Abstract
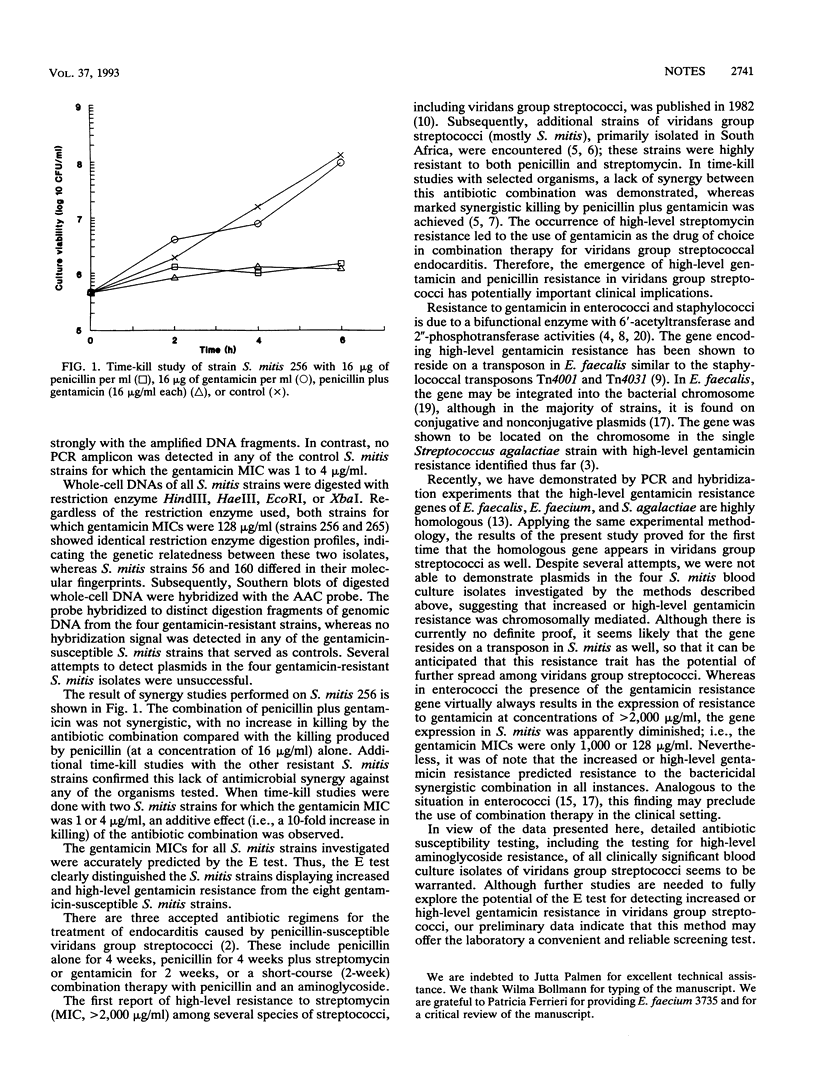
Four blood culture isolates of Streptococcus mitis were found to be resistant to penicillin (MIC, 16 to 32 micrograms/ml) and gentamicin (MIC, 128 or 1,000 micrograms/ml), and the two antibiotics demonstrated a lack of in vitro synergy. As shown by polymerase chain reaction assays, the structural gene known to encode high-level gentamicin resistance in Enterococcus faecalis, Enterococcus faecium, and Streptococcus agalactiae was also present in all four S. mitis strains. Attempts to isolate plasmids were unsuccessful, but an oligonucleotide probe derived from the gentamicin resistance gene hybridized to distinct restriction fragments of genomic DNA, suggesting that the resistance genes in these strains are integrated into the bacterial chromosome.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisno A. L., Dismukes W. E., Durack D. T., Kaplan E. L., Karchmer A. W., Kaye D., Rahimtoola S. H., Sande M. A., Sanford J. P., Watanakunakorn C. Antimicrobial treatment of infective endocarditis due to viridans streptococci, enterococci, and staphylococci. JAMA. 1989 Mar 10;261(10):1471–1477. [PubMed] [Google Scholar]
- Buu-Hoï A., Le Bouguenec C., Horaud T. High-level chromosomal gentamicin resistance in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1990 Jun;34(6):985–988. doi: 10.1128/aac.34.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Wennersten C., Zighelboim-Daum S., Reiszner E., Goldmann D., Moellering R. C., Jr High-level resistance to gentamicin in clinical isolates of Streptococcus (Enterococcus) faecium. Antimicrob Agents Chemother. 1988 Oct;32(10):1528–1532. doi: 10.1128/aac.32.10.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber B. F., Eliopoulos G. M., Ward J. I., Ruoff K. L., Syriopoulou V., Moellering R. C., Jr Multiply resistant viridans streptococci: susceptibility to beta-lactam antibiotics and comparison of penicillin-binding protein patterns. Antimicrob Agents Chemother. 1983 Nov;24(5):702–705. doi: 10.1128/aac.24.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber B. F., Eliopoulos G. M., Ward J. I., Ruoff K., Moellering R. C., Jr Resistance to penicillin-streptomycin synergy among clinical isolates of viridans streptococci. Antimicrob Agents Chemother. 1983 Dec;24(6):871–875. doi: 10.1128/aac.24.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber B. F., Yee Y. High-level aminoglycoside resistance mediated by aminoglycoside-modifying enzymes among viridans streptococci: implications for the therapy for endocarditis. J Infect Dis. 1987 May;155(5):948–953. doi: 10.1093/infdis/155.5.948. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel-Christian S. L., Murray B. E. Characterization of the gentamicin resistance transposon Tn5281 from Enterococcus faecalis and comparison to staphylococcal transposons Tn4001 and Tn4031. Antimicrob Agents Chemother. 1991 Jun;35(6):1147–1152. doi: 10.1128/aac.35.6.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Buu-Hoï A., Delbos F., Bieth G. High-level aminoglycoside resistance in group A, B, G, D (Streptococcus bovis), and viridans streptococci. Antimicrob Agents Chemother. 1982 Jan;21(1):176–179. doi: 10.1128/aac.21.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. T., Malke H., Ferretti J. J. Heterogeneity of the streptokinase gene in group A streptococci. Infect Immun. 1989 Feb;57(2):502–506. doi: 10.1128/iai.57.2.502-506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufhold A., Ferrieri P. Molecular investigation of clinical Enterococcus faecium isolates highly resistant to gentamicin. Zentralbl Bakteriol. 1993 Feb;278(1):83–101. doi: 10.1016/s0934-8840(11)80282-3. [DOI] [PubMed] [Google Scholar]
- Kaufhold A., Podbielski A., Horaud T., Ferrieri P. Identical genes confer high-level resistance to gentamicin upon Enterococcus faecalis, Enterococcus faecium, and Streptococcus agalactiae. Antimicrob Agents Chemother. 1992 Jun;36(6):1215–1218. doi: 10.1128/aac.36.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufhold A., Podbielski A., Johnson D. R., Kaplan E. L., Lütticken R. M protein gene typing of Streptococcus pyogenes by nonradioactively labeled oligonucleotide probes. J Clin Microbiol. 1992 Sep;30(9):2391–2397. doi: 10.1128/jcm.30.9.2391-2397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Korzeniowski O. M., Sande M. A., Wennersten C. B. Species-specific resistance to antimocrobial synergism in Streptococcus faecium and Streptococcus faecalis. J Infect Dis. 1979 Aug;140(2):203–208. doi: 10.1093/infdis/140.2.203. [DOI] [PubMed] [Google Scholar]
- Patterson J. E., Zervos M. J. High-level gentamicin resistance in Enterococcus: microbiology, genetic basis, and epidemiology. Rev Infect Dis. 1990 Jul-Aug;12(4):644–652. doi: 10.1093/clinids/12.4.644. [DOI] [PubMed] [Google Scholar]
- Potgieter E., Carmichael M., Koornhof H. J., Chalkley L. J. In vitro antimicrobial susceptibility of viridans streptococci isolated from blood cultures. Eur J Clin Microbiol Infect Dis. 1992 Jun;11(6):543–546. doi: 10.1007/BF01960811. [DOI] [PubMed] [Google Scholar]
- Rice L. B., Eliopoulos G. M., Wennersten C., Goldmann D., Jacoby G. A., Moellering R. C., Jr Chromosomally mediated beta-lactamase production and gentamicin resistance in Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Feb;35(2):272–276. doi: 10.1128/aac.35.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Gotoh A., Konno M. Purification and characterization of aminoglycoside-modifying enzymes from Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1984 Jun;25(6):754–759. doi: 10.1128/aac.25.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti M., Baiocchi P., Santini C., Brandimarte C., Serra P., Gentile G., Girmenia C., Martino P. Antimicrobial susceptibilities of Streptococcus species that cause septicemia in neutropenic patients. Antimicrob Agents Chemother. 1989 Apr;33(4):580–582. doi: 10.1128/aac.33.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]


