Abstract
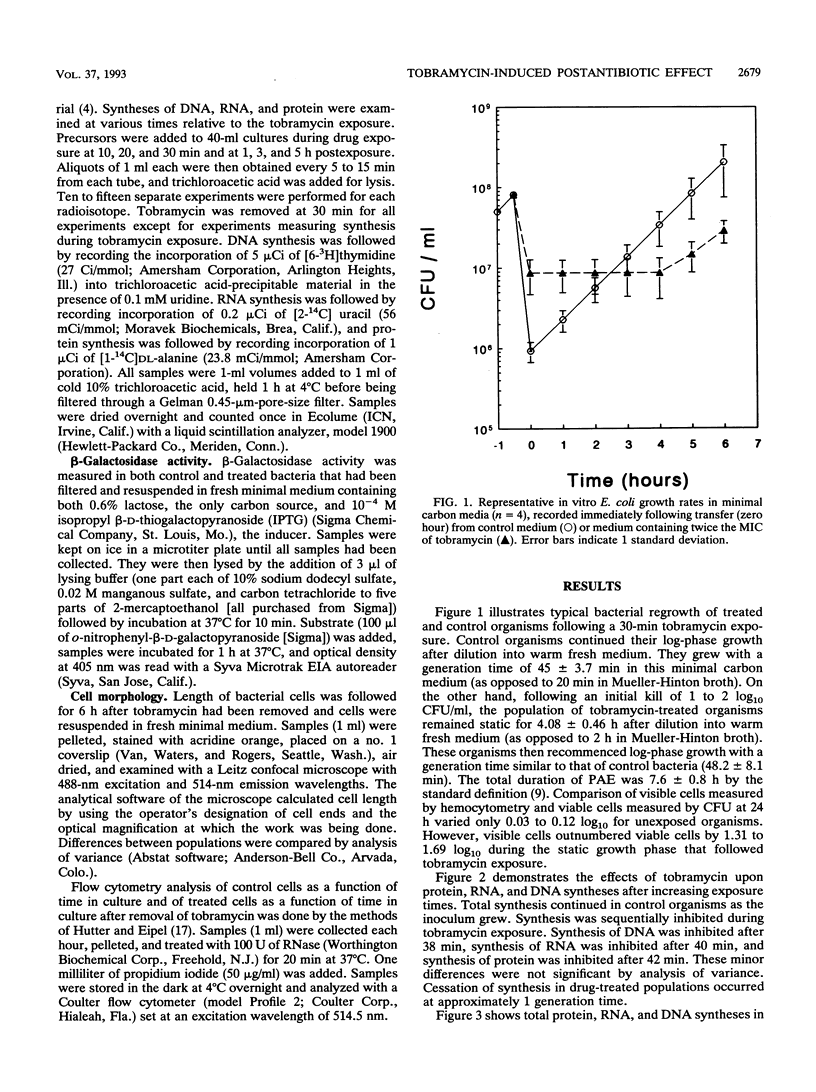
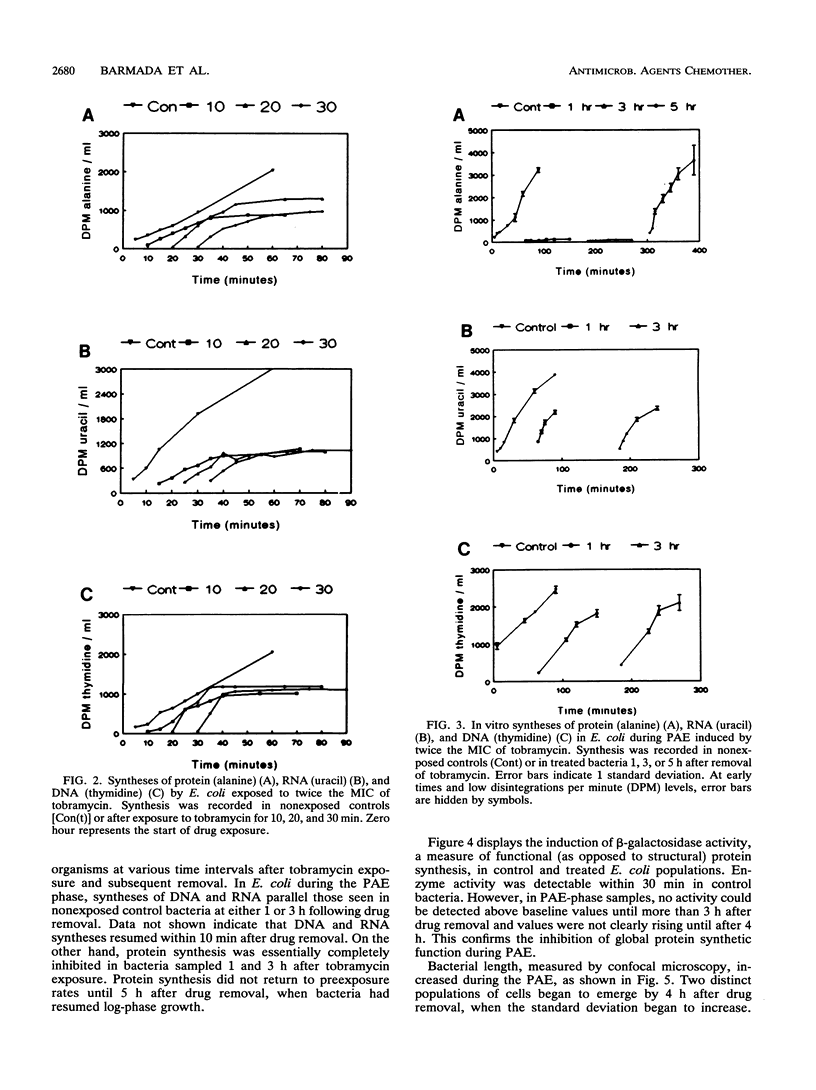
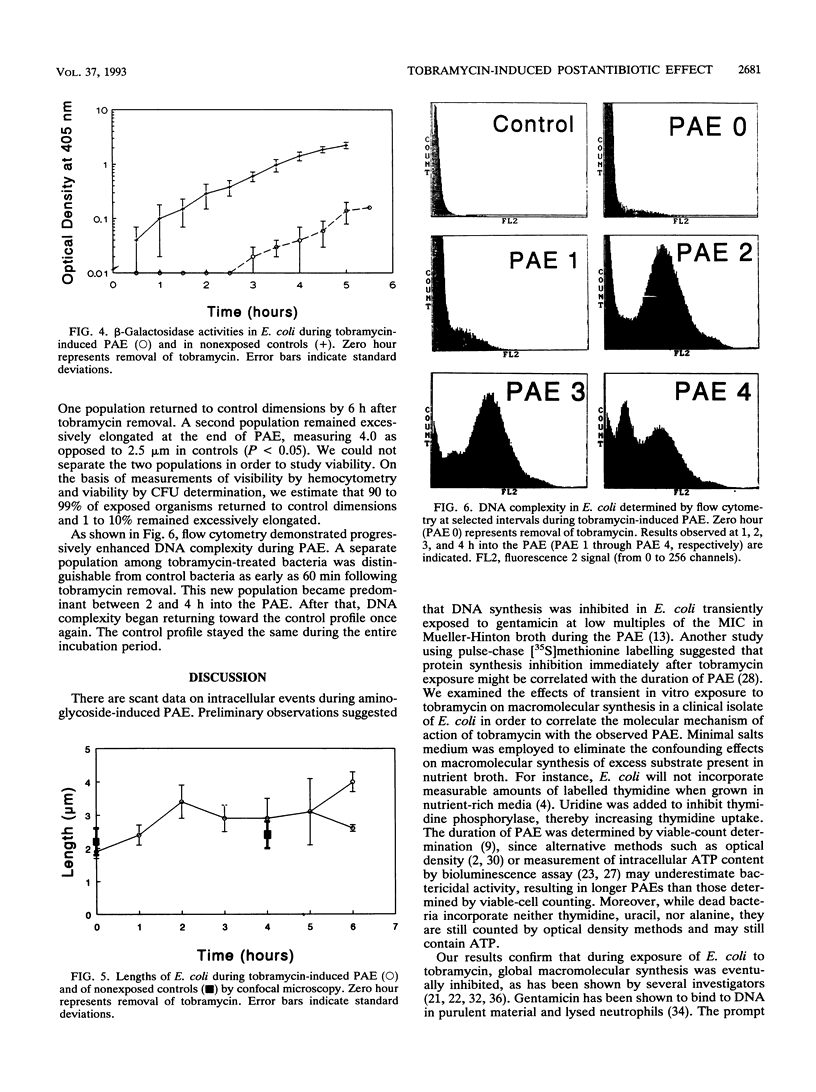
Scant data exist on intracellular events during aminoglycoside-induced postantibiotic effect (PAE). We examined DNA, RNA, and protein syntheses after tobramycin exposure using [3H]thymidine, [14C]uracil, and [14C]alanine incorporation in a clinical Escherichia coli strain. Late-log-phase bacteria in oxygenated minimal salts medium at 37 degrees C were exposed to tobramycin (7.5 micrograms/ml) (twice the MIC) for 30 min. Tobramycin caused a kill of 2 log10 CFU/ml prior to drug removal by filtration and a 5-h PAE, measured by viable counts. Excess amounts of labelled precursors were added to tobramycin-exposed organisms during, immediately after, and at various intervals following exposure. In the presence of tobramycin, DNA, RNA, and protein syntheses were sequentially inhibited within 1 generation time. Following drug removal, both DNA and RNA syntheses promptly resumed, suggesting readily dissociable nonspecific binding to DNA and RNA. However, total protein synthesis did not resume until 4 h later. beta-Galactosidase activity, a measure of functional enzymatic protein synthesis, was also inhibited for 4 h after drug removal. Bacterium length, measured by confocal microscopy, increased during PAE. Two distinct populations eventually emerged: one that returned to control dimensions and one that remained excessively elongated by the end of PAE (2.5 microns versus 4.0 microns; P < 0.05). We hypothesize that only viable cells return to the control morphology. Flow cytometry showed enhanced DNA complexity during PAE, consistent with either impaired cellular protein synthesis in viable cells or perturbations in dying cells. In summary, duration of PAE correlated with inhibition of total and functional protein synthesis but not DNA or RNA synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND N., DAVIS B. D., ARMITAGE A. K. Uptake of streptomycin by Escherichia coli. Nature. 1960 Jan 2;185:23–24. doi: 10.1038/185023a0. [DOI] [PubMed] [Google Scholar]
- Budman D. R., Pardee A. B. Thymidine and thymine incorporation into deoxyribonucleic acid: inhibition and repression by uridine of thymidine phosphorylase of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1546–1550. doi: 10.1128/jb.94.5.1546-1550.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundtzen R. W., Gerber A. U., Cohn D. L., Craig W. A. Postantibiotic suppression of bacterial growth. Rev Infect Dis. 1981 Jan-Feb;3(1):28–37. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- Busse H. J., Wöstmann C., Bakker E. P. The bactericidal action of streptomycin: membrane permeabilization caused by the insertion of mistranslated proteins into the cytoplasmic membrane of Escherichia coli and subsequent caging of the antibiotic inside the cells due to degradation of these proteins. J Gen Microbiol. 1992 Mar;138(3):551–561. doi: 10.1099/00221287-138-3-551. [DOI] [PubMed] [Google Scholar]
- Cabañas M. J., Vázquez D., Modolell J. Dual interference of hygromycin B with ribosomal translocation and with aminoacyl-tRNA recognition. Eur J Biochem. 1978 Jun 1;87(1):21–27. doi: 10.1111/j.1432-1033.1978.tb12347.x. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Post-antibiotic suppressive effect of ciprofloxacin against gram-positive and gram-negative bacteria. Am J Med. 1987 Apr 27;82(4A):58–62. [PubMed] [Google Scholar]
- DUBIN D. T., HANCOCK R., DAVIS B. D. THE SEQUENCE OF SOME EFFECTS OF STREPTOMYCIN IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 13;74:476–489. doi: 10.1016/0006-3002(63)91390-8. [DOI] [PubMed] [Google Scholar]
- Davis B. B. The lethal action of aminoglycosides. J Antimicrob Chemother. 1988 Jul;22(1):1–3. doi: 10.1093/jac/22.1.1. [DOI] [PubMed] [Google Scholar]
- Davis B. D., Chen L. L., Tai P. C. Misread protein creates membrane channels: an essential step in the bactericidal action of aminoglycosides. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6164–6168. doi: 10.1073/pnas.83.16.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan L., Blumenthal R. M., Burnham J. C. Analysis of macromolecular biosynthesis to define the quinolone-induced postantibiotic effect in Escherichia coli. Antimicrob Agents Chemother. 1992 Oct;36(10):2118–2124. doi: 10.1128/aac.36.10.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Aminoglycoside uptake and mode of action--with special reference to streptomycin and gentamicin. I. Antagonists and mutants. J Antimicrob Chemother. 1981 Oct;8(4):249–276. doi: 10.1093/jac/8.4.249. [DOI] [PubMed] [Google Scholar]
- Hancock R. E. Aminoglycoside uptake and mode of action-with special reference to streptomycin and gentamicin. II. Effects of aminoglycosides on cells. J Antimicrob Chemother. 1981 Dec;8(6):429–445. doi: 10.1093/jac/8.6.429. [DOI] [PubMed] [Google Scholar]
- Hutter K. J., Eipel H. E. Flow cytometric determinations of cellular substances in algae, bacteria, moulds and yeasts. Antonie Van Leeuwenhoek. 1978;44(3-4):269–282. doi: 10.1007/BF00394305. [DOI] [PubMed] [Google Scholar]
- Isaksson B., Nilsson L., Maller R., Sörén L. Postantibiotic effect of aminoglycosides on gram-negative bacteria evaluated by a new method. J Antimicrob Chemother. 1988 Jul;22(1):23–33. doi: 10.1093/jac/22.1.23. [DOI] [PubMed] [Google Scholar]
- Kapusnik J. E., Hackbarth C. J., Chambers H. F., Carpenter T., Sande M. A. Single, large, daily dosing versus intermittent dosing of tobramycin for treating experimental pseudomonas pneumonia. J Infect Dis. 1988 Jul;158(1):7–12. doi: 10.1093/infdis/158.1.7. [DOI] [PubMed] [Google Scholar]
- Leggett J. E., Fantin B., Ebert S., Totsuka K., Vogelman B., Calame W., Mattie H., Craig W. A. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis. 1989 Feb;159(2):281–292. doi: 10.1093/infdis/159.2.281. [DOI] [PubMed] [Google Scholar]
- Levy J., Burns J. L., Mendelman P. M., Wong K., Mack K., Smith A. L. Effect of tobramycin on protein synthesis in 2-deoxystreptamine aminoglycoside-resistant clinical isolates of Haemophilus influenzae. Antimicrob Agents Chemother. 1986 Mar;29(3):474–481. doi: 10.1128/aac.29.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga K., Yamaki H., Nishimura T., Tanaka N. Inhibition of DNA replication initiation by aminoglycoside antibiotics. Antimicrob Agents Chemother. 1986 Sep;30(3):468–474. doi: 10.1128/aac.30.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattie H. Kinetics of antimicrobial action. Rev Infect Dis. 1981 Jan-Feb;3(1):19–27. doi: 10.1093/clinids/3.1.19. [DOI] [PubMed] [Google Scholar]
- McDonald P. J., Craig W. A., Kunin C. M. Persistent effect of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J Infect Dis. 1977 Feb;135(2):217–223. doi: 10.1093/infdis/135.2.217. [DOI] [PubMed] [Google Scholar]
- Odenholt I., Holm S. E., Cars O. An in vivo model for evaluation of the postantibiotic effect. Scand J Infect Dis. 1988;20(1):97–103. doi: 10.3109/00365548809117224. [DOI] [PubMed] [Google Scholar]
- Odenholt I., Holm S. E., Cars O. Effects of benzylpenicillin on Streptococcus pyogenes during the postantibiotic phase in vitro. J Antimicrob Chemother. 1989 Aug;24(2):147–156. doi: 10.1093/jac/24.2.147. [DOI] [PubMed] [Google Scholar]
- Odenholt I., Isaksson B., Nilsson L., Cars O. Postantibiotic and bactericidal effect of imipenem against Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 1989 Feb;8(2):136–141. doi: 10.1007/BF01963897. [DOI] [PubMed] [Google Scholar]
- Park M. K., Muhvich K. H., Myers R. A., Marzella L. Hyperoxia prolongs the aminoglycoside-induced postantibiotic effect in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991 Apr;35(4):691–695. doi: 10.1128/aac.35.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. H., Thompson W. L., Luthe M. A., Stern R. C., Grossniklaus D. A., Bloxham D. D., Groden D. L., Jacobs M. R., DiScenna A. O., Cash H. A. Once-daily vs. continuous aminoglycoside dosing: efficacy and toxicity in animal and clinical studies of gentamicin, netilmicin, and tobramycin. J Infect Dis. 1983 May;147(5):918–932. doi: 10.1093/infdis/147.5.918. [DOI] [PubMed] [Google Scholar]
- Rescott D. L., Nix D. E., Holden P., Schentag J. J. Comparison of two methods for determining in vitro postantibiotic effects of three antibiotics on Escherichia coli. Antimicrob Agents Chemother. 1988 Apr;32(4):450–453. doi: 10.1128/aac.32.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOTTS C. R., STANIER R. Y. Mechanism of streptomycin action on bacteria: a unitary hypothesis. Nature. 1961 Nov 18;192:633–637. doi: 10.1038/192633a0. [DOI] [PubMed] [Google Scholar]
- Vaudaux P., Waldvogel F. A. Gentamicin inactivation in purulent exudates: role of cell lysis. J Infect Dis. 1980 Oct;142(4):586–593. doi: 10.1093/infdis/142.4.586. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Turnidge J., Leggett J., Craig W. A. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis. 1988 Feb;157(2):287–298. doi: 10.1093/infdis/157.2.287. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]



