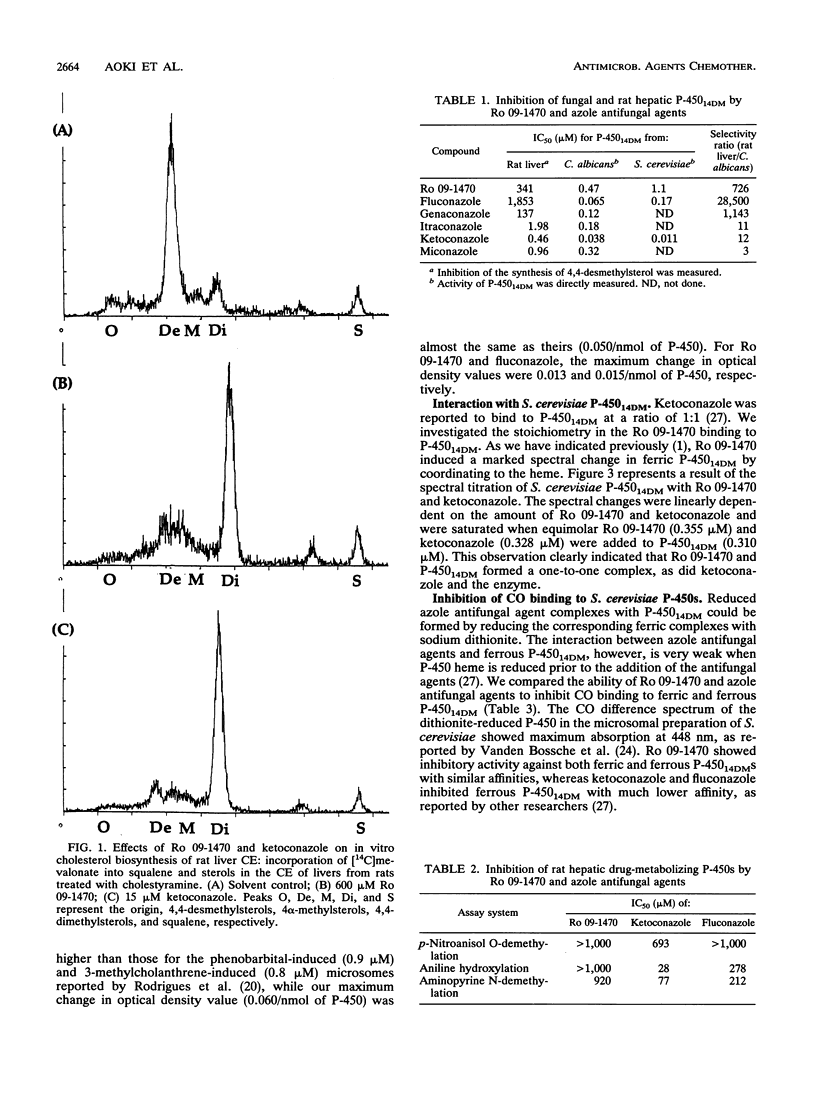
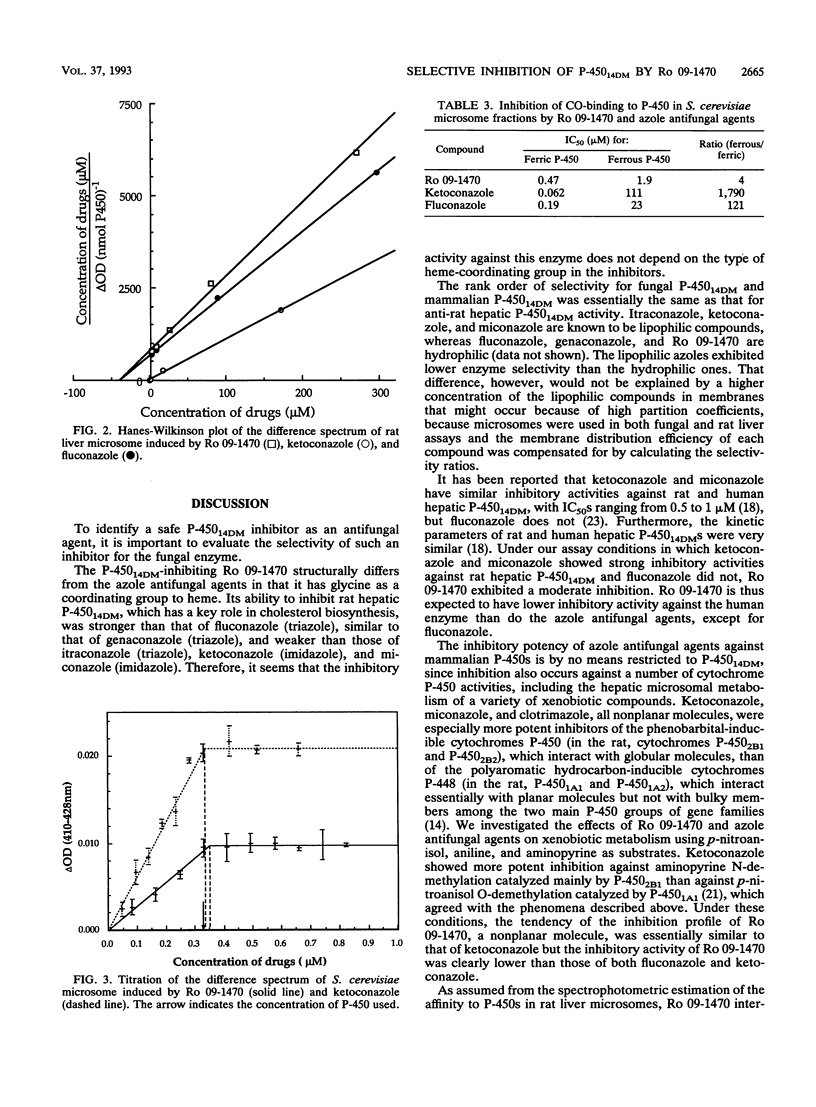
Abstract
Ro 09-1470 is a new antifungal agent that belongs to a series of compounds characterized by a tetrahydropyran skeleton with glycine and alkenyl side chains and that inhibits P-450 lanosterol C-14 demethylase (P-450(14DM)) of fungi (Y. Aoki, T. Yamazaki, M. Kondoh, Y. Sudoh, N. Nakayama, Y. Sekine, H. Shimada, and M. Arisawa, J. Antibiot. 45:160-170, 1992; S. Matsukuma, T. Ohtsuka, H. Kotaki, H. Sawairi, T. Sano, K. Watanabe, N. Nakayama, Y. Itezono, M. Fujiu, N. Shimma, K. Yokose, and T. Okuda, J. Antibiot. 45:151-159, 1992). We have studied the compound's mode of interaction with fungal P-450(14DM) and its selectivity for the fungal versus mammalian P-450 enzymes. Ro 09-1470 bound to the Saccharomyces cerevisiae P-450(14DM) by coordinating to the heme with one-to-one stoichiometry. Unlike the azole compounds, it interacted with both ferric and ferrous heme. It was active also against the P-450(14DM) of Candida albicans. Ro 09-1470 preferentially inhibited the yeast P-450(14DM), showing a 50% inhibitory concentration (IC50) of 0.47 to approximately 1.1 microM, which is much lower than the IC50s for rat hepatic P-450s catalyzing cholesterol biosynthesis (IC50 = 341 microM), p-nitroanisol O-demethylation (> 1,000 microM), aniline hydroxylation (> 1,000 microM), and aminopyrine N-demethylation (920 microM). The degree of selectivity for yeast P-450 was higher than that of ketoconazole.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki Y., Yamazaki T., Kondoh M., Sudoh Y., Nakayama N., Sekine Y., Shimada H., Arisawa M. A new series of natural antifungals that inhibit P450 lanosterol C-14 demethylase. II. Mode of action. J Antibiot (Tokyo) 1992 Feb;45(2):160–170. doi: 10.7164/antibiotics.45.160. [DOI] [PubMed] [Google Scholar]
- Aoyama Y., Okikawa T., Yoshida Y. Evidence for the presence of cytochrome P-450 functional in lanosterol 14 alpha-demethylation in microsomes of aerobically grown respiring yeast. Biochim Biophys Acta. 1981 Sep 24;665(3):596–601. doi: 10.1016/0005-2760(81)90275-7. [DOI] [PubMed] [Google Scholar]
- Aoyama Y., Yoshida Y., Sato R. Yeast cytochrome P-450 catalyzing lanosterol 14 alpha-demethylation. II. Lanosterol metabolism by purified P-450(14)DM and by intact microsomes. J Biol Chem. 1984 Feb 10;259(3):1661–1666. [PubMed] [Google Scholar]
- Aoyama Y., Yoshida Y., Sonoda Y., Sato Y. Metabolism of 32-hydroxy-24,25-dihydrolanosterol by purified cytochrome P-45014DM from yeast. Evidence for contribution of the cytochrome to whole process of lanosterol 14 alpha-demethylation. J Biol Chem. 1987 Jan 25;262(3):1239–1243. [PubMed] [Google Scholar]
- Feldman D. Ketoconazole and other imidazole derivatives as inhibitors of steroidogenesis. Endocr Rev. 1986 Nov;7(4):409–420. doi: 10.1210/edrv-7-4-409. [DOI] [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston J. B., Humphrey M. J., Matthew D. E., Tarbit M. H. Comparison of two azole antifungal drugs, ketoconazole, and fluconazole, as modifiers of rat hepatic monooxygenase activity. Biochem Pharmacol. 1988 Feb 1;37(3):401–408. doi: 10.1016/0006-2952(88)90206-7. [DOI] [PubMed] [Google Scholar]
- Kamataki T., Kitada M., Shigematsu H., Kitagawa H. The involvement of cytochrome P-488 and P-450 in NADH-dependent O-demethylation of p-nitroanisole in rat liver microsomes. Jpn J Pharmacol. 1979 Apr;29(2):191–201. doi: 10.1254/jjp.29.191. [DOI] [PubMed] [Google Scholar]
- Kirsch D. R., Lai M. H., O'Sullivan J. Isolation of the gene for cytochrome P450L1A1 (lanosterol 14 alpha-demethylase) from Candida albicans. Gene. 1988 Sep 7;68(2):229–237. doi: 10.1016/0378-1119(88)90025-x. [DOI] [PubMed] [Google Scholar]
- Lavrijsen K. L., Van Houdt J. M., Van Dyck D. M., Meuldermans W. E., Heykants J. J. Induction potential of fluconazole toward drug-metabolizing enzymes in rats. Antimicrob Agents Chemother. 1990 Mar;34(3):402–408. doi: 10.1128/aac.34.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. F., Ioannides C., Parke D. V. Molecular dimensions of the substrate binding site of cytochrome P-448. Biochem Pharmacol. 1986 Jul 1;35(13):2179–2185. doi: 10.1016/0006-2952(86)90589-7. [DOI] [PubMed] [Google Scholar]
- Matsukuma S., Ohtsuka T., Kotaki H., Shirai H., Sano T., Watanabe K., Nakayama N., Itezono Y., Fujiu M., Shimma N. A new series of natural antifungals that inhibit P450 lanosterol C-14 demethylase. I. Taxonomy, fermentation, isolation and structural elucidation. J Antibiot (Tokyo) 1992 Feb;45(2):151–159. doi: 10.7164/antibiotics.45.151. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Ohba M., Sato R., Yoshida Y., Nishino T., Katsuki H. Involvement of cytochrome P-450 and a cyanide-sensitive enzyme in different steps of lanosterol demethylation by yeast microsomes. Biochem Biophys Res Commun. 1978 Nov 14;85(1):21–27. doi: 10.1016/s0006-291x(78)80005-9. [DOI] [PubMed] [Google Scholar]
- Raucy J. L., Carpenter S. J., Trzaskos J. M. Identification of lanosterol 14 alpha-methyl demethylase in human tissues. Biochem Biophys Res Commun. 1991 May 31;177(1):497–503. doi: 10.1016/0006-291x(91)92011-8. [DOI] [PubMed] [Google Scholar]
- Rine J., Hansen W., Hardeman E., Davis R. W. Targeted selection of recombinant clones through gene dosage effects. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6750–6754. doi: 10.1073/pnas.80.22.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A. D., Lewis D. F., Ioannides C., Parke D. V. Spectral and kinetic studies of the interaction of imidazole anti-fungal agents with microsomal cytochromes P-450. Xenobiotica. 1987 Nov;17(11):1315–1327. doi: 10.3109/00498258709047162. [DOI] [PubMed] [Google Scholar]
- Soucek P., Gut I. Cytochromes P-450 in rats: structures, functions, properties and relevant human forms. Xenobiotica. 1992 Jan;22(1):83–103. doi: 10.3109/00498259209053106. [DOI] [PubMed] [Google Scholar]
- Trzaskos J. M., Bowen W. D., Shafiee A., Fischer R. T., Gaylor J. L. Cytochrome P-450-dependent oxidation of lanosterol in cholesterol biosynthesis. Microsomal electron transport and C-32 demethylation. J Biol Chem. 1984 Nov 10;259(21):13402–13412. [PubMed] [Google Scholar]
- Trzaskos J. M., Henry M. J. Comparative effects of the azole-based fungicide flusilazole on yeast and mammalian lanosterol 14 alpha-methyl demethylase. Antimicrob Agents Chemother. 1989 Aug;33(8):1228–1231. doi: 10.1128/aac.33.8.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Heme ligand replacement reactions of cytochrome P-450. Characterization of the bonding atom of the axial ligand trans to thiolate as oxygen. J Biol Chem. 1982 Mar 25;257(6):3073–3083. [PubMed] [Google Scholar]
- Yoshida Y., Aoyama Y. Interaction of azole antifungal agents with cytochrome P-45014DM purified from Saccharomyces cerevisiae microsomes. Biochem Pharmacol. 1987 Jan 15;36(2):229–235. doi: 10.1016/0006-2952(87)90694-0. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Imai Y., Hashimoto-Yutsudo C. Spectrophotometric examination of exogenous-ligand complexes of ferric cytochrome P-450. Characterization of the axial ligand trans to thiolate in the native ferric low-spin form. J Biochem. 1982 May;91(5):1651–1659. doi: 10.1093/oxfordjournals.jbchem.a133856. [DOI] [PubMed] [Google Scholar]



