Abstract
Male offspring, which cannot reproduce independently, represent a cost of sexual reproduction. This cost is eliminated by the production of hermaphroditic offspring in the self-fertilizing nematode Caenorhabditis briggsae. However, these hermaphrodites can outcross by mating with males. Half the sperm received from males contain no sex chromosome and therefore give rise to male progeny. Mating with males should thus impose the cost of making male offspring. We found that male sperm took immediate precedence over hermaphrodite sperm, resulting in maximized outcrossing, but the appearance of male progeny was delayed after mating. This delay is caused by the male X-bearing sperm outcompeting their nullo-X counterparts. The competitive advantage of X-bearing sperm over nullo-X sperm is limited to sperm from males; it did not occur in a mutant hermaphrodite that produces both types of sperm. The chromosomal effect on sperm competitiveness in C. briggsae, which has not been observed in other species, suggests that the X chromosome has evolved a form of meiotic drive, selfishly increasing the competitiveness of sperm that bear it over those that do not. Thus, the multiple levels of sperm competitiveness found in C. briggsae maximize outcrossing after mating while delaying the cost of making male offspring.
Sexually reproducing organisms generally suffer the cost of making male offspring, which cannot reproduce on their own (1). Self-fertilizing hermaphrodites do not pay the cost of making males because they make only hermaphrodite offspring, but their inbreeding leads to a loss of genetic variation (1, 2). Perhaps in response, many self-fertilizing species retain a capacity to outcross (3). Such is the case with hermaphroditic nematodes in the bacteria-feeding genus Caenorhabditis. Although these hermaphrodites typically self-fertilize, they can also outcross by mating with males.
Outcrossing should reimpose the cost of male offspring on hermaphroditic Caenorhabditis. Because males have only one X chromosome (4, 5), half of their sperm do not receive an X chromosome; therefore, half of the progeny a male sires should be male. In the widely studied Caenorhabditis elegans, mating does indeed impose the cost of male offspring, and this cost is maximized by the precedence of male sperm over hermaphrodite sperm. Because of their competitive superiority, the male sperm fertilize nearly all the offspring produced immediately after mating, and the proportion of males in the progeny jumps to 50% (6, 7). Thus, in C. elegans, the benefits of outcrossing are offset by the cost of making males. Here we describe a novel effect of the male X chromosome on sperm competition in Caenorhabditis briggsae that reduces the cost of making males after outcrossing.
MATERIALS AND METHODS
Our experiments involved mating hermaphrodites to males and checking the offspring for gender and, where appropriate, for morphological markers that indicated paternity. In all experimental crosses, young-adult, virgin hermaphrodites, isolated the preceding day as last-stage juveniles, were placed individually on agar in 30-mm-diameter Petri dishes that were seeded with Escherichia coli, strain OP50 (8), and kept at 20°C. Four wild-type males (strain G16, kindly provided by the Caenorhabditis Genetics Center, St. Paul) were added to each plate and then removed after a 3- or 8-hr mating interval.
Hermaphrodites from either the wild-type strain G16, or a morphologically marked strain were used for all crosses. The morphological marker was a recessive mutation that produces a short and fat, hence “chubby”, phenotype [strain cby-4 (s1272), kindly provided by D. Baillie, Simon Frasier University, Burnaby, BC, Canada]. The outcross progeny of cby-4 hermaphrodites were readily distinguished by their wild-type morphology. Twenty-one wild-type and 26 cby-4 hermaphrodites were mated to males for a 3-hr interval, and 25 more cby-4 hermaphrodites were mated for an 8-hr interval. The mated hermaphrodites were transferred at regular intervals to fresh plates until they either died or stopped laying eggs. Eggs laid on each plate were allowed to hatch, and the emergent worms were grown at 25°C until their body morphology (chubby or wild-type) and/or gender could be scored. For the cby-4 hermaphrodites mated for 8 hr, progeny survivorship was also measured. Immediately after removal of the hermaphrodite, the eggs on each plate were counted and compared with the counts of the grown progeny to determine survivorship.
Progeny were also collected from unmated hermaphrodites isolated on plates as last-stage juveniles to ensure their virginity and transferred regularly to fresh plates. Along with 11 unmated cby-4 hermaphrodites, we collected progeny from 27 unmated mih-3 (s1290) hermaphrodites (from D. Baillie). The mih-3 mutation increases the incidence of chromosomal nondisjunction, resulting in increased loss of one of the two parental sex chromosomes and, therefore, increased male production by unmated hermaphrodites. This process gives rise to one male in six progeny in unmated mih-3 hermaphrodites as compared with one male in 800 progeny from unmated wild-type hermaphrodites (C.W.L., unpublished observations). We determined that most of the male production in mih-3 hermaphrodites was the result of X chromosome loss in the sperm by using artificial insemination to test the genotype of their sperm. We observed 18 males in 100 progeny fertilized by sperm that were taken as spermatids from newly molted mih-3 hermaphrodites and artificially inseminated into 17 cby-4 hermaphrodites (9); therefore, approximately 18% of mih-3 hermaphrodite sperm are nullo-X. Only 1.9% of the oocytes were nullo-X as estimated by artificially inseminating mih-3 hermaphrodites with sperm from males carrying an X-linked mutation, cby-3(bd101)X. (We observed six cby-3 male offspring, which can only result from a nullo-X oocyte fertilized by a male sperm carrying the mutant X chromosome. This number was doubled to account for nullo-X oocytes fertilized by nullo-X sperm and divided by the number of cross progeny, which was estimated by the proportion of males in the offspring after correcting both for males produced by mih-3 self-fertilization and for cby-3 male offspring. Six worms were inseminated, resulting in an estimated 629 outcross progeny.) Thus, the mih-3 mutation results in a greater proportion of nullo-X sperm than nullo-X oocytes. In addition to males, we observed dead eggs and sickly juveniles, indicating the mih-3 nondisjunction defect probably involved not only the sex chromosome, causing males, but also the autosomes, causing death.
We also observed inseminated sperm within mated hermaphrodites. Nine hermaphrodites were fixed immediately after they were witnessed to mate and stained with the DNA-specific label 4′,6-diamidino-2-phenylindole (DAPI) following standard methods (10). Another 13 hermaphrodites were fixed and stained 1 hr after they had mated. Finally, six worms were fixed and stained 24 hr after mating. These preparations were viewed under Nomarski optics and under epifluorescence to locate the sperm by their distinctive condensed nuclei, which stain brightly with DAPI.
RESULTS
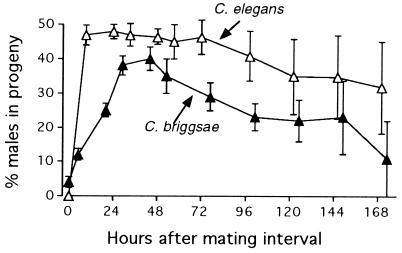
Recently mated C. briggsae hermaphrodites produced mainly hermaphrodite progeny. During the first 24 hr after mating, only a small proportion of the eggs laid by wild-type hermaphrodites developed into male progeny (Fig. 1). Male frequency rose steadily with time and peaked at 40% about 2 days after mating. This delayed appearance of male progeny contrasts with results from mated C. elegans hermaphrodites, whose progeny comprised nearly 50% males immediately after mating (Fig. 1). In C. briggsae, even though the early progeny were hermaphrodite, they were sired by the male, as results from cby-4 matings show (Fig. 2). Thus, the sex ratio of these early outcross progeny was skewed toward hermaphrodites, which composed 86% of the outcross progeny produced during the first 6 hr after mating. The outcross sex ratio reversed with time, especially for the hermaphrodites that mated for only 3 hr. By 45 hr after their 3-hr mating interval, these worms produced progeny of which only 38% were outcross hermaphrodites.
Figure 1.
Males as a percentage of the progeny produced by 21 mated C. briggsae hermaphrodites as a function of time after mating. C. elegans data replotted from LaMunyon and Ward (7). (Error bars = SEM.)
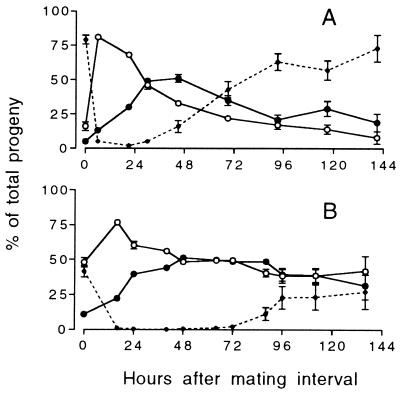
Figure 2.
Mean percentage of outcross progeny produced by mated cby-4 hermaphrodites as a function of time after mating. The hermaphrodites were given either (A) a 3-hr mating interval or (B) an 8-hr mating interval. Dotted line represents self-fertilized progeny. (○) Hermaphrodite outcross progeny; (•) male outcross progeny. (Error bars = SEM; where none are visible, the error is smaller than the symbol.)
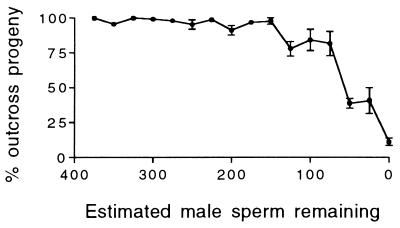
The production of self-fertilized progeny decreased with the length of the mating interval (Fig. 2). When mated for 3 hr, 142 ± 15 (mean ± SEM, here and henceforth) self-fertilized progeny were produced, but only 41 ± 8 such progeny were produced when mated for 8 hr (t = 5.99; P < 0.0001). Some of these self-fertilized progeny were laid during the mating interval (0 time point in Fig. 2), probably before the hermaphrodite had mated. The remaining self-fertilized progeny were produced after the bulk of the cross progeny, indicating that the self sperm were outcompeted by sperm from the male. Fifteen of the 3-hr-mated cby-4 hermaphrodites reverted to laying exclusively self-fertilized progeny, presumably because their supply of male sperm ran out. If each sperm fertilizes an egg, as occurs in C. elegans (6), then in cases where all the male sperm are used, the number of male sperm received is equal to the number of cross progeny produced. In these cases, the number of male sperm remaining in the hermaphrodite at any time point is equal to the number of outcross progeny yet to be produced. A plot of this estimate of the male sperm remaining vs. the percent of the progeny fertilized by male sperm (Fig. 3) shows that male sperm maintain complete precedence over hermaphrodite sperm until only about 100 male sperm remain. Below this level, the hermaphrodite self sperm have the opportunity to fertilize oocytes.
Figure 3.
Percentage of the progeny of mated cby-4 hermaphrodites that were outcross as a function of the number of male sperm estimated to remain in the hermaphrodite reproductive tract. (Error bars = SEM; where none are visible, the error is smaller than the symbol.)
The overall ratio of males to hermaphrodites in the outcross progeny dropped with the length of the mating interval. Whereas the sex ratio was equal after the 3-hr mating (157 ± 10 hermaphrodites to 152 ± 9 males; t = 0.337, P = 0.738), after the 8-hr mating, outcross hermaphrodites significantly outnumbered their male siblings (251 ± 9 hermaphrodites to 181 ± 9 males; t = 5.47, P < 0.0001). This difference can be seen in Fig. 2, where the outcross progeny sex ratio was male biased after the 3-hr mating interval but never after the 8-hr interval.
The observed skews in sex ratio were not a function of differential survivorship. Overall offspring survival for the 8-hr-mated cby-4 hermaphrodites averaged 84%, ranging from 66% to 97%. In the 12 cases with survivorship less than the median of 86.3%, the overall offspring sex ratio (1.4 hermaphrodites per male) was not significantly different from that of 13 cases with survivorship greater than or equal to the median (t = 0.226, P = 0.823). Thus, progeny survivorship did not affect sex ratio. The mortality of progeny was independent of their gender.
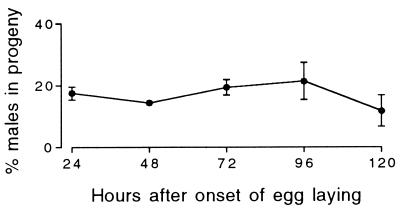
To determine whether the appearance of male progeny was delayed when hermaphrodites make their own nullo-X sperm, we used hermaphrodites from the strain mih-3. The production of male progeny by mih-3 hermaphrodites was not delayed. Rather, it was constant over time, averaging 15.6% (Fig. 4).
Figure 4.
Males as a percentage of the self-fertilized progeny laid by 27 mih-3 hermaphrodites as a function of time after the onset of egg laying. (Error bars = SEM; where none are visible, the error is smaller than the symbol.)
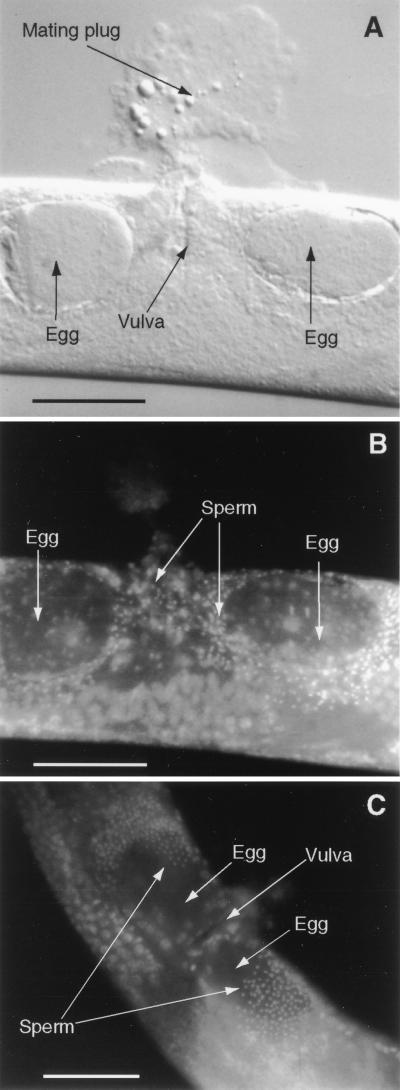
Microscopic examination of mated hermaphrodites revealed that the inseminated sperm moved rapidly to the two sperm storage organs, the spermathecae. These worms all bore a mating plug (Fig. 5A), which, in C. elegans, is known to indicate a successful ejaculation (11). Immediately after insemination, the sperm were dispersed throughout the uterus, including the region directly under the genital opening, or vulva, (Fig. 5B), where they are deposited by the male. One hour after mating, all the sperm were located in the distal ends of the uterus, in or near the spermathecae (Fig. 5C). A similar pattern was observed in six worms fixed and stained 24 hr after mating.
Figure 5.
(A and B) A recently mated C. briggsae hermaphrodite stained with the DNA label DAPI and viewed under both Nomarski (A) and epifluorescence (B) optics. The sperm are readily detected by their bright, compact nuclei. (C) Epifluorescent image of a hermaphrodite that had been similarly fixed and stained 1-hr after it copulated. (Bars = 50 μm.)
DISCUSSION
Male C. briggsae sperm take precedence over hermaphrodite sperm in a manner similar to that found in C. elegans (6, 7). Immediately after worms mate, nearly all the progeny are fertilized by male sperm. In our experiments, self-fertilized progeny were produced after the cross progeny. Because self sperm are made only before oogenesis and not added later (12), they were outcompeted by the male sperm and excluded from fertilizations until the male sperm had been depleted. Thus, male sperm precedence is not simply numerical. Instead, male sperm are competitively superior to hermaphrodite sperm, and displacement lasts until the male sperm run out. Indeed, our results with the cby-4 hermaphrodites show that only 100 male sperm were required for complete displacement of hermaphrodite self-fertilized sperm. Because these are divided between two spermathecae, only 50 sperm per spermatheca can effect precedence. Unmated cby-4 hermaphrodites produced, on average, 224 (± 38 SD) progeny and therefore had at least 100 self sperm per spermatheca in competition with the male sperm. Thus, 50 male sperm can effectively displace at least 100 self sperm.
A second type of sperm precedence occurs in C. briggsae. Immediately after mating, outcross hermaphrodites (the products of X-bearing sperm) were produced in excess of outcross males. Thus, X-bearing male sperm have a fertilization advantage over their nullo-X counterparts. In our experiments, hermaphrodites that received a copious supply of male sperm (those with an 8-hr mating interval) produced mainly outcross hermaphrodite progeny. In these cases, the hermaphrodites received so many X-bearing male sperm that they either ran out of oocytes or died before all the nullo-X male sperm had the opportunity to fertilize oocytes.
What is the nature of this X chromosome fertilization advantage in male sperm? It is not numerical. The 3-hr cby-4 hermaphrodites produced similar numbers of male and hermaphrodite outcross progeny, indicating that males transfer equal numbers of X-bearing and nullo-X sperm. Alternatively, X-bearing male sperm could gain the advantage by activating faster so that they crawl to the spermathecae sooner than nullo-X sperm. However, only 1 hr after mating, all inseminated sperm had activated and crawled to the spermathecae. Any early advantage the X-bearing sperm might gain by rapid activation cannot explain their precedence over nullo-X sperm, which persists for at least 24 hr. During this period eggs are continuously pushed through the spermathecae into the uterus, intermixing the sperm, and disrupting any sperm stratification that might have been established within the first hour after mating. Because the advantage of X-bearing male sperm is neither numerical nor due to differences in activation, it might be the result of adhering better to the spermathecae, inhibiting nullo-X sperm, or fusing preferentially with the oocytes.
While sperm competitiveness clearly varies with the presence of the X chromosome in male sperm, there was no such competitive variation in sperm from mih-3 hermaphrodites. The fraction of males in the progeny was constant over time, averaging 15.6%. Because spermatogenesis precedes oogenesis, all the self sperm were present and in competition before the first oocyte was available for fertilization. Thus, the nullo-X hermaphrodite sperm competed equally with the X-bearing sperm throughout the reproductive period, producing progeny roughly proportional to their number. Therefore, the advantageous effect of the X chromosome on sperm competitiveness does not occur in hermaphrodite sperm; it is specific to male sperm.
The gender specificity of the effect of the X chromosome on sperm competitiveness could arise in several ways. It may be that some part of the X chromosome is expressed during male, but not hermaphrodite, spermatogenesis. Such expression of genes on the X chromosome cannot occur after it segregates to a spermatid because nematode sperm contain no ribosomes (12). However, X chromosome-specific genes may be expressed earlier during meiosis, and if expression continues while the X chromosomes segregate to spermatids, then the products may be enriched in X-bearing sperm. Evidence from C. elegans that such late meiotic gene expression may be possible comes from worms heterozygous for certain sperm defect mutations (13). Some of the sperm that receive the mutant allele are defective, suggesting that these genes are expressed late enough in meiosis that the mutant products segregate with the mutant alleles.
Alternatively, the X chromosome may bear an “imprint,” acquired during spermatogenesis in only one of the parents (14–17). Imprinting is generally used to describe differential effects of the paternal versus maternal genomes in a developing embryo (18). Embryos that receive both copies of an imprinted genetic element from only one parent are defective. This type of imprinting has not been found in C. elegans (19). The effect of the X chromosome described here resembles imprinting in the sense that the X chromosome has a different effect depending on its parental origin, although this effect occurs in the gamete rather than in the embryo. Nonetheless, it could arise from parent-specific modification of the X chromosome, which, for example, might attract factors that increase sperm competitiveness.
Whatever the mechanism, the advantage of the X-bearing sperm suggests that the X chromosome is under a form of meiotic drive, selfishly increasing the competitiveness of sperm that carry it with those that do not. Classically, meiotic drive refers to a distortion of the frequency of alleles in the gametes of heterozygotes, favoring the driven allele (20, 21). In systems that have been studied intensively [i.e., the segregation distorter of Drosophila and the t- haplotype of mice (21–23)], the meiotically driven genetic elements eliminate or cripple the gametes not carrying them. In these systems, the meiotically driven chromosome benefits, but the rest of the genome suffers because meiotic drive typically reduces the total number of viable sperm in heterozygotes and, in some cases, causes homozygote lethality. In contrast, the driven male X chromosome of C. briggsae conveys no detriment because the nullo-X sperm are not disadvantaged: they outcompete hermaphrodite sperm, even though hermaphrodite sperm all bear their own X chromosomes (Fig. 2). In fact, both the X chromosome and the genome as a whole benefit through production of hermaphrodite progeny immediately after outcrossing. It has been shown that early progeny contribute disproportionately to the ability to colonize new habitat rapidly (24), and X chromosome drive ensures that these early progeny are self-fertile.
The X chromosome advantage in C. briggsae male sperm has important evolutionary consequences. Male sperm precedence maximizes outcrossing, an important factor increasing genetic variation in a primarily self-fertilizing species. In C. elegans, outcrossing also results in a cost of making males, because half of the outcross progeny are male. The novel effect of the X chromosome on sperm competitiveness in C. briggsae delays the cost of making males. Although sperm competitiveness varies among laboratory strains of mice (25), and with the presence of intracellular parasites in beetles (26), to our knowledge, this is the clearest evidence of a chromosomal effect on sperm competitiveness. Through the evolution of multiple levels of sperm competitiveness, C. briggsae gains increased outcrossing after mating while reducing the costs of making males in the early, and potentially most important, outcross progeny.
Acknowledgments
We thank P. Muhlrad, J. Nance, P. Sherman, W. Van Voorhies, and an anonymous reviewer for comments on the manuscript. The C. briggsae strains G16 and cby-3(bd101)X were supplied by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. D. Baillie provided the cby-4 (s1272) and mih-3 (s1290) C. briggsae strains. This research was supported by National Institutes of Health Grant GM25243 (S.W.), National Institutes of Health National Research Service Award F32 HD07683-01A1 (C.W.L.), and a grant from the University of Arizona Foundation under the Vice President for Research.
Footnotes
Abbreviation: DAPI, 4′,6-diamidino-2-phenylindole.
References
- 1.Maynard Smith J. The Evolution of Sex. Cambridge, U.K.: Cambridge Univ. Press; 1978. [Google Scholar]
- 2.Waller D M. In: The Statics and Dynamics of Mating System Evolution. Thornhill N W, editor. Chicago: Univ. of Chicago Press; 1993. pp. 97–117. [Google Scholar]
- 3.Ghiselin M T. Q Rev Biol. 1969;44:189–208. doi: 10.1086/406066. [DOI] [PubMed] [Google Scholar]
- 4.Nigon V, Dougherty E C. J Exp Zool. 1949;112:485–503. doi: 10.1002/jez.1401120307. [DOI] [PubMed] [Google Scholar]
- 5.Fodor A, Riddle D L, Nelson F K, Golden J W. Nematologica. 1983;29:203–217. [Google Scholar]
- 6.Ward S, Carrel J S. Dev Biol. 1979;73:304–321. doi: 10.1016/0012-1606(79)90069-1. [DOI] [PubMed] [Google Scholar]
- 7.LaMunyon C W, Ward S. Experientia. 1995;51:817–823. doi: 10.1007/BF01922436. [DOI] [PubMed] [Google Scholar]
- 8.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaMunyon C W, Ward S. Genetics. 1994;138:689–692. doi: 10.1093/genetics/138.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulston J, Hodgkin J. In: The Nematode Caenorhabditis Elegans. Wood W B, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. p. 591. [Google Scholar]
- 11.Barker D M. Anim Behav. 1994;48:147–156. [Google Scholar]
- 12.Kimble J, Ward S. In: The Nematode Caenorhabditis Elegans. Wood W B, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. p. 201. [Google Scholar]
- 13.Argon Y, Ward S. Genetics. 1980;96:413–433. doi: 10.1093/genetics/96.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlow D P. Science. 1995;270:1610–1613. doi: 10.1126/science.270.5242.1610. [DOI] [PubMed] [Google Scholar]
- 15.Crouse H. Genetics. 1960;45:1425–1443. [Google Scholar]
- 16.Lyon M F. Trends Genet. 1993;9:123–128. doi: 10.1016/0168-9525(93)90206-w. [DOI] [PubMed] [Google Scholar]
- 17.Moore T, Haig D. Trends Genet. 1991;7:45–49. doi: 10.1016/0168-9525(91)90230-N. [DOI] [PubMed] [Google Scholar]
- 18.Barlow D P. Trends Genet. 1994;10:194–199. doi: 10.1016/0168-9525(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 19.Haack H, Hodgkin J. Mol Gen Genet. 1991;228:482–485. doi: 10.1007/BF00260643. [DOI] [PubMed] [Google Scholar]
- 20.Lyttle T W. Trends Genet. 1993;9:205–210. doi: 10.1016/0168-9525(93)90120-7. [DOI] [PubMed] [Google Scholar]
- 21.Lyttle T W. Annu Rev Genet. 1991;25:511–557. doi: 10.1146/annurev.ge.25.120191.002455. [DOI] [PubMed] [Google Scholar]
- 22.Silver L M. Annu Rev Genet. 1985;19:179–208. doi: 10.1146/annurev.ge.19.120185.001143. [DOI] [PubMed] [Google Scholar]
- 23.Silver L M. Trends Genet. 1993;9:250–254. doi: 10.1016/0168-9525(93)90090-5. [DOI] [PubMed] [Google Scholar]
- 24.Hodgkin J, Barnes T M. Proc R Soc London Ser B. 1991;246:19–24. doi: 10.1098/rspb.1991.0119. [DOI] [PubMed] [Google Scholar]
- 25.Dewsbury D A. In: Sperm Competition and the Evolution of Animal Mating Systems. Smith R L, editor. Orlando, FL: Academic; 1984. pp. 547–571. [Google Scholar]
- 26.Wade M J, Chang N W. Nature (London) 1995;373:72–74. doi: 10.1038/373072a0. [DOI] [PubMed] [Google Scholar]