Abstract
We investigated the role of the mosquito broad (br) gene in regulating the 20-hydroxyecdysone (20E) effector Vitellogenin (Vg) gene. Injection of double-stranded RNA corresponding to the BR isoform Z2 led to a significant decrease in expression of the Vg gene at 8 and 24 h post-blood meal. Knockdown of Z1 or Z4 resulted in enhanced Vg expression beyond its normal expression time. In vitro studies suggested that the effects of BR require its direct binding to the Vg promoter, as well as protein-protein interaction between BR and the ecdysone receptor complex. The BR isoforms are therefore essential for a proper stage-specific biological response to 20E in the adult female mosquito. In particular, the isoform Z2 is required for 20E-mediated activation of Vg, while isoforms Z1 and Z4 serve as repressors to ensure appropriate termination of Vg expression.
Keywords: Steroid hormone, Transcriptional regulator, C2H2 type zinc-finger, RNA interference, Mosquito
1. Introduction
The insect steroid hormone, 20-hydroxyecdysone (20E), serves as a pivotal temporal cue and directs many critical events in embryogenesis, larval molting, metamorphosis and, in some insects, adult reproduction (Riddiford, 1993; Nijhout, 1994; Raikhel et al., 2005). The functional receptor for 20E is a heterodimer of two nuclear receptors—the ecdysone receptor (EcR) and the homologue of the retinoid X receptor, Ultraspiracle (USP) (Riddiford et al., 2000). The receptor complex acts as a transcription factor recognizing specific DNA sequences (ecdysone response elements) of their target promoters. Binding of 20E and agonist ligands to EcR/USP triggers a conformational change that attracts transcriptional coactivators, leading to transcriptional activation of the target genes (Palli et al., 2005). In response to the 20E titers during Drosophila metamorphosis, EcR/USP directly activates a group of primary-response target genes, including a small set of so-called early genes, such as E74, E75 and Broad (br), which encode intermediate transcriptional regulators (Thummel, 2001). The proteins of these early genes in turn regulate transcription of a larger group of secondary-response genes that ultimately lead to altered cell functions.
A similar 20E regulatory pathway is utilized in the mosquito Aedes aegypti during egg maturation. An increase in the 20E concentration takes place shortly after blood feeding (Hagedorn, 1989). Yolk protein precursors (YPPs) are synthesized in the fat body, a metabolic tissue functionally analogous to the vertebrate liver, under the control of 20E; they are eventually deposited in the developing oocytes. The major YPP gene, Vitellogenin (Vg), is induced about 10,000-fold at its peak expression 20-24 h post-blood meal (PBM), but declines to a background level by 36 h PBM. Sequence analysis of the 5′ regulatory region of Vg reveals the presence of binding sites for EcR/USP, E74, E75 and BR (Kokoza et al., 2001; Chen et al., 2004). Genes encoding these transcriptional factors are upregulated after blood feeding, albeit with distinct patterns, suggesting the direct participation of both the EcR complex and early gene products in regulation of Vg (Pierceall et al., 1999; Wang et al., 2000, 2002; Sun et al., 2002; Chen et al., 2004). Indeed, we have demonstrated that the EcR/USP dimer binds to the Vg promoter shortly after blood feeding and acts synergistically with E74 to activate the Vg expression (Zhu et al., 2003a; Sun et al., 2005).
Br encodes a family of C2H2-type, zinc-finger, DNA-binding proteins. In Drosophila, four BR isoforms (Z1-Z4) share a common N-terminal Bric-a-brac-Tramtrack-Broad (BTB) domain, an evolutionarily conserved protein-protein interaction module (Zollman et al., 1994). Alternative splicing results in isoform-specific C-termini containing one of four pairs of C2H2 zinc fingers that recognize different DNA sequences (DiBello et al., 1991; Bayer et al., 1996). Transcripts of four BR isoforms are present in the fat body of A. aegypti females (Chen et al., 2004). Their expression is elevated after blood feeding, corresponding to the peak of 20E titers. In cell transfection assays, BR isoforms by themselves have no marked effects on the Vg promoter. However, Z1 and Z4 suppress the EcR/USP-mediated 20E activation of Vg, while Z2 enhances such activation. Z3 appears to have no effect in the same experiment (Chen et al., 2004). To explore the functions of BR in adult mosquitoes in vivo, we used isoform-specific RNA interference (RNAi) to knock down Br expression. Here, we show that BR isoforms play differential roles to ensure the appropriate temporal pattern of the Vg expression in the fat body after a blood meal. Additionally, in vitro studies suggest that the effects of BR require their direct binding to the Vg promoter. Our results also imply that BR proteins regulate Vg transcription via protein-protein interaction with the EcR/USP complex.
2. Materials and methods
2.1. Animals
Mosquitoes, A. aegypti, were raised as described by Hays and Raikhel (1990). Larvae were fed a standard diet (Lea, 1964) and adults were fed on a 10% sucrose solution continuously by wick. Vitellogenesis was initiated by feeding 3-5-day-old (post-emergence) females on white laboratory rats. All dissections were performed in Aedes physiological saline (Hagedorn et al., 1977) at room temperature.
2.2. RNA interference assay
Synthesis of double-stranded RNAs (dsRNAs) and microinjection were performed as described previously (Zhu et al., 2003b). dsRNAs corresponding to the BR isoform-specific region (Z1, nt 1466-1796; Z2, nt 1504-1717; Z3, nt 1540-1957; Z4, nt 1453-1953), where no other significant homology was found by Blast search (NCBI), were prepared using the HiScribe RNAi transcription Kit (New England Biolabs). The integrity of the dsRNAs was assessed by means of gel electrophoresis, and concentration was determined by spectrophotometry. One day after emergence, mosquito females were each microinjected intra-thoracically with approximately 0.5 μg of dsRNA. The injected mosquitoes were allowed a period of 5 days for recovery and then were fed blood. The transcription of br and Vg was analyzed using real-time PCR as described previously (Chen et al., 2004). Each sample was analyzed in triplicate and normalized to the internal control, β-actin mRNA.
2.3. Cell culture and transient transfection assay
The EcR-deficient Drosophila melanogaster L57-3-11 cell line, provided generously by Dr. Lucy Cherbas (Indiana University), was transfected according to the instructions of Hu et al. (2003). The coding region sequences of the four BR-C isoforms were inserted into the pAc5.1/V5/HisA (Invitrogen) vector and fused to a V5 and a His tag (Chen et al., 2004). The construction of plasmids pVg2.1-luc, pAc5-AaEcR and pAc5-AaUSP has been described elsewhere (Wang et al., 1998).
Western-blot analyses were carried out with a fraction of the transfected L57-3-11 cells. A 20-μl aliquot of each sample was separated by means of SDS-PAGE and tested independently with antibodies against AaEcR, AaUSP and V5 tag (Invitrogen).
2.4. Electrophoretic mobility shift assay (EMSA)
EMSAs for BR were performed as described previously (Chen et al., 2004). For competition reactions, unlabelled oligonucleotides were added in excess of 50-fold.
2.5. Immunoprecipitation
Transfected cells were harvested 40 h after transfection in ice-cold NP-40 lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% NP-40) supplemented with protease inhibitors. Assays were performed using the Immunoprecipitation Kit (Roche) following the manufacturer’s instructions. Antibodies against AaEcR were used for this analysis. The resulting immune complexes were precipitated by addition of protein G-agarose beads. After extensive washing, the complexes were separated by means of SDS-PAGE and analyzed by means of Western blotting with anti-V5 antibody (Invitrogen).
3. Results
3.1. BR isoforms play important roles in controlling temporal expression of the Vg gene
The increase of Br transcripts after blood feeding, induced by a rise in the 20E concentration, suggests that BR isoforms are required for the 20E-activated production of YPP (Chen et al., 2004). To test this hypothesis, we synthesized dsRNAs corresponding to the isoform-specific regions of BR and injected them independently into newly emerged female mosquitoes. dsRNA corresponding to a bacterial malE gene was injected in a similar manner to act as a negative control. Five days after injection, these mosquitoes were given a blood meal. mRNA levels of br decreased considerably, both before and after blood feeding, in the fat body of female mosquitoes injected with br dsRNAs, but not in the control mosquitoes (Fig. 1).
Fig. 1.
Knockdown of br expression in the adult female mosquito. dsRNAs corresponding to individual BR isoforms were injected into thoraces of female mosquitoes as described in Section 2. mRNA expression of Z1 (A), Z2 (B), Z3 (C) and Z4 (D) was measured by means of real-time PCR at the indicated time points after blood feeding. Each sample was analyzed in triplicate and normalized to the internal control, β-actin mRNA. WT, uninjected A. aegypti Rockefeller/UGAL strain; Mal RNAi, injected with double-stranded RNA complementary to bacterial malE; Z1-Z4, injected with dsRNA corresponding to the isoform-specific regions of the mosquito BR isoforms.
After confirmation of the knockdown of br transcripts, we then looked into their effects on the expression of the Vg gene. In control mosquitoes, the Vg mRNA levels started to rise after blood feeding, peaked at about 24 h PBM, declined thereafter and returned to the background levels by 36 h PBM (Fig. 2A). The knockdown of Z2 was associated with a significant decrease in the Vg mRNA levels at 8 h (P < 0.05) and 24 h PBM. The reduction of Z3 resulted in a substantial decrease in the Vg mRNA levels at 24 h PBM. Conversely, injection of dsRNA corresponding to Z1 or Z4 not only markedly augmented the Vg transcription at 24 h PBM, but also led to a considerable increase in Vg expression at 36 h PBM (Fig. 2A). Importantly, enhanced Vg transcription was detected even at 48 h PBM in the fat body of mosquitoes with reduced Z1 and Z4 expression (Fig. 2B). Injection of a combination of dsRNA corresponding to both Z1 and Z4 caused a slightly stronger transcription of Vg at 24 h PBM than injection of Z1 or Z4 dsRNA alone, suggesting that these isoforms act cooperatively in temporal regulation of the Vg expression (Fig. 2B). Together, these data indicate that the br gene is required for the proper expression pattern of Vg. Moreover, isoforms Z2 and Z3 likely function as transcriptional activators of the Vg gene, and Z1 and Z4 as its repressors.
Fig. 2.

The BR isoforms are required for proper Vg expression in the fat body after blood ingestion. dsRNAs corresponding to individual (A) or a combination of two (B) BR isoforms were injected into the thoraces of newly emerged female mosquitoes. The transcripts of Vg in female mosquitoes were then measured using real-time RT-PCR at the indicated time after blood feeding and were normalized to β-actin expression. Please note the different Y-axis scale used in Panel B for animals at 48 h PBM. WT, uninjected A. aegypti Rockefeller/UGAL strain; Mal, injected with double-stranded RNA complementary to bacterial malE; Z1, Z2, Z3 and Z4, injected with BR isoform-specific dsRNA; Z1/Z4, injected with a mixture of dsRNA for Z1 and Z4. Representative data (mean ± S.E.M.) from at least three independent experiments are shown.
3.2. BR isoforms are implicated in regulation of the usp gene
Binding sites for BR isoforms are also found in regulatory regions of the usp gene (Wang, Zhu and Raikhel, unpublished data), encoding an obligatory component of the EcR complex, which is involved in the primary and secondary control of the Vg gene. The two mosquito USP isoforms, USP-A and USP-B, are differentially expressed and regulated by the 20E ligand (Wang et al., 2000). usp-a is inhibited by 20E and is thus expressed in postvitellogenic fat bodies, while the usp-b transcript levels increase 5-8-fold during the vitellogenic stage and are activated by 20E. Injection of dsRNA corresponding to the Z1 isoform resulted in modestly enhanced transcription of usp-a at 24 h and its dramatic activation at 36 h PBM, suggesting that Z1 is a stage-specific repressor of usp-a expression (Fig. 3A). Downregulation of Z2 and Z3 led to a substantial decrease in the usp-a expression at 36 h PBM, indicating their possible roles in stage-specific activation of usp-a. Lessening of Z4 had no marked effect on the usp-a expression (Fig. 3A). Injection of dsRNA corresponding to the Z1 isoform resulted in enhanced transcription of usp-b at 24 and 36 h PBM, suggesting that Z1 is a stage-specific repressor of usp-b expression (Fig. 3B). Reduction of Z4 prolonged the induced usp-b transcription to 36 h PBM, at a level comparable to its normal peak at 24 h PBM, implying that Z4 could serve as an additional stage-specific regulator of usp-b expression (Fig. 3B). Downregulation of Z2 and Z3 had no obvious effects on the usp-b promoter. These results thus indicate that the br gene has a profound role in modulating the stage-specific 20E response.
Fig. 3.

RNAi-mediated reduction in mosquito BR leads to abnormal expression of the USP gene. dsRNAs corresponding to individual BR isoforms were injected into thoraces of newly emerged female mosquitoes. The usp-a (A) and usp-b (B) transcripts in the fat body were then measured using real-time RT-PCR at the indicated time after blood feeding as described in Section 2. WT, uninjected A. aegypti Rockefeller/UGAL strain; Mal, injected with double-stranded RNA complementary to bacterial malE; Z1, Z2, Z3 and Z4, injected with BR isoform-specific dsRNA.
3.3. Regulatory functions of BR require their binding to the Vg promoter
Consistent with results of the RNAi experiment, potential binding sites of the BR isoforms have been identified in the Vg 5′ upstream regulatory region. Furthermore, cell transfection assays have demonstrated that the Z1, Z2 and Z4 isoforms modulate transactivation of the Vg promoter by the EcR/USP dimer (Chen et al., 2004). To elucidate the mechanism underlying the regulation by BR, we systematically removed the BR binding sites on the Vg promoter by means of site-directed mutagenesis and exploited the derivative pVg-luc reporter plasmids in transfection assays. Since the Z3 isoform only marginally increased the effect of EcR/USP in the presence of 20E in our previous study (Chen et al., 2004), we did not test the Z3 binding sites in this experiment. When AaEcR/AaUSP and the Z1 isoform were co-expressed in L57 cells, Z1 significantly dampened activation of the Vg promoter by AaEcR/AaUSP in the presence of 20E (Fig. 4A). In contrast, the dose-dependent repression by Z1 was mostly relieved when the pVg-luc reporter construct was deprived of the only Z1 binding site on the Vg promoter.
Fig. 4.
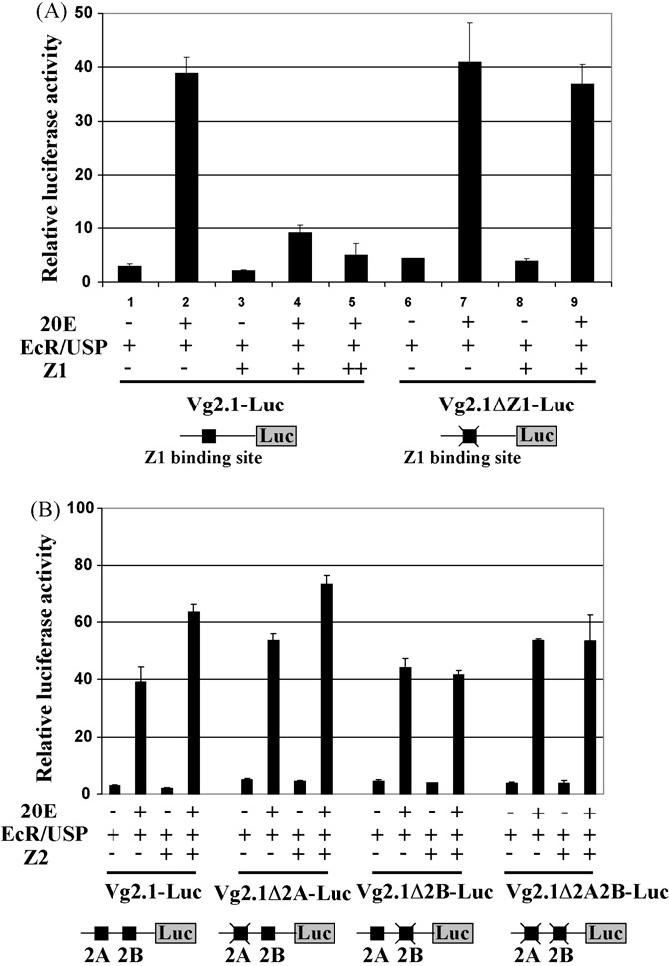
The BR binding sites on the Vg promoter are essential for transcriptional regulation by the BR isoforms. Drosophila L57 cells were transfected with 100 ng phRL-CMV (Renilla luciferase control vector), 100 ng of different reporter plasmids and 100 ng expression vectors for AaEcR-B, AaUSP-B, AaBR-Z1 (A) and AaBR-Z2 (B). A schematic diagram of the reporter constructs is depicted at the bottom of each panel. Transfections with increasing amounts of AaBR-Z1 are indicated as “+” (100 ng) and “++” (300 ng). After transfection, cells were cultured in medium with or without 1 × 10-6 M of 20E. Data represent the ratios of firefly luciferase to Renilla luciferase activity (relative luciferase activity). Values are means of triplicate independent transfection experiments, with error bars representing the S.D. of the mean.
A similar experiment was carried out to test the two binding sites of BR-Z2 (2A and 2B) separated apart in the Vg promoter. After the 2A sequence was mutated in the reporter plasmid, the EcR/USP-mediated activation of the Vg promoter became slightly stronger (P < 0.01). Nonetheless, co-expression of the Z2 protein enhanced the activation of the Vg promoter (P < 0.01) despite the loss of the 2A site. However, when 2B was destroyed, alone or in combination with 2A, Z2 lost its effect on the Vg promoter in the transfection assays (Fig. 4B). These results imply that the isoforms Z1 and Z2 directly bind to the regulatory region of Vg and modulate its transcription after a blood meal.
Seven binding sites of Z4 (4A-4G) are scattered in a 2.1 kb upstream region of Vg, exhibiting different binding affinity to Z4 (Chen et al., 2004). Starting from pVg2.1-luc, a series of mutations were introduced to remove potential Z4 binding sites, individually and in combination. Even when seven binding sites were destroyed altogether in the reporter plasmid, the repression by Z4 on Vg promoter was not completely relieved (Fig. 5A). Since the Z4 recognition sequences are rich in A/T base pairs, we could not rule out that additional Z4 binding sites exist on the Vg promoter. Instead, we constructed three BR-Z4 mutants, in which four cystidines (at positions of 495, 498, 525 and 528) in the two zinc-finger DNA binding domains were changed into alanines (Fig. 5B). The first and second zinc fingers were disrupted individually in ZF-A and ZF-B, and collectively in ZF-AB. As expected, DNA binding affinity of the mutated Z4 proteins was reduced drastically in the EMSA experiment (Fig. 5C). The suppression on the EcR-mediated activation was severely reduced when Z4 was replaced by its mutants in the cell transfection assay, indicating that the intact DNA binding domain of Z4 is essential for its effect on the Vg promoter (Fig. 5D).
Fig. 5.
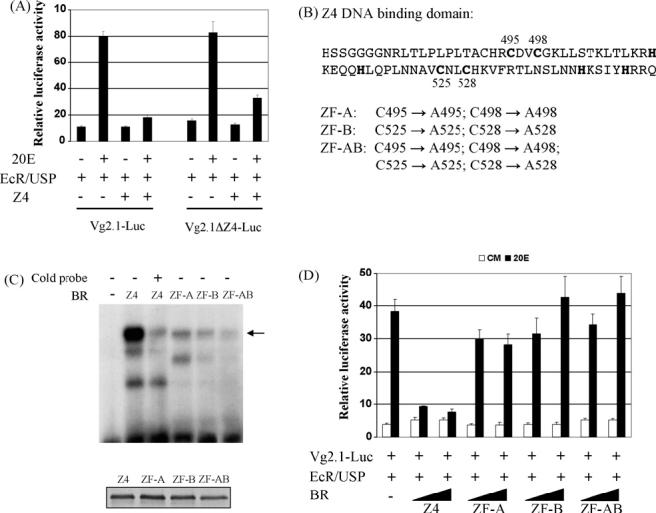
Regulation of the Vg promoter requires intact DNA binding domains of BR. (A) Drosophila L57 cells were co-transfected with expression vectors for AaEcR-B, AaUSP-B, AaBR-Z4, and indicated reporter plasmids. In pVg2.1ΔZ4-Luc, all seven identified potential Z4 binding sites were removed by point mutagenesis. After transfection, cells were cultured in medium with or without 1 × 10-6 M of 20E. Data represent the ratios of firefly luciferase to Renilla luciferase activity (relative luciferase activity). (B) Point mutations were introduced into the Z4 DNA binding domain. The cystidines targeted in the zinc fingers are highlighted in the amino acid sequence. (C) EMSA was performed using 32P-labeled oligonucleotide containing consensus Drosophila BR-Z4 binding site and in vitro-synthesized mosquito Z4 and its mutants. A 100-fold molar excess of unlabeled probe was added as specific competitor. When the Z4 proteins were synthesized, an in vitro coupled transcription and translation (TNT) reaction was performed in parallel in the presence of 35S-methionine. The autoradiograph of the TNT products is shown at the bottom, indicating that comparable amounts of individual Z4 proteins were added in the EMSA experiment. (D) Drosophila L57 cells were co-transfected with pVg2.1-Luc reporter, expression vectors for AaEcR-B and AaUSP-B, and indicated AaBR-Z4 constructs. The solid triangle represents increasing amounts of the Z4 expression plasmids.
3.4. BR binds the EcR complex in vitro
The fact that BR modulates activity of the EcR complex on the Vg promoter prompted us to test whether a protein-protein interaction exists between BR and the EcR/USP dimer. Individual BR isoforms (as fusions to the V5 epitope tag) were co-expressed with AaEcR-B and AaUSP-B in Drosophila L57 cells. Whole cell extracts of these cells were then subjected to immunoprecipitation assays. The three BR isoforms we tested separately (Z1, Z2 and Z4) were precipitated using antibodies against mosquito EcR in a 20E-independent manner (Fig. 6). Together, these results suggest that BR regulates Vg expression via protein-protein association with EcR/USP on the Vg promoter.
Fig. 6.
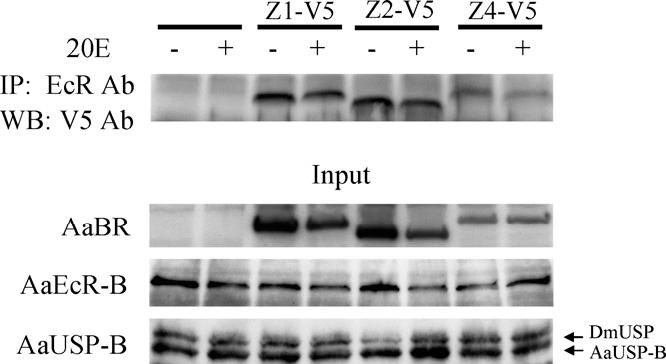
BR is physically associated with EcR/USP in vitro. L57 cells were co-transfected with expression vectors for AaEcR-B, AaUSP-B and individual BR-V5 fusion proteins. Cell extracts were incubated with antibodies against EcR, and the precipitated proteins were subjected to Western-blot analysis with V5 antibody. At the bottom, AaEcR-B, AaUSP-B and AaBR proteins in the inputs were examined by mean of immunoblotting using antibodies against AaEcR, DmUSP and V5 tag, respectively. When the experiment was conducted using 20E, 1 × 10-6 M 20E was added into the binding and washing buffers.
4. Discussion
The role of br during metamorphosis is well understood in holometabolous insects, such as Drosophila and moths (Riddiford et al., 2003). Extensive genetic analyses in Drosophila have shown that br is a critical mediator of the ecdysone response during metamorphosis (Belyaeva et al., 1980; Kiss et al., 1988; Karim et al., 1993). The br mutants exhibit abnormal regulation of many intermolt, early and late gene activities (Karim et al., 1993; Zhou and Riddiford, 2002). More recently, it has been demonstrated that br is expressed during each nymphal molt of the direct-developing insect Oncopeltus fasciatus (Erezyilmaz et al., 2006).
Much less is known about the role of br in adult insects. In adult Drosophila, genetic analysis has shown that br is a key control gene during oogenesis (Deng and Bownes, 1997). The different expression patterns of BR isoforms regulate the number of eggs produced by determining whether the cells in each individual egg chamber become committed to apoptosis or to progress through normal development (Terashima and Bownes, 2004). Moreover, Z1 and Z3 overexpression were demonstrated to suppress yp gene expression in egg chambers, while Z2 and Z4 had no effect (Terashima and Bownes, 2004). Our study revealed that the mosquito BR proteins modulate stage-specific expression of Vg, a 20E target gene, during vitellogenesis in the mosquito female fat body, providing additional evidence that br has evolutionarily conserved roles in the insect ecdysone response.
Our functional analyses of br by means of isoform-specific RNAi in adult female mosquitoes demonstrated that BR is required for the robust and precisely timed expression of the Vg gene after a blood meal. Moreover, individual BR isoforms were shown to play distinct roles in such regulation. We observed Z2 to serve as a transcription activator, essential for the optimal Vg expression at 8 and 24 h PBM. Z3 appeared to be functionally similar to Z2, but its effect was limited to 24 h PBM. On the contrary, Z1 and Z4 acted as negative regulators to attenuate the Vg expression at 24 and 36 h, and even at 48 h PBM once the 20E titers in the female mosquitoes had reached the maximum at about 20 h PBM. The results of in vivo studies are in line with effects of BR on the Vg promoter in the cell transfection assays. Importantly, these functions of BR isoforms also appear to be consistent with their expression patterns in the mosquito fat body. Transcription of Z2 exhibited a sharp rise after the onset of vitellogenesis and reached two peaks, one at 8 h and one at 24 h PBM. Conversely, the Z3 transcripts remained at a relatively low level throughout the entire vitellogenic period, increasingly slightly at 24 h PBM. mRNA levels of both Z1 and Z4 were relatively low during the early vitellogenic stage, but started to rise after 16 h PBM and peaked at 24 h (Chen et al., 2004). In summary, individual BR isoforms participated in the regulation of Vg in response to the 20E pulse after blood ingestion through a coordinated and well-orchestrated effort.
It has been shown in D. melanogaster that different BR isoforms can have opposite regulatory functions on the same gene. For example, the larval fat-body-specific expression of the Fbp-1 gene is activated by the Z3 isoform, but repressed by the Z2 protein (Mugat et al., 2000). Interestingly, functions of an individual isoform are promoter dependent. While Z3 is required for activation of the Fbp-1 gene, it acts as a repressor for the transcription of a salivary-gland-specific gene Sgs4 (Bayer et al., 1996). A similar phenomenon is also observed in the mosquito 20E response. Our preliminary study suggested that both Z1 and Z4 isoforms are required to upregulate expression of the fat-body-specific lipophorin receptor gene, which is activated at 30-36 h PBM when the 20E titers in the female adults drop to the basal level (Jun and Raikhel, unpublished result). Therefore, it appears that mere existence of the BR proteins cannot explain their diverse properties in terms of transcription regulation. The activities of the BR isoforms probably rely on their binding sequences in the context of native target promoters and/or proteins associated with BR at different scenarios.
The presence of zinc-finger DNA binding domains in BR proteins suggested that they work as transcription regulators and interact directly with cis-acting regulatory elements in target genes. Indeed, there are numerous binding sites for BR isoforms in the mosquito Vg promoter. EMSA assays confirmed that BR can bind these sites in vitro. When binding sites for Z1 and Z2 were mutagenized in the pVg-Luc reporter construct, the BR proteins lost their influences on the Vg promoter in cell transfection assays. Similarly, mutations in the Z4 zinc-finger domain also relieved repression of the Vg promoter by this isoform. Thus, these data collectively imply that the BR proteins act through their binding sites in the Vg promoter, and that direct DNA-protein interactions are indispensable for appropriate activation of this ecdysone effector gene. It is worth noting that the upregulation of usp gene after blood feeding is also modulated by the BR isoforms. This observation not only indicates that BR has profound effects on the vitellogenic 20E response, but also raises the possibility that Vg is under direct and indirect control of BR.
Intriguingly, BR by itself had no marked effect on the Vg promoter in the cell transfection assay. Instead, the BR isoforms either repressed or enhanced transcription of the Vg promoter activated by the EcR complex (Chen et al., 2004). The functional interaction between BR and EcR/USP may stem from their physical association that we found in vitro. Proteins harboring the BTB domain have been shown to play crucial roles in specifying the overall transcriptional activity of nuclear receptors in response to their cognate ligands. The human promyelocytic leukemia zinc finger (PLZF) protein is a transcriptional repressor belonging to the BTB family. PLZF interacts directly with the Vitamin D3 receptor (VDR) and represses VDR-dependent gene activation (Ward et al., 2001). The ligand-independent physical contact involves the DNA-binding domain of VDR and the BTB domain of PLZF. Likewise, PLZF has been found to bind the retinoic acid receptor (RAR) and to interfere with the transcriptional activity of the RAR-RXR heterodimer (Martin et al., 2003). The zinc finger domain of PLZF and the ligand binding domain of RAR are sufficient for the interaction of these two proteins, while participation of RXR is not required. Unlike BR, DNA binding of PLZF in both cases is not required for its repressive functions. Interestingly, a chromosomal translocation joining the PLZF gene to RARα is associated with a rare syndrome of acute promyelocytic leukemia (Chen et al., 1994). The PLZF-RARα binds to retinoic acid response element as a heterodimeric partner of RXR, interfering with RARα functions by exerting a dominant negative effect (Licht et al., 1996). The functional interactions between members of the nuclear receptor and BTB families thus represent a regulatory mechanism well-conserved in vertebrates and invertebrates.
Regulation of Vg after blood feeding is complicated, requiring the participation of a lot of transcriptional factors. Another early gene product, E74B, has been shown to work synergistically with the EcR/USP dimer in activating the Vg promoter (Sun et al., 2005). This collaboration demands 20E-dependent protein interaction between E74B and EcR/USP, and their direct binding to the Vg promoter. In light of the modulatory effects of BR, we believe that BR and E74B bind to the EcR/USP complex and, presumably by altering conformation of the EcR/USP complex, bring to the Vg promoter transcriptional cofactors that change local chromosomal structure or formation of the transcription apparatus. The requirement of DNA binding increases local concentrations of these proteins on the Vg promoter and also provides an additional layer of specificity for such protein interactions. Future studies should determine which domains of BR and EcR/USP are involved in the protein interaction, and whether EcR/USP sequentially or simultaneously binds BR and E74B on the Vg promoter. It should also be of interest to elucidate the molecular details of how BR regulates a target gene when the EcRE is not present in the promoter.
Acknowledgement
This work was supported by a NIH grant AI-36959 to A.S. Raikhel.
References
- Bayer CA, Holley B, Fristrom JW. A switch in broad-complex zinc-finger isoform expression is regulated posttranscriptionally during the metamorphosis of Drosophila imaginal discs. Dev. Biol. 1996;177:1–14. doi: 10.1006/dbio.1996.0140. [DOI] [PubMed] [Google Scholar]
- Belyaeva ES, Aizenzon MG, Semeshin VF, Kiss II, Koczka K, Baritcheva EM, Gorelova TD, Zhimulev IF. Cytogenetic analysis of the 2B3-4-2B11 region of the X-chromosome of Drosophila melanogaster. I. Cytology of the region and mutant complementation groups. Chromosoma. 1980;81:281–306. doi: 10.1007/BF00285954. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhu J, Sun G, Raikhel AS. The early gene Broad is involved in the ecdysteroid hierarchy governing vitellogenesis of the mosquito Aedesaegypti. J. Mol. Endocrinol. 2004;33:743–761. doi: 10.1677/jme.1.01531. [DOI] [PubMed] [Google Scholar]
- Chen Z, Guidez F, Rousselot P, Agadir A, Chen SJ, Wang ZY, Degos L, Zelent A, Waxman S, Chomienne C. PLZF-RAR alpha fusion proteins generated from the variant t(11;17)(q23;q21) translocation in acute promyelocytic leukemia inhibit ligand-dependent transactivation of wild-type retinoic acid receptors. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1178–1182. doi: 10.1073/pnas.91.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WM, Bownes M. Two signalling pathways specify localised expression of the Broad-Complex in Drosophila eggshell patterning and morphogenesis. Development. 1997;124:4639–4647. doi: 10.1242/dev.124.22.4639. [DOI] [PubMed] [Google Scholar]
- DiBello PR, Withers DA, Bayer CA, Fristrom JW, Guild GM. The Drosophila Broad-Complex encodes a family of related proteins containing zinc fingers. Genetics. 1991;129:385–397. doi: 10.1093/genetics/129.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erezyilmaz DF, Riddiford LM, Truman JW. The pupal specifier broad directs progressive morphogenesis in a direct-developing insect. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6925–6930. doi: 10.1073/pnas.0509983103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn HH, Turner S, Hagedorn EA, Pontecorvo D, Greenbaum P, Pfeiffer D, Wheelock G, Flanagan TR. Postemergence growth of the ovarian follicles of Aedes aegypti. J. Insect Physiol. 1977;23:203–206. doi: 10.1016/0022-1910(77)90030-0. [DOI] [PubMed] [Google Scholar]
- Hagedorn HH. Physiological roles of hemolymph ecdysteroids in adult insect. In: Koolman J, editor. Ecdysone, From Chemistry to Mode of Action. Georg Thieme Medical Publishers, Inc.; 1989. pp. 279–289. [Google Scholar]
- Hays AR, Raikhel AS. A novel protein produced by the vitellogenic fat-body and accumulated in mosquito oocytes. Rouxs Arch. Dev. Biol. 1990;199:114–121. doi: 10.1007/BF02029559. [DOI] [PubMed] [Google Scholar]
- Hu X, Cherbas L, Cherbas P. Transcription activation by the ecdysone receptor (EcR/USP): identification of activation functions. Mol. Endocrinol. 2003;17:716–731. doi: 10.1210/me.2002-0287. [DOI] [PubMed] [Google Scholar]
- Karim FD, Guild GM, Thummel CS. The Drosophila Broad-Complex plays a key role in controlling ecdysone-regulated gene expression at the onset of metamorphosis. Development. 1993;118:977–988. doi: 10.1242/dev.118.3.977. [DOI] [PubMed] [Google Scholar]
- Kiss I, Beaton AH, Tardiff J, Fristrom D, Fristrom JW. Interactions and developmental effects of mutations in the Broad-Complex of Drosophila melanogaster. Genetics. 1988;118:247–259. doi: 10.1093/genetics/118.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoza VA, Martin D, Mienaltowski MJ, Ahmed A, Morton CM, Raikhel AS. Transcriptional regulation of the mosquito vitellogenin gene via a blood meal-triggered cascade. Gene. 2001;274:47–65. doi: 10.1016/s0378-1119(01)00602-3. [DOI] [PubMed] [Google Scholar]
- Lea AO. Studies on the dietary and endocrine regulation of autogenous reproduction in Aedes taeniorhynchus (Wied.) J. Med. Entomol. 1964;39:40–44. doi: 10.1093/jmedent/1.1.40. [DOI] [PubMed] [Google Scholar]
- Licht JD, Shaknovich R, English MA, Melnick A, Li JY, Reddy JC, Dong S, Chen SJ, Zelent A, Waxman S. Reduced and altered DNA-binding and transcriptional properties of the PLZF-retinoic acid receptor-alpha chimera generated in t(11;17)-associated acute promyelocytic leukemia. Oncogene. 1996;12:323–336. [PubMed] [Google Scholar]
- Martin PJ, Delmotte MH, Formstecher P, Lefebvre P. PLZF is a negative regulator of retinoic acid receptor transcriptional activity. Nucl. Recept. 2003;1:6. doi: 10.1186/1478-1336-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugat B, Brodu V, Kejzlarova-Lepesant J, Antoniewski C, Bayer CA, Fristrom JW, Lepesant JA. Dynamic expression of broad-complex isoforms mediates temporal control of an ecdysteroid target gene at the onset of Drosophila metamorphosis. Dev. Biol. 2000;227:104–117. doi: 10.1006/dbio.2000.9879. [DOI] [PubMed] [Google Scholar]
- Nijhout HF. Insect Hormones. Princeton University Press; 1994. [Google Scholar]
- Palli SR, Hormann RE, Schlattner U, Lezzi M. Ecdysteroid receptors and their applications in agriculture and medicine. Vitam. Horm. 2005;73:59–100. doi: 10.1016/S0083-6729(05)73003-X. [DOI] [PubMed] [Google Scholar]
- Pierceall WE, Li C, Biran A, Miura K, Raikhel AS, Segraves WA. E75 expression in mosquito ovary and fat body suggests reiterative use of ecdysone-regulated hierarchies in development and reproduction. Mol. Cell. Endocrinol. 1999;150:73–89. doi: 10.1016/s0303-7207(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Brown M, Belles X. Hormonal control of reproductive processes. In: Gilbert L, Gill S, Iatrou K, editors. Comprehensive Insect Physiology, Biochemistry, Pharmacology and Molecular Biology. Volume 3. Endocrinology. Elsevier Press; 2005. [Google Scholar]
- Riddiford LM. Hormones and Drosophila development. In: Bate M, Arias AM, editors. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; 1993. pp. 899–939. [Google Scholar]
- Riddiford LM, Cherbas P, Truman JW. Ecdysone receptors and their biological actions. Vitam. Horm. 2000;60:1–73. doi: 10.1016/s0083-6729(00)60016-x. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Hiruma K, Zhou X, Nelson CA. Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster. Insect Biochem. Mol. Biol. 2003;33:1327–1338. doi: 10.1016/j.ibmb.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Sun G, Zhu J, Li C, Tu Z, Raikhel AS. Two isoforms of the early E74 gene, an Ets transcription factor homologue, are implicated in the ecdysteroid hierarchy governing vitellogenesis of the mosquito, Aedes aegypti. Mol. Cell. Endocrinol. 2002;190:147–157. doi: 10.1016/s0303-7207(01)00726-2. [DOI] [PubMed] [Google Scholar]
- Sun G, Zhu J, Chen L, Raikhel AS. Synergistic action of E74B and ecdysteroid receptor in activating a 20-hydroxyecdysone effector gene. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15506–15511. doi: 10.1073/pnas.0503501102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima J, Bownes M. Translating available food into the number of eggs laid by Drosophila melanogaster. Genetics. 2004;167:1711–1719. doi: 10.1534/genetics.103.024323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel CS. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev. Cell. 2001;1:453–465. doi: 10.1016/s1534-5807(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Wang SF, Miura K, Miksicek RJ, Segraves WA, Raikhel AS. DNA binding and transactivation characteristics of the mosquito ecdysone receptor-Ultraspiracle complex. J. Biol. Chem. 1998;273:27531–27540. doi: 10.1074/jbc.273.42.27531. [DOI] [PubMed] [Google Scholar]
- Wang SF, Li C, Zhu J, Miura K, Miksicek RJ, Raikhel AS. Differential expression and regulation by 20-hydroxyecdysone of mosquito ultraspiracle isoforms. Dev. Biol. 2000;218:99–113. doi: 10.1006/dbio.1999.9575. [DOI] [PubMed] [Google Scholar]
- Wang SF, Li C, Sun G, Zhu J, Raikhel AS. Differential expression and regulation by 20-hydroxyecdysone of mosquito ecdysteroid receptor isoforms A and B. Mol. Cell Endocrinol. 2002;196:29–42. doi: 10.1016/s0303-7207(02)00225-3. [DOI] [PubMed] [Google Scholar]
- Ward JO, McConnell MJ, Carlile GW, Pandolfi PP, Licht JD, Freedman LP. The acute promyelocytic leukemia-associated protein, promyelocytic leukemia zinc finger, regulates 1,25-dihydroxyvitamin D-3-induced monocytic differentiation of U937 cells through a physical interaction with vitamin D-3 receptor. Blood. 2001;98:3290–3300. doi: 10.1182/blood.v98.12.3290. [DOI] [PubMed] [Google Scholar]
- Zhou X, Riddiford LM. Broad specifies pupal development and mediates the ‘status quo’ action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development. 2002;129:2259–2269. doi: 10.1242/dev.129.9.2259. [DOI] [PubMed] [Google Scholar]
- Zhu J, Miura K, Chen L, Raikhel AS. Cyclicity of mosquito vitellogenic ecdysteroid-mediated signaling is modulated by alternative dimerization of the RXR homologue Ultraspiracle. Proc. Natl. Acad. Sci. U.S.A. 2003;100(a):544–549. doi: 10.1073/pnas.0235695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen L, Raikhel AS. Posttranscriptional control of the competence factor betaFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 2003;100(b):13338–13343. doi: 10.1073/pnas.2234416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollman S, Godt D, Prive GG, Couderc JL, Laski FA. The BTB domain, found primarily in zinc finger proteins, defines an evolutionarily conserved family that includes several developmentally regulated genes in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 1994;91:10717–10721. doi: 10.1073/pnas.91.22.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]



