Disorders of ovulation account for about 30% of infertility and often present with irregular periods (oligomenorrhoea) or an absence of periods (amenorrhoea). Many of the treatments are simple and effective, so couples may need only limited contact with doctors. This makes it easier for a couple to maintain a private loving relationship than in the stressful, more technological environment of assisted conception. However, not all causes of anovulation are amenable to treatment by ovulation induction. Anovulation can sometimes be treated with medical or surgical induction, but it is the cause of the anovulation that will determine whether ovulation induction is possible. The various options are discussed later in this article.
Causes suitable for ovulation induction
Hypothalamic-pituitary causes
Hypogonadotrophic hypogonadism is characterised by a selective failure of the pituitary gland to produce luteinising hormone and follicle stimulating hormone. The commonest cause is excessive exercise, being underweight, or both. Women who have a low body mass index (weight (kg)/(height (m)2)) (for example, < 20) or who exercise excessively—for example, gymnasts, marathon runners, ballerinas—may develop amenorrhoea because of a physiological reduction in the hypothalamic production of gonadotrophin releasing hormone. Women who are underweight for their height when they get pregnant are more likely to have “small for dates” babies; and children of women who have eating disorders are more likely to be admitted to hospital with failure to thrive.
Sheehan's syndrome (panhypopituitarism), caused by infarction of the anterior pituitary venous complex (usually after massive postpartum haemorrhage or trauma), and Kallman's syndrome (amenorrhoea with anosmia caused by congenital lack of hypothalamic production of gonadotrophin releasing hormone) are rare. Children treated for a craniopharyngioma or some forms of leukaemia may have hypogonadotrophic hypogonadism secondary to cerebral irradiation, which may affect the hypothalamus or the pituitary.
Table 1.
Causes of anovulation suitable for ovulation induction treatment
| Hypothalamic |
| • Low concentration of gonadotrophin realeasing hormone (hypogonadotrophic hypogonadism) |
| • Weight or exercise related amenorrhoea |
| • Kallman's syndrome |
| • Stress |
| • Idiopathic |
| Pituitary |
| • Hyperprolactinaemia |
| • Pituitary failure (hypogonadotrophic hypogonadism) |
| • Sheehan's syndrome |
| • Craniopharyngioma or hypophysectomy |
| • Cerebral radiotherapy |
| Ovarian |
| • Polycystic ovaries |
| Other endocrine |
| • Hypothyroidism |
| • Congenital adrenal hyperplasia |
Hyperprolactinaemia is usually caused by a pituitary microadenoma. This leads to a reduction in the production of pituitary luteinising hormone and follicle stimulating hormone. Although the commonest presentation is secondary amenorrhoea, some women may present with galactorrhoea. A smaller number may have headaches or disturbed vision that may indicate a macroadenoma, which needs urgent investigation and treatment. A microadenoma is easily treated with drugs with a subsequent resumption of menses and fertility.
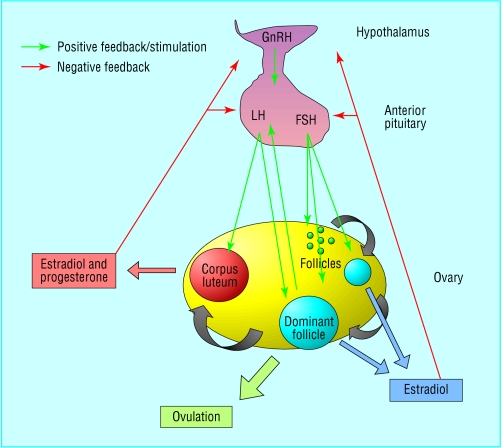
Figure 1.
Hypothalmic-pituitary-ovarian axis (FSH=follicle stimulating hormone; GnRH=gonadotrophin releasing hormone; LH=luteinising hormone)
Ovarian causes
Polycystic ovary syndrome is the commonest cause (70%) of anovulatory subfertility. The primary abnormality seems to be an excess of androgen production within the ovary that leads to the recruitment of large numbers of small preovulatory follicles, which fail to respond to normal concentrations of follicle stimulating hormone. Thus, a dominant follicle is rarely produced. Women with polycystic ovary syndrome commonly present in their late teens or early 20s with hirsutism, acne, or irregular periods (cycle length > 35 days). Even if they ovulate, the chance of conception for these women is reduced because fewer ovulatory events occur in a given time frame. Only a third of women with polycystic ovary syndrome are obese, but obesity increases the likelihood of a woman with the syndrome developing anovulation.
Figure 2.
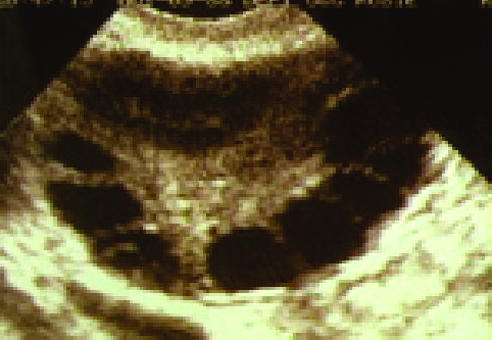
Transvaginal scan of a polycystic ovary. Typically 10 or more follicles of <10 mm in diameter (“string of pearls”) are in a single transverse or longitudinal section through the ovary. Stromal density and ovarian volume increase
Causes unsuitable for ovulation induction
Premature ovarian failure (premature menopause)
Unfortunately this is an irreversible condition. The only treatment option that can result in conception is the use of donated eggs with in vitro fertilisation. Patients will need hormone replacement therapy to alleviate menopause symptoms and to reduce loss of bone density (see www.daisynetwork.org.uk).
Table 2.
Causes of anovulation not suitable for ovulation induction treatment
| Ovarian failure |
| • Idiopathic |
| • Radiotherapy or chemotherapy |
| • Surgical removal |
| • Genetic |
| • Autoimmune |
| Chromosomal |
| • Turner's syndrome (45,X) |
| • Androgen insensitivity syndrome (46,XY) |
Genetic abnormalities
The commonest genetic abnormality is Turner's syndrome (45,X), in which underdeveloped (streak) ovaries result in primary ovarian failure (premature menopause). With adequate oestrogen replacement the uterus can grow large enough for the woman to conceive using donated eggs with in vitro fertilisation. Some translocations and deletions of the X chromosome also cause ovarian failure. Information about Turner's syndrome can be found on the Turner Syndrome Support Society's website at www.tss.org.uk
Ten per cent of primary amenorrhoea is caused by androgen insensitivity syndrome (formerly testicular feminisation). These women have a 46,XY karyotype and intra-abdominal gonads that are testes but have developed as phenotypically female because of the absence of, or non-functionality of androgen receptors. The vagina usually ends blindly and, as there is no uterus, pregnancy is impossible. The gonads should be removed because of an increased risk of malignant change. Explaining the nature of the problem to the patient needs care and sensitivity, and longer term psychological support may be needed.
Table 3.
Investigations for anovulation
| Investigation | When done | Interpretation |
|---|---|---|
| Progesterone | Mid-luteal phase of cycle (for example, day 21 of 28 day cycle or day 28 of 35 day cycle) | >30 nmol/l confirms ovulation; if 10-30 nmol/l check when sample taken in relation to cycle length |
| Follicle stimulating hormone | Early follicular phase | >10 IU/l indicates reduced ovarian reserve; >40 IU/l indicates ovarian failure; <5 IU/l may indicate pituitary or hypothalamic problem |
| Luteinising hormone | Early follicular phase | >10 IU/l indicates polycystic ovaries; <5 IU/l may indicate pituitary or hypothalamic problem |
| Testosterone | Any time in cycle | >2.4 nmol/l indicates polycystic ovaries >5 nmol/l suggests congenital adrenal hyperplasia; check DHEAS and 17-OHP |
| Prolactin | Any time in cycle (but not after exercise or stress) | >1000 IU/l indicates pituitary adenoma; needs repeating |
| Thyroid stimulating hormone | Any time in cycle if woman has symptoms or signs of hypothyroidism or has hyperprolactinaemia | High thyroid stimulating hormone indicates hypothyroidism |
| Transvaginal ultrasound scan | Oligomenorrhoea or amenorrhoea; raised luteinising hormone or testosterone | Identifies polycystic ovaries |
| MRI/CT of pituitary | abcboxtIf two prolactin levels >1000 IU/l | Identifies macroadenomas |
| Karyotype | Primary amenorrhoea and premature menopause | Identifies karyotypic abnormalities—for example, Turner's syndrome (45,X), translocations, and androgen insensitivity syndrome (46,XY) |
| Body mass index | Oligomenorrhoea or amenorrhoea | Body mass index >30 suggests polycystic ovary syndrome; body mass index <20 suggests hypogonadotrophic hypogonadism |
CT=computed tomogram; DHEAS=dihydroepiandrosterone sulphate; MRI=magnetic resonance imaging scan; 17-OHP=17-hydroxyprogesterone
Diagnosis of anovulatory subfertility
Hypogonadotrophic hypogonadism
Regardless of the underlying cause, the concentrations of luteinising hormone, follicle stimulating hormone, and estradiol will be low. A careful history (surgery, radiotherapy, massive haemorrhage, lack of smell, exercise, and eating habits) and a body mass index measurement will reveal the cause.
Hyperprolactinaemia
A serum prolactin concentration of > 1000 IU/l is diagnostic and usually indicates a microadenoma. Magnetic resonance imaging or computed tomography should be arranged to detect whether a macroadenoma is present. Patients with a macroadenoma must have their visual fields checked. The luteinising hormone and follicle stimulating hormone concentrations are usually at the lower end of the normal range with a low estradiol concentration.
Polycystic ovary syndrome
A transvaginal ultrasound scan of the pelvis will confirm the diagnosis. In 80% of women with polycystic ovary syndrome the testosterone concentration will exceed the normal upper limit of 2.4 nmol/l, making this a sensitive and specific endocrine test for this condition. Luteinising hormone concentrations are raised (> 10 IU/l) in 45-70% of women with the syndrome.
Management of anovulation
Treating specific causes
Change of weight
Women with polycystic ovary syndrome who are overweight (body mass index > 30) should be advised to lose weight. Together with exercise, weight loss (even as little as 5% of body mass) reduces insulin and free testosterone levels, resulting in improved menstrual regularity, ovulation, and pregnancy rates. If a woman is obese when she is pregnant she is more likely to miscarry. Women who are underweight (body mass index < 20) should be encouraged to gain weight, and no infertility treatment should be offered until their body mass has returned to the lower limits of normal.
Hyperprolactinaemia
Bromocriptine is safe and commonly used. Treatment should start with a dose of 1.25 mg (taken with food) at night for the first fortnight and then increased to 2.5 mg for another fortnight. The prolactin level should be checked, and if the level is below 1000 IU/l, the dose should be maintained. The side effects of bromocriptine (postural hypotension, nausea, vertigo, headache) can make it unacceptable to the patient. Cabergoline and quinagolide are newer long acting dopamine agonists with fewer side effects. Once prolactin levels have returned to below 1000 IU/l the woman's periods should return and 70-80% of women will ovulate.
Hypothyroidism
In hypothyroidism thyrotropin releasing hormone may stimulate prolactin secretion in addition to thyrotropin releasing hormone from the anterior pituitary. Correction of the hypothyroidism with thyroxine replacement allows thyroid stimulating hormone and prolactin levels to return to normal, releasing the suppression to gonadotrophin secretion and ovulation.
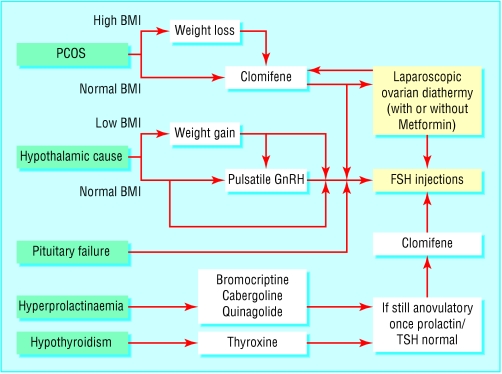
Figure 3.
Hormone relationships that may affect fertility (BMI=body mass index; FSH=follicle stimulating hormone; GnRH=gonadotrophin releasing hormone; PCOS=polycystic ovary syndrome; TSH=thyroid stimulating hormone)
Medical induction
Pulsatile gonadotrophin releasing hormone
Treatment with gonadotrophin releasing hormone that is started in a specialised hospital setting may be suitable for women who have a purely hypothalamic cause for their amenorrhoea, for example women with recovered weight related amenorrhoea but who are still not ovulating. The woman wears a small mechanical syringe pump that can deliver a pulse of gonadotrophin releasing hormone subcutaneously every 90 minutes, and this usually leads to unifollicular ovulation. Local reactions may occur at the injection site. Conception rates are similar to those in the normal population at around 20-30% per cycle and 80-90% after 12 months' use.
Figure 4.
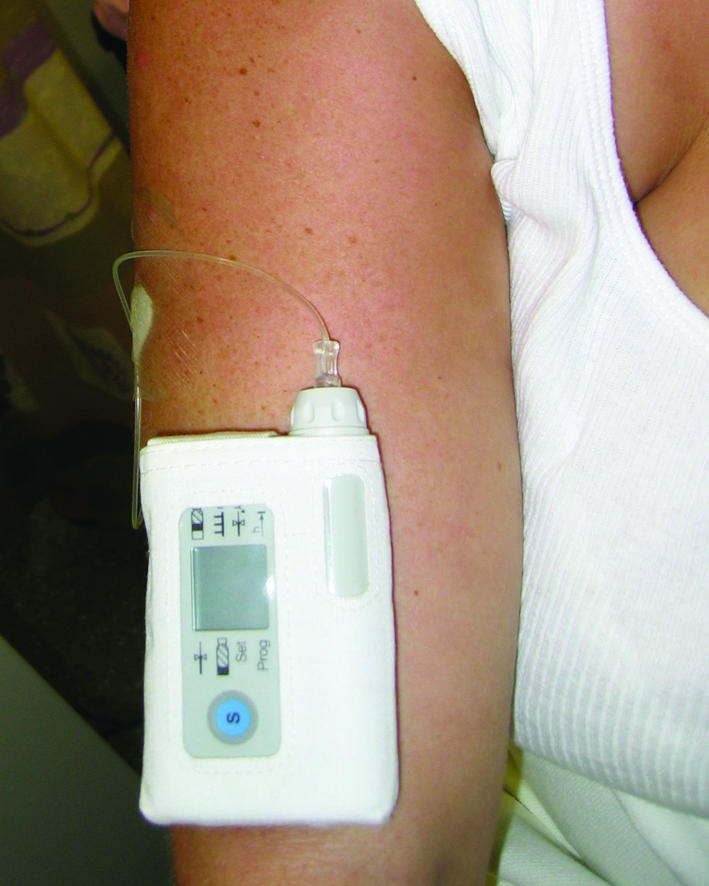
Patient wearing a gonadotrophin releasing hormone pump
Antioestrogen treatment: Clomifene
Clomifene acts by blocking oestrogen receptors in the pituitary leading to an increased production of follicle stimulating hormone, which then stimulates development of one or more dominant follicles. These drugs can be used only in conditions in which the hypothalamic-pituitary axis is functioning—for example, polycystic ovary syndrome. Ovulation induction with clomifene should be undertaken only in circumstances that allow access to ovarian ultrasound monitoring, because of the risk of multiple follicle development and the small but real risk of ovarian hyperstimulation syndrome (Royal College of Obstetricans and Gynaecologists' guidelines, No 3). Seventy per cent of women with polycystic ovary syndrome will ovulate in response to clomifene, with a conception rate of 40-60% at six months. The incidence of twins is around 10%, and triplets 1%.
The aim of ovulation induction is regular ovulation of one egg per cycle to avoid multiple pregnancy
After publication of a study that showed an increased risk of ovarian cancer in women who used clomifene for longer than 12 months the Committee on Safety of Medicines in the United Kingdom has recommended that women should not take clomifene for longer than six months
Metformin
Increasingly, studies report that metformin at doses of 1500 mg a day (in a similar way to weight loss) may improve menstrual regularity by reducing insulin and free testosterone concentrations in both lean and obese women with polycystic ovary syndrome who are not ovulating. However, caution is needed because metformin is not licensed for this indication, and the results of convincing trials are still awaited.
Follicle stimulating hormone injections
Treatment with follicle stimulating hormone is used in women with hypothalamic-pituitary causes of anovulation, and for women with polycystic ovary syndrome who have failed to respond to or conceive using clomifene. As the most serious complications of this therapy are ovarian hyperstimulation syndrome and high order multiple pregnancy, it is essential that this treatment is monitored by reproductive specialists with access to ultrasonography and tertiary care facilities.
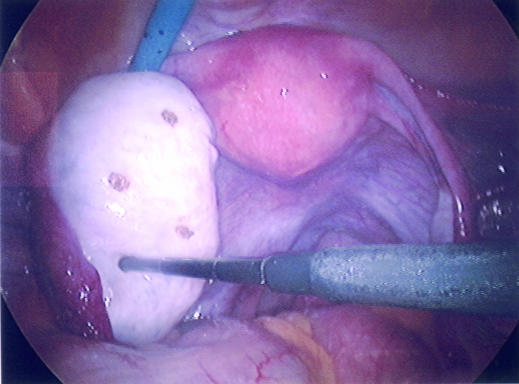
Figure 5.
Ovary showing small holes made in the cortex at laparoscopy using a diathermy point to encourage ovulation in a patient with polycystic ovary syndrome
Surgical induction
Laparoscopic ovarian diathermy or “drilling” has replaced wedge resection of the ovaries in women with polycystic ovary syndrome. At laparoscopy, five to six diathermy or laser punctures are made in the ovary. Success rates are comparable with follicle stimulating hormone administration, with lower risks of multiple pregnancy or ovarian hyperstimulation syndrome, but complications can arise from surgery and adhesion formation. If too much ovarian tissue is destroyed there is a potential risk of premature ovarian failure in the future, although this risk is still being evaluated.
Table 4.
Practice points
| • Absence of or inadequate ovulation is a common cause of infertility and in many cases can be treated effectively |
| • Amenorrhoea and, more commonly, oligomenorrhoea indicate that ovulation is not occurring, so a serum progesterone test is unhelpful |
| • Weight is important for the success of ovulation induction and outcome of pregnancy. The woman should achieve a body mass index of 20-29 before starting ovulation induction treatment |
| • Most couples in whom the only cause of subfertility is anovulation can overcome ovulation problems (60-98% cumulative conception rate at six months), but couples with concomitant male factor or tubal subfertility should be treated with appropriate assisted conception techniques |
| • Ovulation induction should be undertaken in a secondary or tertiary care setting. Couples must be warned of the risk of multiple pregnancy (5-10%) and ovarian hyperstimulation syndrome (<1%) |
| • The cumulative conception rate is lower for women with polycystic ovary syndrome than for those who have hypothalamic amenorrhoea |
Diane Hamilton-Fairley is a consultant obstetrician and gynaecologist at Guy's and St Thomas's NHS Trust.
The ABC of subfertility is edited by Peter Braude, professor and head of department of women's health, Guy's, King's, and St Thomas's School of Medicine, London and Alison Taylor, consultant in reproductive medicine and director of the Guy's and St Thomas's assisted conception unit. The series will be published as a book in the winter.
Competing interests: None declared.