one of the most active of all the DNA gyrase inhibitors tested in the DNA supercoiling reaction of Escherichia coli DNA gyrase; 50% inhibitory concentrations (in micrograms per milliliter) of 0.03 (cyclothialidine), 0.06 (novobiocin), 0.06 (coumermycin A1), 0.66 (norfloxacin), 0.88 (ciprofloxacin), and 26 (nalidixic acid) were found. In addition, DNA gyrases from gram-positive species were inhibited equally as well as DNA gyrase from E. coli. Cyclothialidine also inhibited the in vitro DNA replication directed from oriC of E. coli. Among the bacterial species tested, only Eubacterium spp. were inhibited by cyclothialidine, suggesting that it can enter the cells of Eubacterium and exert antibacterial activity through interference with the DNA gyrase within the cells, although its penetration into most bacterial cells appears to be poor. These results provide a basis for cyclothialidine to be a lead structure for novel antibacterial agents with DNA gyrase inhibitory activities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. A., Sekimizu K., Funnell B. E., Kornberg A. Extensive unwinding of the plasmid template during staged enzymatic initiation of DNA replication from the origin of the Escherichia coli chromosome. Cell. 1986 Apr 11;45(1):53–64. doi: 10.1016/0092-8674(86)90537-4. [DOI] [PubMed] [Google Scholar]
- Barrett J. F., Gootz T. D., McGuirk P. R., Farrell C. A., Sokolowski S. A. Use of in vitro topoisomerase II assays for studying quinolone antibacterial agents. Antimicrob Agents Chemother. 1989 Oct;33(10):1697–1703. doi: 10.1128/aac.33.10.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Chamberland S., Bayer A. S., Schollaardt T., Wong S. A., Bryan L. E. Characterization of mechanisms of quinolone resistance in Pseudomonas aeruginosa strains isolated in vitro and in vivo during experimental endocarditis. Antimicrob Agents Chemother. 1989 May;33(5):624–634. doi: 10.1128/aac.33.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras A., Maxwell A. gyrB mutations which confer coumarin resistance also affect DNA supercoiling and ATP hydrolysis by Escherichia coli DNA gyrase. Mol Microbiol. 1992 Jun;6(12):1617–1624. doi: 10.1111/j.1365-2958.1992.tb00886.x. [DOI] [PubMed] [Google Scholar]
- Ferro A. M., Higgins N. P., Olivera B. M. Poly(ADP-ribosylation) of a DNA topoisomerase. J Biol Chem. 1983 May 25;258(10):6000–6003. [PubMed] [Google Scholar]
- Filutowicz M., Jonczyk P. The gyrB gene product functions in both initiation and chain polymerization of Escherichia coli chromosome replication: suppression of the initiation deficiency in gyrB-ts mutants by a class of rpoB mutations. Mol Gen Genet. 1983;191(2):282–287. doi: 10.1007/BF00334827. [DOI] [PubMed] [Google Scholar]
- Filutowicz M. Requirement of DNA gyrase for the initiation of chromosome replication in Escherichia coli K-12. Mol Gen Genet. 1980 Jan;177(2):301–309. doi: 10.1007/BF00267443. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. R., Ulrich R. G., Wang T. S., Korn D. Monoclonal antibodies against human DNA polymerase-alpha inhibit DNA replication in permeabilized human cells. J Biol Chem. 1985 Jan 10;260(1):134–138. [PubMed] [Google Scholar]
- Oara H., Terasima T. Lethal effect of mitomycin-C on cultured mammalian cells. Gan. 1972 Jun;63(3):317–327. [PubMed] [Google Scholar]
- Otter R., Cozzarelli N. R. Escherichia coli DNA gyrase. Methods Enzymol. 1983;100:171–180. doi: 10.1016/0076-6879(83)00053-1. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Clark D. C., Wotton S. Indirect method for assessing the penetration of beta-lactamase-nonsusceptible penicillins and cephalosporins in Escherichia coli strains. Antimicrob Agents Chemother. 1976 Aug;10(2):215–218. doi: 10.1128/aac.10.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg U., Grosse F. Purification and characterization of DNA topoisomerase II from calf thymus associated with polypeptides of 175 and 150 kDa. Eur J Biochem. 1986 Nov 3;160(3):451–457. doi: 10.1111/j.1432-1033.1986.tb10061.x. [DOI] [PubMed] [Google Scholar]
- Shalit I., Berger S. A., Gorea A., Frimerman H. Widespread quinolone resistance among methicillin-resistant Staphylococcus aureus isolates in a general hospital. Antimicrob Agents Chemother. 1989 Apr;33(4):593–594. doi: 10.1128/aac.33.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. L., Mitscher L. A., Sharma P. N., O'Donnell T. J., Chu D. W., Cooper C. S., Rosen T., Pernet A. G. Mechanism of inhibition of DNA gyrase by quinolone antibacterials: a cooperative drug--DNA binding model. Biochemistry. 1989 May 2;28(9):3886–3894. doi: 10.1021/bi00435a039. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Orr E. DNA gyrase: affinity chromatography on novobiocin-Sepharose and catalytic properties. Nucleic Acids Res. 1981 Aug 11;9(15):3589–3603. doi: 10.1093/nar/9.15.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. A., Gootz T. D., Barrett J. F. Biochemical characteristics and physiological significance of major DNA topoisomerases. Antimicrob Agents Chemother. 1989 Dec;33(12):2027–2033. doi: 10.1128/aac.33.12.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagai N., Tawara K. Quinolone antibacterial-agent-induced cutaneous phototoxicity: ear swelling reactions in Balb/c mice. Toxicol Lett. 1991 Oct;58(2):215–223. doi: 10.1016/0378-4274(91)90176-7. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Watanabe J., Kuramitsu S., Maruyama H. B. Comparison of the activity of ionophores with other antibacterial agents against anaerobes. Antimicrob Agents Chemother. 1981 Apr;19(4):519–525. doi: 10.1128/aac.19.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo I., Vizán J. L., Rodríguez-Sáinz M. C., Moreno F. An unusual mechanism for resistance to the antibiotic coumermycin A1. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8860–8864. doi: 10.1073/pnas.88.19.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ende A., Baker T. A., Ogawa T., Kornberg A. Initiation of enzymatic replication at the origin of the Escherichia coli chromosome: primase as the sole priming enzyme. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3954–3958. doi: 10.1073/pnas.82.12.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]



