Abstract
Insulin-dependent diabetes mellitus results from T cell-mediated destruction of insulin-producing, pancreatic islet β cells. How this destruction takes place has remained elusive—largely due to the slow kinetics of disease progression. By crossing a transgenic mouse carrying a β cell-specific T cell receptor onto the NOD.scid background, we produced a simplified but robust and accelerated model of diabetes. This mouse produces CD4+ T cells bearing transgenic T cell receptor but is devoid of CD8+ T cells and B cells. More importantly, this mouse develops a rapid diabetes, which has allowed us to record and quantify β cell death. We have determined that β cells within the inflamed islets die by apoptosis.
Nonobese diabetic (NOD) mice naturally develop an insulin-dependent diabetes mellitus (IDDM) with remarkable similarity to that of human IDDM patients (reviewed in refs. 1–3). As a result, NOD mice have become an invaluable tool for studying the underlying immunobiology of IDDM and the complex genetics that control it (4). Through their study, we now know that IDDM results from the selective destruction of insulin-producing, pancreatic β cells. This destruction is coordinated by β cell antigen-specific, CD4+ T cells that produce proinflammatory cytokines (5, 6).
How β cells are killed is not well understood. This is due to the fact that β cell death is difficult to detect in vivo, due to the slow kinetics of the inflammatory process and the rapid clearance of dead cells from the host. The lymphocytic infiltration, termed insulitis, takes place over several months; early lesions contain predominantly mild insulitis with little loss of β cells. By the time NOD mice show sustained hyperglycemia, >90% of the total β cell mass is destroyed, making analysis impossible. Therefore, it has been difficult to establish a reproducible window in which to study β cell death in vivo.
As a result, a number of groups have sought to study β cell death in vitro, testing, for example, whether several proinflammatory cytokines can kill β cells (for review see ref. 7). No consensus was reached on how β cells died, but interleukin (IL)-1β, alone or in combination with interferon-γ and tumor necrosis factor (TNF)-α, could inhibit the glucose-induced secretion of insulin and induced the production of the toxin, nitric oxide, by β cells (8–14). This led to the speculation that local production of IL-1β may play an important role in inducing β cell death (15). While a number of studies have examined the role of cytokines—notably IL-2, IL-10, IL-12, TNF-α, and interferon-γ—to enhance islet pathology and speed β cell death in vivo (16–23), they have not provided any direct assessment of how β cells are affected and if they, in fact, die. Moreover, it is difficult to appraise how well these cytokine models reflect the natural disease process.
We have taken a different approach to studying β cell death. We believed that to realize how β cells die in IDDM, in a physiologically meaningful way, we needed (i) to perform our studies in NOD strain mice, (ii) to assess β cell death in situ, and (iii) to develop a rapid diabetes model that relied on islet cell-specific T cells and not cytokines as the biological mediator. To this end, we made use of the BDC2.5 T cell receptor (TCR) transgenic mouse line (24). This transgenic line of NOD mice carries the rearranged TCR-α and TCR-β chain genes of a CD4+ T cell clone, isolated from a diabetic NOD mouse (25), that is islet cell antigen-specific (26) and capable of transferring diabetes to neonatal NOD mice (27). The BDC2.5 TCR transgenic mice developed an early and severe insulitis but did not have an accelerated rate of diabetes (24). Herein, we now report that crossing the transgenes onto the NOD.scid background hastens not only the development of insulitis but also diabetes. Moreover, by compressing the transition time from the inception of insulitis to development of overt disease to a matter of days, we have been able to capture and analyze β cell death in situ and show, for the first time, that β cells in infiltrated islets die by apoptosis.
MATERIALS AND METHODS
Mice.
BDC2.5 TCR transgenic mice were described previously (24). Mice used in these experiments were housed under specific pathogen-free conditions and were backcrossed to NOD/lt for 17–19 generations and then crossed to NOD.scid mice. The NOD.scid mice are bred under pathogen-free conditions at Washington University from an original breeding stock kindly provided by Ed Leiter (The Jackson Laboratory).
Flow Cytometry.
Three-color flow cytometric analysis was performed as described (24). Flow cytometry was performed on either a FACScan or FACSvantage (Becton Dickinson). We purchased anti-CD4-phycoerythrin (PE; Caltag), anti-CD8α-Quantum Red (Sigma), anti-B220 (PharMingen), and goat anti-mouse IgM (Jackson ImmunoReseach). mAb to the β chain of the transgenic TCR, anti-Vβ4 (28), was the gift of K. Tomonari (Fukui Medical School, Japan) and was conjugated with fluorescein isothiocyanate (FITC). List mode data was collected on 1 × 105 cells and reanalyzed on a PC using winmdi (version 2.1.4) software written by J. Trotter (http: //facs.scripps.edu).
Diabetes.
Diabetes was assessed by measurement of venous blood using a Bayer Glucometer Elite one-step blood glucose meter (Bayer, Elkhart, IN). Animals were considered diabetic after two consecutive measurements ≥250 mg/dl (13.75 mM). Onset of diabetes was dated from the first consecutive reading.
Immunohistochemistry.
Mice were killed by cervical dislocation or CO2 asphyxiation. The entire pancreata were removed and fixed in 10% neutral-buffered formalin at 4°C for at least 20 h but not more than 26 h. Pancreata were then embedded in paraffin, and sections (2 μm) were collected on poly l-lysine coated slides (VWR Scientific).
Sections were deparaffinized in xylene and alcohol and stained with hematoxylin and eosin for general morphology. Parallel sections were stained using the terminal dideoxynucleotidetransferase (TdT)-mediated X-dUTP nick and end labeling (TUNEL) procedure (modified from ref. 29) to detect apoptosis followed by immunohistochemistry for insulin to detect β cells. Nuclear counterstains were omitted in TUNEL stains, but immediately adjacent sections were stained with anti-insulin and hematoxylin. Sections were digested with proteinase K (20 μg/ml for 15 min), endogenous peroxidase was blocked in hydrogen peroxide (3% for 5 min), and residual avidin or biotin was quenched by sequential incubation in avidin (20 μg/ml for 20 min) or biotin (10 μg/ml for 20 min). Sections were then preincubated in 1.5 mM CoCl2 TdT buffer (Boehringer Mannheim). TdT (Boehringer Mannheim) was then used to label nicked and fragmented DNA with biotin-conjugated dUTP at 37°C for 100 min (1.5 mmol of CoCl2, 0.6 μl of TDT, and 1.5 μl of biotin-dUTP per 100 μl of buffer; 30 μl per 4 cm2). Slides were washed in 2× standard saline citrate (SSC; 15 min) and incubated in 2% BSA (ELISA grade; Sigma), followed by streptavidin–horseradish peroxidase (Caltag; 1:1000, 30 min at 37°C). The slides were developed with diaminobenzidine (DAB; Pierce) for 2 min. Immunohistochemistry for insulin was then performed using a two-step protocol. Endogenous peroxidase activity was blocked, and slides were incubated with rabbit antiserum to insulin (Dako; 1:500 in 5% normal mouse serum for 30 min). After wash steps, staining was revealed with horseradish peroxidase-conjugated anti-rabbit Ig (Dako; 1:500 in 5% NMS for 30 min), developed with aminoethylcarbazole (AEC; Pierce) for 10 min, and mounted in Crystal/Mount (Biomeda, Foster City, CA). Cells showing nuclear DAB precipitates (TUNEL+) surrounded by a distinct cytoplasmic rim of AEC precipitate (insulin) containing granules were considered β cells undergoing apoptosis. Apoptotic β cells were clearly labeled by anti-insulin within discrete β granules and contained sharply demarcated TUNEL+ nuclei.
For each pancreas, multiple parallel sections were analyzed in five levels, each 0.5 mm apart, to minimize sample error. On average, 100 islets were analyzed per pancreas. Insulitis was graded by a quadripartite scale: 0; no insulitis; 1, peri-insulitis; 2, moderate insulitis with disrupted islet architecture containing insulin-producing β cells; and 3, complete destructive insulitis with few or no insulin+ cells. An insulitic score was computed for each pancreas by summing the insulitis grade for each islet and dividing the sum by the total number of islets examined.
T Cell Transfer.
For in vitro activation spleen and mesenteric lymph node cells from BDC2.5 TCR transgenic mice were cultured at 105 cells per ml with 2 × 104 mouse islet cells per ml in 20 ml of DMEM supplemented with 5% FCS (HyClone), 10 mM Hepes, 5 × 10−5 M 2-mercaptoethanol, penicillin (100 units/ml), streptomycin sulfate (100 μg/ml), and glutamine (2 mM) in a 25-cm2 tissue culture flask, upright, in a humidified incubator at 37°C and 5% CO2. After 3 days, the contents of the flask were transferred to a 75-cm2 flask, and culture medium was added to 75 ml supplemented with 50 units/ml of recombinant IL-2. Cells were analyzed by flow cytometry on day 6 or 7 and transferred by intravenous injection to recipient mice (≈106 T cells per mouse). For experiments with T cells from diabetic mice, spleen and mesenteric lymph node cells from diabetic BDC2.5/NOD.scid mice were isolated, washed, analyzed by flow cytometry, and transferred as above.
RESULTS
BDC2.5/NOD.scid Mice Contain a Monospecific CD4+ T Cell Population.
We had previously described the generation of BDC2.5 TCR transgenic NOD mice (24). These mice carry the rearranged TCR genes from a CD4+ diabetogenic NOD T cell. Molecular analysis of the T cells derived from these mice demonstrated that transgenic TCR-β (Vβ4) chain expression was absolute and precluded the expression of endogenous encoded TCR-β proteins. The allelic exclusion of endogenous TCR-α chains by the transgenic TCR-α (a Vα1 family member) was somewhat more variable but was sufficiently high to result in a dramatically enriched population of islet cell-reactive CD4+ T cells and to accelerate insulitis. Additionally, the original N3 generation mice, housed under conventional conditions, became diabetic at 4 months of age.
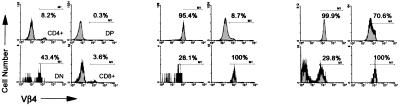
By crossing the BDC2.5 TCR transgenic mice onto the NOD.scid background, we effectively eliminated all T and B lymphocytic populations except for CD4+ T cells that were rescued by, and expressed exclusively, the BDC2.5 TCR. The thymi (Fig. 1) of prediabetic BDC2.5/NOD.scid animals had normal T lymphocytic cellularity (121 × 106 ± 20 × 106 thymoctyes at day 21) and showed a marked skewing of thymocytes toward the CD4+ subset. This, together with the exclusive expression of the Vβ4 on the CD4+CD8− thymocytes, indicated good positive selection of the BDC2.5 TCR in the NOD.scid background.
Figure 1.
Transgenic TCR expression selects CD4+ T cells in the thymi of BDC2.5/NOD.scid mice. Three-color flow cytometric analysis of thymi from negative littermate (NOD.scid/+; Left), TCR transgenic (BDC2.5/NOD.scid/+; Center), and TCR/NOD.scid transgenic (BDC2.5/NOD.scid; Right) mice was performed using anti-CD4, anti-CD8α, and anti-Vβ4 TCR mAb. (Upper) Two-color dot plots of CD4 versus CD8. (Lower) Vβ4 TCR expression on gated CD4+CD8− (CD4+), CD4+CD8+ (DP), CD4−CD8+ (CD8+) and CD4− CD8− (DN) subpopulations.
The splenic T cell population from BDC2.5/NOD.scid mice was devoid of both TCR-αβ and TCR-γδ expressing CD8+ T cell subpopulations (Fig. 2; and data not shown) but contained normal numbers of CD4+ T cells (3–10 × 106 CD4+ T cells per spleen in 21-day-old mice), all of which expressed physiological normal levels of the BDC2.5 TCR and CD4 coreceptor.
Figure 2.
Lack of CD8+ T cells in the spleen of BDC2.5/NOD.scid mice. Three-color flow cytometric analysis of splenocytes from negative littermate (NOD.scid/+; Left), TCR transgenic (BDC2.5/NOD.scid/+; Center), and TCR/NOD.scid (BDC2.5/NOD.scid; Right) mice was performed using anti-CD4, anti-CD8α, and anti-Vβ4 TCR mAb. (Top) Two-color CD4 versus CD8 dot plots. (Middle) Vβ4 TCR expression on gated CD4+CD8− (CD4+), CD4−CD8+ (CD8+) and CD4−CD8− (DN) subpopulations. (Bottom) Two-color dot plots, B220 versus sIgM confirms scid phenotype of B cells in bottom right panel.
Accelerated Insulitis and Diabetes.
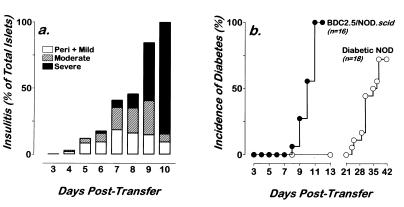
The BDC2.5/NOD.scid mice developed a remarkably accelerated insulitis commencing between day 14 and day 16 of life (Fig. 3a). While we had seen incidences of periductal insulitis in the BDC2.5/NOD.scid/+ littermates at this age, the BDC2.5/NOD.scid mice consistently showed a more advanced and acute insulitis. This insulitis progressed rapidly becoming severe by days 22–30. Examination of the islet histology revealed the presence of CD4+ T cells, F4/80+ macrophage, RB6+ neutrophils, and natural killer cells in the infiltrates. However, CD8+ T cells were not seen in the lesions or in the spleens or lymph nodes of these animals (data not shown). Additionally, 25 BDC2.5/NOD.scid mice aged 22–30 days were examined and found negative for evidence of sialoadenitis.
Figure 3.
Rapid insulitis and diabetes in BDC2.5/NOD.scid mice. (a) Insulitis time course in BDC2.5/NOD.scid (filled bars) and BDC2.5/NOD.scid/+ littermates (open bars). Multiple hematoxylin and eosin pancreas sections from 3–6 mice in each time group were analyzed for insulitis. Eighty to 100 islets from each mouse were scored for no insulitis (0), peri- and mild insulitis (1), moderate insulitis (2), or severe insulitis (3), and then each mouse was given a mean score of insulitis. (b) The kinetics of diabetes was determined by measurement of blood glucose using a glucometer. Animals were considered diabetic after two consecutive readings ≥250 mg/dl (13.75 mM). •, BDC2.5/NOD.scid mice; ○, BDC2.5/NOD.scid/+ littermates. No diabetic BDC2.5/NOD.scid mouse lived past 33 days of age unless treated with insulin.
BDC2.5/NOD.scid mice became diabetic and did so rapidly. The first overt diabetic BDC2.5/NOD.scid mice (blood glucose ≥250 mg/dl) were observed on day 22 of life, and, by day 30, all BDC2.5/NOD.scid mice were diabetic (Fig. 3b). The onset of diabetes was not accompanied by appearance or expansion of CD8+ T cells in the spleen or pancreas. All BDC2.5/NOD.scid mice died from complications of diabetes on or before 33 days of age. In contrast, the BDC2.5/NOD.scid/+ mice did not become diabetic until ≥110 days of age (Fig. 3b). The current incidence of diabetes in our pathogen-free colony of BDC2.5 and BDC2.5/NOD.scid/+ mice is 10–15% at 6 months of age (Fig. 3b; and data not shown).
These results reinforced earlier observations that CD4+ T cells bearing a TCR specific for peripherally expressed self antigens were not subject to intra- or extrathymic deletion or anergy (24, 30, 31). More importantly, however, these results illustrated that BDC2.5 TCR+, CD4+ T cells were capable of orchestrating an accelerated onset diabetes in the complete absence of CD8+ T cells and B lymphocytes. The profound differences in onset rate between the BDC2.5/NOD.scid mice and the BDC2.5/NOD.scid/+ and BDC2.5/NOD mice are currently being investigated and will be reported elsewhere.
BDC2.5/NOD.scid T Cells and Accelerated Diabetes Transfer.
When CD4+ T cells recovered from the spleens and mesenteric lymph nodes of overtly diabetic BDC2.5/NOD.scid mice were reintroduced into adult NOD.scid mice, they transfer both insulitis and diabetes efficiently. A single, intravenous injection of 106 T cells resulted in infiltration of lymphocytes at the islet margins by day 4 and in severe and destructive insulitis at 9 to 10 days (Fig. 4a). The initial onset of diabetes was on day 8, and, by day 11, all recipients are diabetic with blood glucose ≥500 mg/dl (Fig. 4b). This represented a markedly enhanced rate of disease progression. In fact, it took 2–4 × 107 splenic T cells from overtly diabetic NOD mice 4–5 weeks to transfer disease to NOD.scid recipient mice (Fig. 4b). Moreover, published data for several diabetic T cell clones, including BDC2.5, showed that multiple injections over a several week period was needed to produce disease in NOD.scid (32, 33).
Figure 4.
T cells from diabetic BDC2.5/NOD.scid mice efficiently transfer insulitis and diabetes to NOD.scid recipients. NOD.scid mice received 106 T cells from BDC2.5/NOD.scid mice by intravenous injection. Recipients were then followed for evidence of insulitis and diabetes. (a) Insulitis was assessed from multiple hematoxylin and eosin sections of pancreas. Insulitis was scored as peri- and mild insulitis (open bars), moderate (hatched bars), or severe (filled bars). Insulitis is represented as the composite of 40–100 islets per animal and 3–6 mice per time point. (b) Cumulative incidence of diabetes in 16 NOD.scid recipients of 106 T cells from diabetic BDC2.5/NOD.scid mice. Diabetes was assessed by measurement of blood glucose using a glucometer. Mice were considered diabetic after two consecutive readings ≥250 mg/dl (13.75 mM). Diabetes was dated to the first sequential readings.
Apoptotic β Cells.
The rapid evolution from insulitis to diabetes in BDC2.5/NOD.scid mice and in NOD.scid recipients of BDC2.5/NOD.scid-derived T cells provided us with well-defined windows in which to study and analyze β cell death in vivo—between days 21–28 for BDC2.5/NOD.scid mice and days 6–9 for NOD.scid recipients.
We examined pancreata from prediabetic BDC2.5/NOD.scid mice aged 21–28 days. Pancreata were analyzed by immunohistochemistry for evidence of insulitis, necrosis, and apoptosis. Insulitis and necrosis was assessed by conventional hematoxylin and eosin staining, while β cell apoptosis was appraised using double labeling, first to reveal the characteristic DNA double strand breaks of apoptotic cell death using TUNEL (34) and, second, by anti-insulin to identify islet β cells.
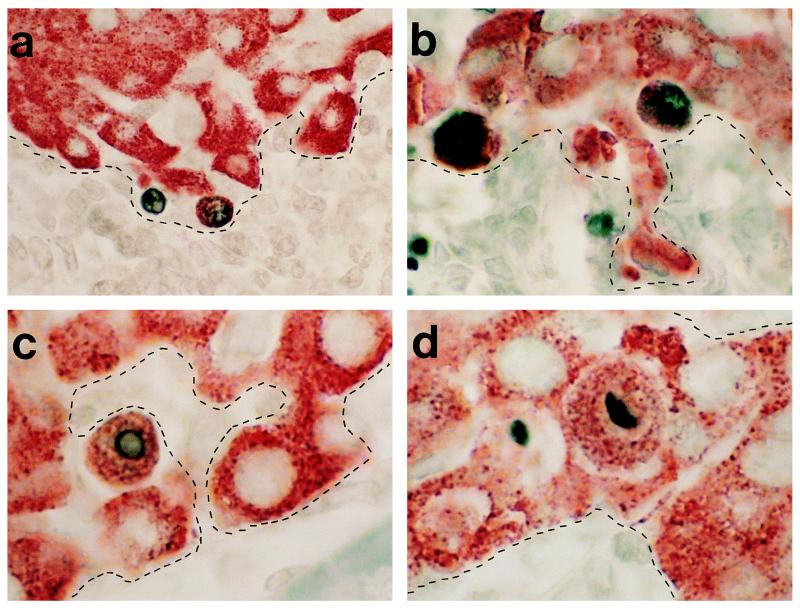
We consistently found TUNEL+ β cells in infiltrated islets of these mice (Fig. 5 a–d), but failed to find histological evidence for β cell necrosis in infiltrated islets of BDC2.5/NOD.scid mice (data not shown). Parallel hematoxylin and eosin staining revealed that β cell apoptosis occurred in areas of lymphocytic infiltration (the borders of infiltration, as determined from parallel hematoxylin stains, are depicted by dashed lines in Fig. 5 a–d). Age-matched BDC2.5/NOD.scid/+ mice showed little or no significant evidence of TUNEL+ lesions during this time period. These data provided the first direct in situ evidence in support of apoptosis as an important mechanism of β cell death as a result of autoimmune infiltration of pancreatic islets.
Figure 5.
β cell apoptosis in BDC2.5/NOD.scid mice. Photomicrographs of TUNEL+ β cells at site of leukocytic infiltration. TUNEL+ nuclei stained with DAB of β cells (insulin containing granule stained with AEC) at the interface of leukocytic infiltration. The dashed lines demarcate the interface of insulitis as determined from parallel hematoxylin-stained sections. (a) ×250 magnification; (b) ×400 magnification; and (c and d) ×500 magnification.
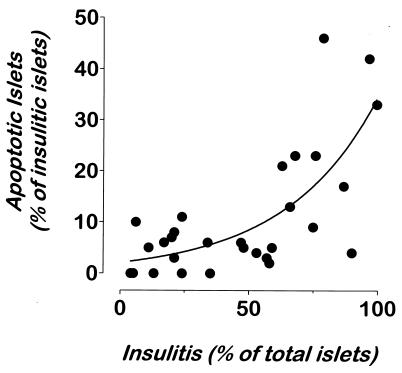
To quantify apoptosis, we analyzed NOD.scid recipients of either T cells from diabetic BDC2.5/NOD.scid mice or T cells from BDC2.5/NOD mice activated with islet cells for 1 week in culture. Both direct transfer and in vitro-activated T cells gave similar results. In five independent experiments, 53 NOD.scid mice received 106 transgenic T cells. Randomized groups of animals were killed at daily intervals from day 3 posttransfer, and their pancreata were analyzed for evidence of apoptosis by TUNEL. Pancreata 3–4 days posttransfer did not show signs of either insulitis or apoptosis (n = 6). Peri-insular to moderate insulitis was first seen at day 5 and 6, with the severity of the insulitis rising abruptly during days 7–10. On day 5, one of three mice had moderate insulitis with apoptotic β cells clearly evident in infiltrated islets (12% of infiltrated islets showed one or more apoptotic β cells). Fig. 6 depicts the composite analysis of the 31 NOD.scid recipients evaluated 6–9 days posttransfer. A clear correlation exists between the severity of insulitis and the appearance of discernible TUNEL+ β cells. Six of 11 mice had signs of moderate to severe insulitis on day 6, and all six of these had islets with TUNEL+ β cells. The incidence of apoptotic β cell death peaked on days 7 and 8, where 20 of 23 animals showed evidence of apoptotic β cells (Fig. 6). On day 9, three of six recipient mice were overtly diabetic and did not contain significant β cell mass to warrant further analysis. However, the three normoglycemic animals had heavily infiltrated pancreatic islets with evidence of apoptotic β cells. The remaining mice were diabetic on day 10 (n = 6) and did not possess intact β cell mass.
Figure 6.
Presence of apoptotic β cells correlates with the level of insulitis. Pancreata from 31 normoglycemic NOD.scid recipients of T cells from diabetic BDC2.5/NOD.scid mice were analyzed for insulitis and apoptosis. Multiple sections from five distinct layers from each pancreas were stained by TUNEL and anti-insulin reagents and with hematoxylin. Islets were scored independently for insulitis and apoptosis. On average, 100 islets were scored per mouse. A second-order relationship (solid curve) was found using the graphpad software (r = 0.8963).
Apoptosis was limited to islets with insulitis. Of the 4500 islets examined in 35 recipient mice, 1917 were insulitic and 2583 were intact (Table 1). Only four of the intact islets (0.4%) showed β cell apoptosis as evidenced by a lone TUNEL+ β cell; this was in marked contrast to adjacent insulitic islets where 233 TUNEL+ islets (12.2%) with 519 total apoptotic β cells were seen. Thus, an extremely significant association between insulitis and TUNEL+ β cells existed (P < 0.0001; χ2).
Table 1.
Apoptotic β cells are found in insulitic islets
| Islets examined | Insulitic islets | Intact islets |
|---|---|---|
| Total islets | 1917 | 2583 |
| Islets with apoptotic β cells | 233 | 4 |
| Islets with apoptotic β cells, % | 12.2* | 0.2 |
Islets (4500) were examined from 35 NOD.scid mice receiving 106 T cells from diabetic BDC2.5/NOD.scid mice. Randomized groups of recipients were killed between day 3 and day 9 posttransfer, and pancreata were examined by TUNEL and anti-insulin staining to quantify apoptotic β cells.
P < 0.0001, χ2 test (with Yates’ correction for continuity).
DISCUSSION
By crossing the BDC2.5 TCR transgenes onto the NOD.scid background, we have created a simplified but robust model for IDDM. These mice, which are devoid of functional CD8+ T cells and contain only a monospecific, β cell-responsive CD4+ T population, become diabetic far more rapidly than either normal NOD mice or the original BDC2.5 TCR transgenic mice. The accelerated course of disease allowed us to determine by direct in situ histological staining of unmanipulated pancreatic tissue that pancreatic β cells die by apoptosis as a result of lymphocytic infiltration.
Until now, it had not been possible to identify the mode of β cell death. We succeeded, however, because the BDC2.5/NOD.scid mice have a dramatically compressed time of development of diabetes making increased numbers of dying β cells available for analysis. Based on the number of β cells in an NOD mouse and the fact that 90% of them must be destroyed to produce overt diabetes (35–37), we calculated the rate of β cell loss over time in our transfer model as 60,000 β cells per day over 5 days (time of first insulitis to time of mean onset of disease), assuming that the rate of death is linear and that the clearance rate is constant. We observed ≈75 apoptotic β cells per mouse (based on Table 1 and corrected for total number of islets per mouse versus islets sampled). Since we know the rate of β cell death and the observed number of apoptotic β cells per mouse, we could easily calculate the clearance rate. We found that the average apoptotic β cells was cleared in 1.7 min. This is similar to the rate of clearance of apoptotic thymocytes upon negative selection (38). If we apply these calculations to NOD diabetes, we find that the death rate is much slower, most likely ≈4300 cells per day if we assume an ≈10-week disease course (insulitis beginning sometime between the 6th and 8th week of life, with the first overt diabetes between week 16 or 18). If the clearance rate is the same, the expected frequency of apoptotic β cells per mouse at any one time is slightly under 4. If we were to score ≈100 islets per mouse as we did above, we would have expected to see less than a single apoptotic β cell per animal, since the rate of apoptosis is only slightly greater than the rate of clearance. It is hardly surprising then that apoptosis was not seen before.
Apoptosis was specific to β cells in infiltrated islets only (see Table 1). Additionally, β cell apoptosis did not correlate with the glucose tolerance of the animals just before analysis (H.L.H., unpublished results), in that both glucose-tolerant and -intolerant mice showed comparable levels of β cell apoptosis confined solely to infiltrated islets, therefore ruling out the possibility that the β cell death was the result of a nonspecific “exhaustion” of β cells struggling to maintain normoglycemia in the face of a vigorous and destructive insulitis.
How then is the apoptotic signal delivered to β cells? In general, apoptosis is induced in target cells by the direct delivery of the perforin-containing, cytotoxic granules by cytotoxic T cells (CD8+ and some Th1-like, CD4+ T cells) and NK cells or through the activation of the Fas (CD95) pathway by Fas-ligand (CD95L) expressing cells (39–41). Additionally, TNF-α has been shown to induce apoptosis through the activation of the TNF/TNF-α receptor (TNF-αR)/TNF receptor-associated death domain (TRADD) pathway (42, 43), while IL-1 and inducible nitric oxide synthase have been shown to trigger apoptosis of some target cells through the production of nitric oxide (44). In BDC2.5/NOD.scid mice, the apoptosis is limited to the β cells at or near the interface between the lymphocytic infiltrate and intact islet cell tissue, indicating that either direct cell–cell contact or localized production of cytokines could mediate β cell killing. In the lymphocytic choriomeningitis virus transgenic model of IDDM, the role for perforin-mediated cytotoxicity of β cell has been clearly demonstrated (45); this is not surprising given the longstanding demonstrations that lymphocytic choriomeningitis virus clearance and lymphocytic choriomeningitis virus-mediated diabetes are exclusively CD8+ T cell-dependent. However, it is unlikely that antigen-specific killing is at work in the BDC2.5/NOD.scid mice, since these mice lack CD8+ T cells and the target β cells lack expression of major histocompatibility complex class II (46) required for direct antigen presentation to CD4+ T cells. Therefore, our results favor apoptosis through the Fas/Fas-L or TNF-α/TNF-αR pathways or by the IL-1/inducible nitric oxide synthase pathway. We found that the T cells from diabetic BDC2.5/NOD.scid mice are dramatically skewed toward the Th1 phenotype (J.D.K., unpublished data). It is known that Fas-L is up-regulated on Th1 T cells (47) and may be expressed on NK cells as well. Moreover, β cells can be induced to express Fas (48) and therefore could serve as targets for Fas-L+, CD4+, Th1 T cells. Recently, it has been shown that the Fas pathway is required for the progression of experimental autoimmune encephalomyelitis (EAE; J. H. Russell, personal communication). However, our recent data using lpr islets indicate that the Fas/FasL interaction does not constitute a major player as these islets serve as efficient targets in transfer experiments (S.V.P. and J.D.K., unpublished work).
As for TNF-α and IL-1β, when neutralizing mAbs were used to ascertain whether these and other cytokines could mediate β cell apoptosis, we attained equivocal results. Anti-IL-1 or anti-IFN-γ mAb treatment of NOD.scid recipient mice plainly had no effect on the transfer of disease by BDC2.5/NOD.scid T cells under conditions shown to inhibit the function of these cytokines. The treatment of recipients with anti-TNF-α mAb, however, did forestall but did not prevent the transfer of diabetes. However, the role of TNF-α in β cell death is difficult to interpret in these experiments, since anti-TNF-α treatment affected T cell migration as well (S.V.P. and J.D.K., unpublished work).
We have developed a dramatically simplified and acute model of insulin-dependent diabetes mellitus by introducing the BDC2.5 TCR transgenes onto the NOD.scid genetic background. Unmanipulated BDC2.5/NOD.scid mice develop a rapid insulitis and frank diabetes in the functional absence of CD8+ TCR-αβ+ T cells, δγ T cells, and B cells. The compressed time frame of the disease allowed the capture of β cell death in situ, where we found clear evidence of β cell apoptosis in infiltrated islets. Having determined how β cells die in IDDM, we are now capable of determining how apoptosis is induced, which should allow us to eventually intercede in this process.
Acknowledgments
We wish to thank Drs. O. Kanagawa, J. Russell, P. Lacy, and E. Unanue for critical reading of this manuscript and Mr. Aaron Mackey for help with the discussion. We wish to thank Ms. Mary Lee Chivetta, Olga Strots, and Stephanie Klees for excellent technical assistance and Ms. Kathy Frederick for help in establishing and maintaining our pathogen-free mouse colony. This work is supported by the generous start-up funding from the Department of Pathology and by a U.S. Public Health Service/Juvenile Diabetes Foundation Program Project Grant 1 P01 AI/DK 39676. H.L.H. is supported by a predoctoral fellowship from the Howard Hughes Medical Institute. J.D.K. is the recipient of Career Development Award from the American Diabetes Association.
Footnotes
Abbreviations: NOD, nonobese diabetic; IDDM; insulin-dependent diabetes mellitus; IL, interleukin; TNF, tumor necrosis factor; TCR, T cell receptor; TdT, terminal dideoxynucleotidetransferase; TUNEL, TdT-mediated X-dUTP nick and end labeling.
References
- 1.Bach J F. Endocr Rev. 1994;15:516–542. doi: 10.1210/edrv-15-4-516. [DOI] [PubMed] [Google Scholar]
- 2.André I, Gonzalez A, Wang B, Katz J, Benoist C, Mathis D. Proc Natl Acad Sci USA. 1996;93:2260–2263. doi: 10.1073/pnas.93.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tisch R, McDevitt H. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 4.Vsye T J, Todd J A. Cell. 1996;85:311–318. doi: 10.1016/s0092-8674(00)81110-1. [DOI] [PubMed] [Google Scholar]
- 5.Katz J D, Benoist C, Mathis D. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 6.Healey D, Ozegbe P, Arden S, Chandler P, Hutton J, Cooke A. J Clin Invest. 1995;95:2979–2985. doi: 10.1172/JCI118006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham J M, Green I C. Growth Regul. 1994;4:173–180. [PubMed] [Google Scholar]
- 8.Mandrup-Poulsen T, Zumsteg U, Reimers J, Pociot F, Morch L, Helqvist S, Dinarello C A, Nerup J. Cytokine. 1993;5:185–191. doi: 10.1016/1043-4666(93)90003-n. [DOI] [PubMed] [Google Scholar]
- 9.Comens P G, Wolf B A, Unanue E R, Lacy P E, McDaniel M L. Diabetes. 1987;36:963–970. doi: 10.2337/diab.36.8.963. [DOI] [PubMed] [Google Scholar]
- 10.Corbett J A, Kwon G, Turk J, McDaniel M L. Biochemistry. 1993;32:13767–13770. doi: 10.1021/bi00213a002. [DOI] [PubMed] [Google Scholar]
- 11.Corbett J A, Wang J L, Sweetland M A, Lancaster J R, Jr, McDaniel M L. J Clin Invest. 1992;90:2384–2391. doi: 10.1172/JCI116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh N, Eizirik D L, Sandler S. Autoimmunity. 1994;18:285–290. doi: 10.3109/08916939409009530. [DOI] [PubMed] [Google Scholar]
- 13.Zawalich W S, Diaz V A. Diabetes. 1986;35:1119–1123. doi: 10.2337/diab.35.10.1119. [DOI] [PubMed] [Google Scholar]
- 14.Eizirik D L, Tracey D E, Bendtzen K, Sandler S. Diabetologia. 1991;34:445–448. doi: 10.1007/BF00403185. [DOI] [PubMed] [Google Scholar]
- 15.Helqvist S. Dan Med Bull. 1994;41:151–166. [PubMed] [Google Scholar]
- 16.Trembleau S, Penna G, Bosi E, Mortara A, Gately M K, Adorini L. J Exp Med. 1995;181:817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heath W R, Allison J, Hoffmann M W, Schonrich G, Hammerling G, Arnold B, Miller J F. Nature (London) 1992;359:547–549. doi: 10.1038/359547a0. [DOI] [PubMed] [Google Scholar]
- 18.Sarvetnick N, Shizuru J, Liggitt D, Martin L, McIntyre B, Gregory A, Parslow T, Stewart T. Nature (London) 1990;346:844–847. doi: 10.1038/346844a0. [DOI] [PubMed] [Google Scholar]
- 19.Sarvetnick N, Liggitt D, Pitts S L, Hansen S E, Stewart T A. Cell. 1988;52:773–782. doi: 10.1016/0092-8674(88)90414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart T A, Hultgren B, Huang X, Pitts-Meek S, Hully J, MacLachlan N J. Science. 1993;260:1942–1946. doi: 10.1126/science.8100367. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi Y, Herrera P, Muniesa P, Huarte J, Belin D, Ohashi P, Aichele P, Orci L, Vassalli J D, Vassalli P. J Exp Med. 1992;176:1719–1731. doi: 10.1084/jem.176.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wogensen L, Lee M S, Sarvetnick N. J Exp Med. 1994;179:1379–1384. doi: 10.1084/jem.179.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moritani M, Yoshimoto K, Tashiro F, Hashimoto C, Miyazaki J, Ii S, Kudo E, Iwahana H, Hayashi Y, Sano T, Itakura M. Int Immunol. 1994;6:1927–1936. doi: 10.1093/intimm/6.12.1927. [DOI] [PubMed] [Google Scholar]
- 24.Katz J D, Wang B, Haskins K, Benoist C, Mathis D. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 25.Haskins K, Portas M, Bradley B, Wedmann D, Lafferty K J. Diabetes. 1988;37:1444–1448. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 26.Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. Proc Natl Acad Sci USA. 1989;86:8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haskins K, McDuffie M. Science. 1990;249:1433–1436. doi: 10.1126/science.2205920. [DOI] [PubMed] [Google Scholar]
- 28.Tomonari K, Lovering E, Spence S. Immunogenetics. 1990;31:333–339. doi: 10.1007/BF02115007. [DOI] [PubMed] [Google Scholar]
- 29.Gavrieli Y, Sherman Y, Ben-Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goverman J, Woods A, Larson L, Wiener L P, Hood L, Zaller D M. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 31.Lafaille J J, Nagashima K, Katsuki M, Tonegawa S. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 32.Peterson J D, Haskins K. Diabetes. 1996;45:328–336. doi: 10.2337/diab.45.3.328. [DOI] [PubMed] [Google Scholar]
- 33.Christianson S W, Shultz L D, Leiter E H. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Sasson S A, Sherman Y, Gavrieli Y. Methods Cell Biol. 1995;46:29–39. [PubMed] [Google Scholar]
- 35.Andersson, A. & Hellerstrom, C. (1972) Diabetes 21, Suppl. 2, 546–554. [DOI] [PubMed]
- 36.Dean P M. Diabetologia. 1973;9:115–119. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- 37.Cahill G F, Jr, Kahn C R. In: Pancreatic Islet Cells in Diabetes Mellitus: A Clinical Perspective. Hanahan D, McDevitt H O, Cahill G F Jr, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. pp. 3–16. [Google Scholar]
- 38.Surh C D, Sprent J. Nature (London) 1994;372:100–103. [Google Scholar]
- 39.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 40.Nagata S, Suda T. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 41.Nagata S, Golstein P. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 42.Hsu H, Shu H B, Pan M G, Goeddel D V. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 43.Hsu H, Xiong J, Goeddel D V. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 44.McDaniel M L, Kwon G, Hill J R, Marshall C A, Corbett J A. Proc Soc Exp Biol Med. 1996;211:24–32. doi: 10.3181/00379727-211-43950d. [DOI] [PubMed] [Google Scholar]
- 45.Kagi D, Odermatt B, Ohashi P S, Zinkernagel R M, Hengartner H. J Exp Med. 1996;183:2143–2152. doi: 10.1084/jem.183.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McInerney M F, Rath S, Janeway C A., Jr Diabetes. 1991;40:648–651. doi: 10.2337/diab.40.5.648. [DOI] [PubMed] [Google Scholar]
- 47.Ramsdell F, Seaman M S, Miller R E, Picha K S, Kennedy M K, Lynch D H. Int Immunol. 1994;6:1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 48.Stassi G, Todaro M, Richiusa P, Giordano M, Mattina A, Sbriglia M S, Lo Monte A, Buscemi G, Galluzzo A, Giordano C. Transplant Proc. 1995;27:3271–3275. [PubMed] [Google Scholar]