Short abstract
The United Kingdom is trying to bring more non-governmental funding into NHS research through partnerships with the commercial and voluntary sectors. But it is still unclear exactly how the reforms in England will work and how trusts will resolve the tensions between patients' needs, financial viability, and the commercial exploitation of research findings
Essential research into health services is often unattractive to commercial sector funders and requires government support.1 Although appreciable NHS funding has been earmarked for this purpose, critics have argued that much of the budget has disappeared into the general funds for service delivery.2 In 2000, the Department of Health announced a major overhaul of health related research within the NHS aimed at providing a clearer strategic direction.3 The reforms signal an increased role for the commercial sector in the identification of strategic objectives, setting priorities, and in the delivery and exploitation of clinical research. This paper provides an overview of the new funding and organisational arrangements for NHS research in England.
Organisation of funding of health research
Funding of health research in the United Kingdom is complex. Although most health related research is funded from the commercial sector, the Department of Health is a major contributor (table). In 2002-03 the Department of Health contributed £540m to research on health.4 The current pattern of funding (fig 1) reflects the work of the Culyer review, which was carried out because of concerns over the financial viability of large teaching hospitals after the introduction of the internal market in the NHS. In 1994, the Culyer taskforce recommended that Department of Health funding of health related research should be allocated to support research and development activity in NHS trusts and be clearly identifiable and distinct from funding for clinical services.5 Some 77% (£418m in 2002/03) of research funding is currently allocated to hospital and primary care trusts; over two thirds of this is to London trusts and most to teaching hospitals (see fig A on bmj.com).6,7 For some hospitals the so called Culyer funds constitute 10% or more of their budget and have had the effect of cushioning special health authorities and teaching hospitals from the full effects of the internal market. Most of the funding is used to support the additional clinical service costs associated with research and is embedded in clinical service budgets. Researchers have often noted ruefully that research and development funds do not translate into funding for de novo research ideas and project support.8
Table 1.
UK expenditure on research and development in 20002
| Expenditure (£m) | |
|---|---|
| For profit sector (industry) | 3000 |
| Voluntary sector | 540 |
| Department of Health | 500 |
| Higher Education Funding Council for England | 190 |
| Medical Research Council | 300 |
Fig 1.
Department of Health spending on research and development, 2002-3
Figure 1.
Credit: SUE SHARPLES
The fact that most research and development funding is embedded in the clinical budgets of large hospital trusts has limited the Department of Health's ability to set the strategic direction for health services research. The government has attempted to coordinate research strategy through bilateral agreements and concordats with the research councils (most notably the Medical Research Council), the Higher Education Funding Council for England, and large medical research charities, but despite these arrangements decisions about what research should be funded are predominantly made by these partner institutions, not the Department of Health. The new reforms aim to bring NHS research into line with the wider strategic objectives of a mixed economy of provision in all aspects of health care and research.3
Mechanisms for delivering reform
New bodies, partnerships, and networks
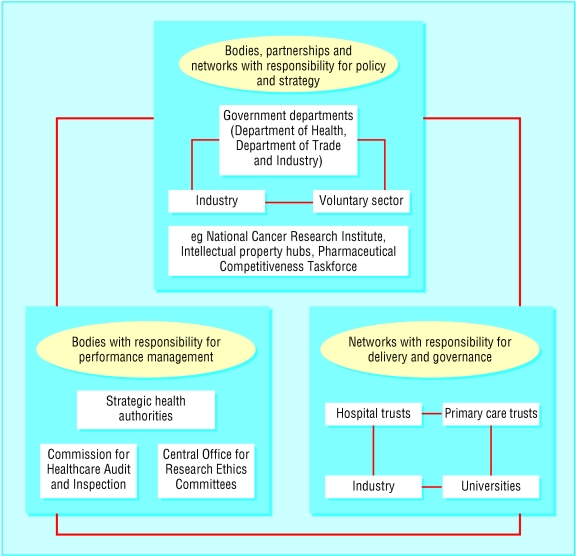
The government has created several new bodies, partnerships, and networks to further the research strategy and coordinate activity between stakeholders in the NHS, academia, industry, and the voluntary sector. Some of these structures are dedicated to developing policy and strategy and others to implementation and delivery of research. In addition, responsibility for regulation and performance has been given to a range of organisations including the Department of Health, strategic health authorities, and Commission for Healthcare Audit and Inspection. As figure 2 shows, the exact nature of roles and the inter-relationships between the structures and the mechanisms for accountability to the secretary of state for health and parliament are unclear.
Fig 2.
Plurality of stakeholders in NHS research and development. Bodies, partnerships, and networks in the development of policy and strategy for research and development, delivery of health related research and development, and evaluation and performance management
An important strategic body is the Pharmaceutical Industry Competitiveness Taskforce. This is a partnership between the Department of Health and industry that has predominantly trade objectives of ensuring the continued competitiveness of the United Kingdom's pharmaceutical industry.9 The taskforce has identified three obstacles to competitive research and development in the United Kingdom10: speed of start up time for new clinical trials, quality of research, and cost of research.
The taskforce's recommendations for overcoming these impediments are being incorporated into the Department of Health's research and development policy through other newly established formal research networks such as those for cancer. In cancer, research priorities are now set by the National Cancer Research Institute, which includes representatives of the government, industry, and voluntary sector.11,12 Two national cancer networks support the institute.
Firstly, a National Cancer Research Network has been set up to ensure that clinical trials run smoothly and increase recruitment of participants. It is creating local research networks that map directly onto 34 cancer service networks across England. London has five cancer research networks, each with annual budgets of up to £500 000.
Secondly, the National Translational Cancer Research Network aims to ensure that novel scientific discoveries can be translated quickly from the laboratory to routine clinical treatment. It has set up a network of 10 centres based in centres of academic and clinical excellence, each centre receiving £1m funding over five years to build infrastructure and workforce capability. In addition to these aims, one of the network's major activities is to establish a national tumour tissue bank to enable the commercial and academic use of tissue collected from NHS patients.13 The network approach is likely to be adopted for other clinical and research areas under the national service strategy, such as cardiovascular disease and mental illness.
Another important strategic partnership with industry is the Public Sector Research Exploitation Fund. With funds totalling £10m, this has been established by the Department of Trade and Industry “to enable public sector bodies carrying out research to have access to the skills and expertise needed to evaluate the commercial potential of their work and to take steps to bring ideas towards exploitation.”14,15 The initiative has led to the creation of 12 intellectual property networks or IP hubs, which provide a support structure for NHS organisations as they seek to identify and maximise the economic potential of their intellectual property.16
Although the hubs are still in their infancy—existing as notional networks of hospital and primary care trusts—most will become external commercial organisations with which NHS organisations will contract. To this end, the hubs will take advantage of new legislation that supports the strategy of moving towards public-private partnerships in NHS research and development by enabling NHS organisations to form spin-off companies. Section 5 of the Health and Social Care Act 2001 gives NHS organisations freedom to work with external investors in developing and marketing their intellectual property. NHS trusts and their employees will be able to have shares in spin-off companies created to take commercial advantage of the intellectual property generated through their research.
Changes to funding
In order to support the development of these new networks, the funding streams established under the Culyer reforms are being replaced with two new streams: support for science and priorities and needs.17,18 Support for science funding “will be allocated to NHS and non-NHS providers of NHS services to meet the costs of supporting health R&D.”3 Given these aims, it seems likely that the scope of support for science will be similar to existing funding, providing financial stability for large hospital trusts while the mixed economy in research infrastructure is being established.
The aim of the priorities and needs funding stream is different. Its purpose is to provide research programmes for the strategic priorities—for example, the National Service Frameworks and the broader needs of the NHS.18,19 If the developments in cancer research are indicative of what lies ahead, the private sector will have an important role in identifying and implementing research priorities in other disease groups. Priorities and needs funding is still being developed, but its use in supporting collaborative networks for delivering research and development makes it likely that the funding will be shifted out of the budgets of NHS organisations. The recasting of the national research and development programme into three key funding competitions—service delivery and organisation, health technology and assessment, and new and emerging applications of technology—provides another financial mechanism for directing money towards strategy and new partnerships with industry.
Research governance framework
The Department of Health has introduced a new regulatory framework—the research governance strategy—to ensure compliance with the relevant professional, ethical, legal, and scientific standards and oversee the emerging mixed economy in health related research and development. Trusts are required to take greater organisational control over their research activities, including monitoring and auditing of projects, and there will be new roles for the Commission for Healthcare Audit and Inspection and the strategic health authorities. Procedures for ethical review of research will be overseen on behalf of the Department of Health by a new body, the United Kingdom Ethics Committee Authority. These strategic developments are analysed in the accompanying article.20
Issues arising from the reform process
The new partnerships have the potential of providing much needed strategic direction. However, the increased participation of industry and trade and commerce objectives sets up a conflict of interest, both within the institution and at the level of individual researchers, where the duty of care to research subjects or patients may be compromised by financial incentives and other pressures. NHS trusts are already under pressure to host the research activities of the new bodies irrespective of the costs, the financial arrangements, or the legitimacy of the research. The proposals to establish NHS bodies as foundation trusts or corporate bodies with a duty to maximise surpluses, including through commercial ventures, will increase the conflicts of interest.21
Summary points
Almost 80% of Department of Health's research and development funding is allocated to NHS trusts, limiting the department's ability to set strategic direction
Radical overhaul of strategy, delivery systems, and funding streams aims to bring more non-governmental funding into all aspects of healthcare research
Research strategy is being developed through new networks and bodies between NHS, academia, industry, and the voluntary sector
Accountability arrangements for the work of the networks and bodies is complex and unclear
NHS funding will be allocated through two new funding streams: support for science and NHS priorities and needs
Several issues arise from the strategy, most notably the potential for conflicts of interest
The new arrangements could result in the NHS acting as a laboratory for commercial research. One potential danger is that without strong mechanisms for parliamentary accountability public money could be skewed towards commercial research products and away from healthcare needs. It may be difficult to ensure that the needs and views of patients and clinicians inform the process of research prioritisation. Experience in the United States suggests that managing such conflicts is not simple.22,23 The lack of clear direct parliamentary accountability in the new bodies and networks will make it extremely difficult to distinguish between research for the public good and research solely for commercial gain.
Supplementary Material
 An additional figure is available on bmj.com
An additional figure is available on bmj.com
We thank Naomi Pfeffer, Azeem Majeed, and Alan Thompson for their comments.
Contributors and sources: NMcN has worked in NHS research and development for over four years and manages UCLH Research and Development Directorate and policy implementation. SK has been in UCLH research and development for over three years and is also a part time doctoral student in sociolegal studies in the Centre for Analysis of Risk and Regulation at the London School of Economics. SK has primary responsibility for implementing research governance, and she and NMcN have lead responsibilities on the development of research governance policies and procedures at UCLH. AMP has been responsible for the development and implementation of research and development strategy at UCLH since 1998. Her research interests are in public health policy and health services research. The search strategy used for the paper consisted of a Medline search and web based searching of the Department of Health and other government sites.
Competing interests: None declared.
References
- 1.House of Lords Select Committee on Science and Technology. Priorities in medical research. Vol 1. London: HMSO, 1988. [PMC free article] [PubMed]
- 2.Harrison A, New B. Public interest, private decisions: health-related research in the UK. London: King's Fund, 2002.
- 3.Department of Health. Research and development for a first class service. London: DoH, 2000. www.doh.gov.uk/research/documents/rd3/first_class_service.pdf (accessed 3 July 2003).
- 4.Department of Health website. www.doh.gov.uk/research/ (accessed 3 July 2003).
- 5.Culyer A. Supporting research and development in the NHS. Report of the Department of Health Research and Development Task Force. London: HMSO, 1994.
- 6.Arnold E, Morrow S, Thuriaux B, Martin B. Implementing the Culyer reforms in North Thames: final report. science and technology policy research. London: Technopolis, 1999.
- 7.Harrison A, New B. The finance of research and development in health care. Health Care UK 2001: the King's Fund review of health policy. London: King's Fund, 2001: 26-44.
- 8.McCollum C. UK National Health Service R&D funding—a bureaucratic nightmare. Lancet 2003;361: 1906. [DOI] [PubMed] [Google Scholar]
- 9.Pharmaceutical Industry Competitiveness Taskforce. Final report. London: DoH, 2001.
- 10.Pharmaceutical Industry Competitiveness Taskforce. Clinical research report. London: DoH, 2002.
- 11.Department of Health. NHS Cancer Plan. London: DoH, 2000.
- 12.National Cancer Research Institute. Strategic analysis 2002. London: NCRI, 2002. http://www.ncri.org.uk/documents/publications/reportdocs/NCRI_Strategic_Analysis_2002.pdf (accessed 2 July 2003).
- 13.National Cancer Tissue Resource. www.ntrac.org.uk/Initiatives/NCTR/NCTR.asp (accessed 8 July 2003).
- 14.Office of Science and Technology. Public sector research exploitation fund—basic guidelines. www.ost.gov.uk/enterprise/knowledge/psrefundguide.pdf (accessed 5 August 2003).
- 15.Department of Health. Science and innovation strategy. London: DoH, 2001.
- 16.Department of Health. The NHS as an innovative organisation: a framework and guidance on the management of intellectual property in the NHS. London: DoH, 2002.
- 17.Department of Health. NHS R&D funding consultation paper: NHS support for science. London: DoH, 2000.
- 18.Department of Health. NHS R&D funding consultation paper: NHS priorities and needs funding. London: DoH, 2000.
- 19.Department of Health. NHS priorities and needs R&D funding: a position paper. London: DoH, 2001.
- 20.Kerrison S, McNally N, Pollock AM. United Kingdom research governance strategy. BMJ 2003;327: 553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walt G, Brugha R, Haines A Working with the private sector: the need for institutional guidelines BMJ 2002;325: 432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korn D. Conflicts of interest in biomedical research. JAMA 2000;284: 2234-8. [DOI] [PubMed] [Google Scholar]
- 23.Morin K, Rakatansky H, Riddick FA Jr, Morse LJ, O'Bannon JM 3rd, Goldrich MS, et al. Managing conflicts of interest in the conduct of clinical trials JAMA 2002;287: 78-84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.