Abstract
Cholesterol and other sterols are important components of biological membranes and are known to strongly influence the physical characteristics of lipid bilayers. Although this has been studied extensively in fully hydrated membranes, little is known about the effects of cholesterol on the stability of membranes in the dry state. Here, we present a Fourier transform infrared spectroscopy study on the effects of cholesterol on the phase behavior of dry liposomes composed of phosphatidylcholines with different degrees of fatty acid unsaturation or of mixtures of phosphatidylcholine with a plant galactolipid. In addition, we have analyzed the H-bonding of cholesterol, galactose, and a combination of the two additives to the P=O and C=O groups in dry phosphatidylcholine bilayers. The data indicate a complex balance of interactions between the different components in the dry state and a strong influence of fatty acid unsaturation on the interactions of the diacyl lipids with both cholesterol and galactose.
INTRODUCTION
Cholesterol (Chol) and other sterols are essential constituents of the membranes of eukaryotic cells, and the plasma membranes of mammalian cells contain up to 50 mol % Chol. Sterols perform different roles in membranes with perhaps the most important one being to modulate the physicochemical properties of the lipid bilayer (see Ohvo-Reikila et al. (1) and Yeagle (2) for reviews), including the formation of liquid-ordered domains, or rafts (3–5). Incorporation of Chol into phosphatidylcholine (PC) bilayers results in a broadening of the cooperative gel to liquid-crystalline phase transition by disordering the gel phase and ordering the liquid-crystalline phase of the host lipids. At a sufficiently high Chol content, the phase transition may be completely abolished (6). These effects are strongest with lipids containing completely saturated fatty acyl chains and become progressively weaker with an increasing degree of unsaturation, being completely absent with lipids containing polyunsaturated, long-chain fatty acids (7–9).
Interestingly, it has been shown that in freeze-dried liposomes made from fully saturated PC, the addition of Chol leads to a marked reduction in the gel to liquid-crystalline phase transition temperature (Tm) (10). Investigations into the effects of Chol on the stability of liposomes during drying have only rarely been reported so far, and in these studies no clear effects on solute leakage were found (11,12). However, the available data suggest that the membrane-stabilizing effect of the lyoprotectant trehalose is modulated by the presence of Chol in the bilayers (10,13).
Chol could influence the interactions of phospholipids with sugars in two opposing ways, namely by increasing the lipid spacing due to its intercalation between the lipids, thereby disrupting intermolecular interactions between phospholipids (14) and presumably increasing the ability of sugars to interact with lipid headgroups and by competing with the sugars for H-bonding interactions with the lipid headgroups.
Chol is situated in a lipid bilayer with its rigid sterol ring structure positioned between the fatty acyl chains and its acyl chain extending toward the bilayer center, parallel to the fatty acyl chains of the diacyl lipids (see Yeagle (2) for a review). In contrast, the Chol OH group is localized in the membrane interfacial region, allowing interactions with the ester carbonyl (15,16), the phosphate and choline groups, as well as with water molecules (15). Recent molecular dynamics simulations indicate that in the fully hydrated state there is no apparent competition between trehalose and Chol for interaction sites on PC molecules (17).
Although a modulation of the effect of trehalose on the phase behavior of fully saturated PC bilayers by Chol in the dry state has been shown (10,13), possible H-bonding interactions of Chol with dry lipids and the influence of sugars on such interactions have not been reported. Also, the effects of lipid unsaturation and of the presence of glycolipids have so far not been investigated. Here, we have used Fourier transform infrared spectroscopy (FTIR) to characterize the effects of Chol on the gel to liquid-crystalline phase transition of dry membranes containing different molecular species of PC or the plant glycolipid digalactosyldiacylglycerol (DGDG). In addition, we have analyzed the interactions of Chol and galactose (Gal) with different parts of PC headgroups separately and in combination, providing the first description of this complex interaction network to our knowledge.
MATERIALS AND METHODS
Materials
The PCs POPC (16:0/18:1, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine), DSPC (18:0/18:0, 1,2-distearoyl-sn-glycero-3-phosphatidylcholine), DOPC (18:1/18:1, 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine), and DLPC (18:3/18:3, dilinolenoyl-sn-glycero-3-phosphatidylcholine) were obtained from Avanti Polar Lipids (Alabaster, AL). The chloroplast glycolipid DGDG was purchased from Lipid Products (Redhill, Surrey, UK) and Gal and Chol from Sigma (Deisenhofen, Germany).
Sample preparation
Liposomes were composed of different mole fractions of PCs, DGDG, and Chol. Lipids (10 mg) dissolved in chloroform were mixed, and the solvent was evaporated under a stream of N2. To ensure complete removal of the solvent, the lipids were stored under vacuum overnight. The dry lipid film was hydrated in 200 μl of distilled water, or 22.2 mM Gal, corresponding to the amount of sugar present in liposomes containing 20% DGDG. Unilamellar liposomes were formed from the hydrated lipids using a hand-held extruder (Avestin, Ottawa, Canada) with two layers of 100-nm-pore filters (18). Liposome suspensions (50 μl) were spread on CaF2 windows and dried at 0% relative humidity in desiccators at 28°C for 24 h in the dark (19). Samples prepared in this way contain below 0.02 g H2O g−1 dry weight (20).
FTIR spectroscopy
A window with the dry sample was placed in a cuvette holder, fixed in a vacuum chamber connected to a temperature control unit (Specac Eurotherm, Worthington, UK), and placed in the infrared beam. Temperature was monitored by a fine thermocouple fixed on the window next to the sample. Samples were kept in the sample holder under vacuum at 30°C for 30 min to remove residual moisture absorbed during handling. This was verified by the absence of a water band in the FTIR spectra at 1650 cm−1. Temperature was then decreased to −30°C, and after 10 min equilibration the temperature was increased at a rate of 1°C min−1. Two spectra with 4 cm−1 resolution were recorded and coadded every minute with a Perkin Elmer (Foster City, CA) GX 2000 FTIR spectrometer. Spectra were analyzed using Spectrum 5.0.1 software. After normalization of absorbance and baseline correction, the wavenumber of the CH2 symmetric stretching vibration (νCH2s) around 2850 cm−1 and the P=O asymmetric stretching (νP=Oas) vibration (1300–1200 cm−1) were determined by the automatic peak identification routine. The gel to liquid-crystalline phase transition temperature (Tm) was determined as the midpoint of the shift in νCH2s with temperature (21). The carbonyl stretching peak (νC=O) in the infrared region between 1800 cm−1 and 1680 cm−1 was analyzed above the Tm of the respective lipids by peak deconvolution and curve fitting using the peak-fitting module of OriginPro 7.0 (OriginLab, Northampton, MA) as described in detail recently (20,22).
RESULTS
Influence of Chol on the physical properties of dry bilayers containing POPC or POPC/DGDG
Alterations in the physical state of the hydrophobic interior of dry liposomes due to the presence of Chol were studied by FTIR spectroscopy. The frequency of νCH2s of lipid hydrocarbon chains is related to the degree of conformational disorder of the chains, and an increase in temperature induces an increasing number of gauche conformers resulting in an upshift in band frequency during lipid melting (23).
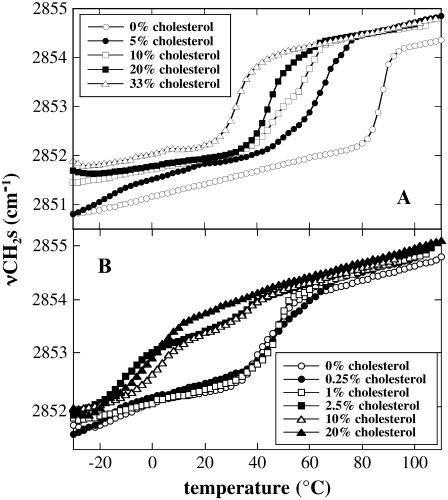
Fig. 1 shows the melting curves of dry liposomes composed of either pure POPC or 80% POPC/20% DGDG with the addition of different amounts of Chol. The ratio between the two diacyl lipids was held constant in all samples, so that the addition of Chol only changed the ratio between diacyl lipids and sterol. This leads to a lower fraction of diacyl lipids in the membranes with increasing sterol content. As an example, the composition of membranes containing 80% POPC/20% DGDG and 20% Chol was 64 mol % POPC/16 mol % DGDG/20 mol % Chol. For the sake of brevity and clarity, however, only the ratio of the diacyl lipids in the absence of Chol is subsequently shown, along with the appropriate Chol content.
FIGURE 1.
Lipid melting curves of dry liposomes prepared from POPC (A) or 80% POPC/20% DGDG (B). The temperature-dependent increase in the position of the symmetric CH2 stretching band (νCH2s) of fatty acyl chains was determined by FTIR spectroscopy. The diacyl lipids were mixed with different fractions of Chol as indicated.
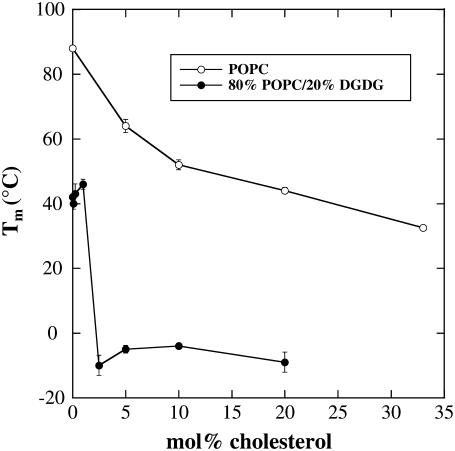
The transition temperature from the gel to the liquid-crystalline phase (Tm) can be determined as the midpoint of the lipid melting curves shown in Fig. 1. For pure POPC liposomes, Tm was 88°C and it decreased to 40°C in membranes containing 20% DGDG, in good agreement with our previous data (24). Addition of increasing amounts of Chol gradually decreased Tm in pure POPC liposomes (Fig. 1 A), reaching 33°C at 33 mol % Chol. Higher concentrations of Chol (43 and 50 mol %) did not lead to a further reduction in Tm (data not shown), probably due to the formation of transbilayer Chol dimers (25). It has been shown that Chol is soluble up to 67 mol % in fully hydrated bilayers of various saturated and monounsaturated PCs, forming Chol crystals at higher concentrations (26) and that at 25 mol % Chol all hydrocarbon chains are interacting with sterol molecules while sterol-sterol interactions are minimized (27). It seems reasonable to assume that Chol solubility could be significantly decreased in dry bilayers, but to the best of our knowledge no published data on Chol solubility in dry membranes are available. However, at 33 mol % there was no evidence of Chol crystals in dry DPPC samples (10).
Chol increases the motional freedom of the fatty acyl chains of lipids at temperatures below Tm and increases their order in the liquid-crystalline phase in fully hydrated membranes (2). Under these conditions, the Chol content of the membranes has only a small influence on Tm, but the difference between fatty acid mobility in the gel and liquid-crystalline phases is diminished. At sufficiently high Chol concentrations, no phase transition is found anymore (6,28). In the dry state, however, Chol reduced the Tm of POPC liposomes and the motional freedom of the lipid acyl chains was only moderately increased both in the gel and liquid-crystalline phases (Fig. 1 A).
A similar picture emerged when the effect of Chol on the phase behavior of liposomes containing 80% POPC/20% DGDG was studied (Fig. 1 B). Chol led to a strong decrease in Tm and to a slight increase in νCH2s in both the gel and liquid-crystalline state. The increase in νCH2s in the gel phase, however, was less pronounced in membranes containing POPC and DGDG than in membranes containing pure POPC, resulting in an approximately equal position of νCH2s in the gel phase of POPC and POPC/DGDG membranes containing a high fraction of Chol. It is also interesting to note that at intermediate Chol concentrations (2.5 and 10 mol %) evidence for a high and a low temperature transition could be found, whereas at 20 mol % Chol only one phase transition below 0°C was evident.
To better illustrate the effects of Chol on Tm of bilayers composed of pure POPC, or 80% POPC and 20% DGDG, Tm is plotted versus the mol fraction of Chol in Fig. 2. It is apparent that Chol decreased Tm gradually for POPC liposomes and stepwise for those containing DGDG. Up to 1 mol % Chol, Tm for 80% POPC/20% DGDG membranes was 40°C. In a narrow concentration range (1–2.5 mol %), Tm dropped to ∼ −10°C, with a second transition still in the temperature range of the pure diacyl lipids (Fig. 1 B). A further increase in Chol content had no influence on the low temperature transition but abolished the high temperature transition, indicating that all diacyl lipids interacted with Chol.
FIGURE 2.
Lipid phase transition temperature (Tm) of dry liposomes prepared from POPC or 80% POPC/20% DGDG as a function of the Chol content of the membranes. Tm was determined as the midpoint of the melting curves shown in Fig. 1. The error bars indicate mean ± SE from measurements on three samples. Where no error bars are visible, they are smaller than the symbols.
PCs can establish H-bonds with the OH group of Chol through their P=O and C=O groups (29). There are, however, only limited experimental data on such H-bonding interactions in fully hydrated membranes (see Ohvo-Reikila et al. (1) for a review) and, to the best of our knowledge, no published reports for dry membranes.
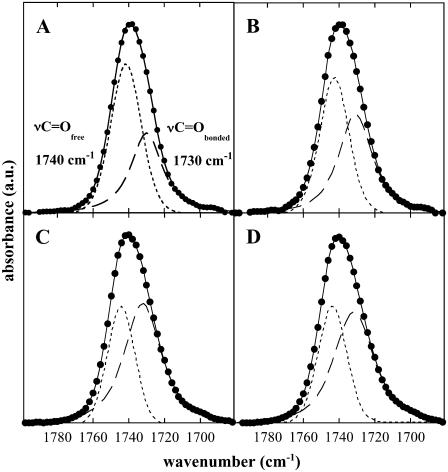
The C=O groups of diacyl lipids are localized at the polar-apolar interface of lipid bilayers and give rise to an infrared band in the spectral region of 1700–1800 cm−1. Under fully hydrated conditions the C=O peak is shifted downfield in comparison with the position in the dry state, indicating H-bonding interactions with water molecules (30–32). The νC=O band of diacyl lipids can be decomposed into two components, with the higher wavenumber band component (1740–1742 cm−1) assigned to the free C=O groups and the lower wavenumber component (∼1730 cm−1) attributed to the νC=O of conformers involved in H-bonds (33,34). These two conformer populations can be quantified by deconvolution of the νC=O peak (20,22). Assuming that the relative area of the bands after deconvolution is proportional to the respective conformer population (νC=Ofree and νC=Obonded), the ratio between the relative areas (AC=Obonded/AC=Ofree) can be used to estimate the H-bonding to the lipid C=O groups (20,22).
Fig. 3 shows the νC=O contours and the fitted bands from dry liposomes composed of pure POPC or 80% POPC/20% DGDG in the absence (Fig. 3, A and C) and in the presence (Fig. 3, B and D) of Chol. The band components of νC=Ofree and νC=Obonded change their relative area and intensity depending on the diacyl lipid and Chol composition.
FIGURE 3.
Infrared spectra in the carbonyl stretching region (νC=O) of dry POPC (A and B) or 80% POPC/20% DGDG (C and D) liposomes. Samples in A and C contained only the diacyl lipids, whereas samples in B and D contained 33 mol % and 20 mol % Chol, respectively. Spectra were recorded at 90°C. The peaks were deconvoluted and fitted into two band components designated as νC=Ofree at ∼1740 cm−1 (upfield peak, no H-bonding) and as νC=Obonded at ∼1730 cm−1 (lowfield peak, H-bonded). The curve of the original peak comprises both the measured and the fitted curves.
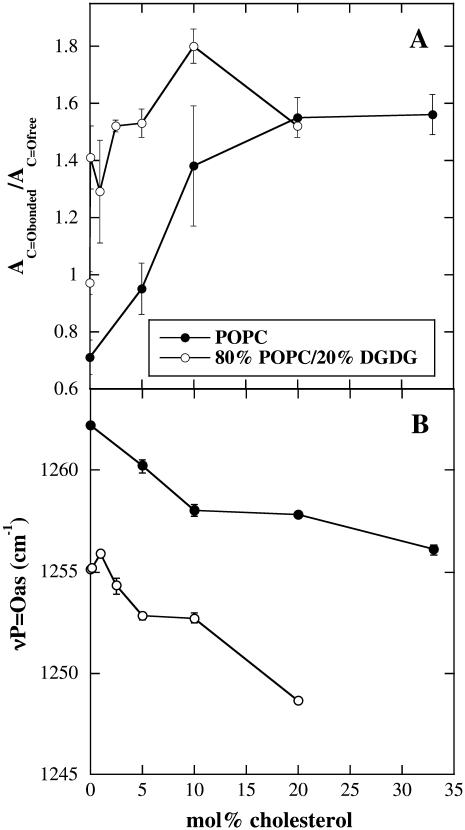
As reported previously for dry egg PC (20,22), there was also a population of H-bonded conformers in the C=O groups of dry POPC (Fig. 3 A). Since the water content in our samples was always very low (<0.02 g H2O/g dry weight; (22)), as indicated by the position of the νP=Oas peak (Fig. 4 B) at 1262 cm−1, we assume that this νC=Obonded population mainly derives from charge-pair interactions between C=O and choline groups (35). The ratio AC=Obonded/AC=Ofree for pure POPC liposomes was 0.71, indicating that most C=O groups were nonbonded. In liposomes containing 20% DGDG this ratio was higher (0.97; Fig. 3 C), indicating H-bonding between C=O groups and the galactolipid headgroups. This has been shown, albeit in a nonquantitative manner, in previous publications (30,36). The addition of high amounts of Chol resulted in a strong increase in the AC=Obonded/AC=Ofree ratio, to 1.56 in pure POPC membranes and to 1.52 in 80% POPC/20% DGDG membranes (Fig. 3, B and D, respectively).
FIGURE 4.
Effect of different mol fractions of Chol on (A) the ratio of the fitted peak areas of the two νC=O band components (AC=Obonded/AC=Ofree) described in Fig. 3 and on (B) the position of the νP=Oas vibration. In addition to the indicated fractions of Chol, liposomes contained either pure POPC or 80% POPC/20% DGDG as indicated. Spectra were obtained from dry liposomes at 90°C. The error bars indicate mean ± SE from measurements on three samples.
Increasing concentrations of Chol up to 20 mol % led to a gradual increase in the population of H-bonded C=O conformers in 100% POPC liposomes, whereas for membranes containing 80% POPC/20% DGDG, the AC=Obonded/AC=Ofree ratio was already increased from 0.97 to 1.43 at 0.25 mol % Chol (Fig. 4 A). A further increase in Chol content only had a minor influence on the population of H-bonded C=O conformers in the presence of DGDG.
The P=O group of phospholipids is another potential partner for H-bonding interactions. The position of the νP=Oas vibration is sensitive to H-bonding (32,37) and is shifted from ∼1260 cm−1 in dry PCs to 1225–1230 cm−1 in fully hydrated membranes (30,36). Similarly, the addition of soluble sugars or glycolipids leads to a downfield shift in νP=Oas in dry membranes, due to P=O⋯HO H-bonding (see Hincha et al. (38) for a recent review). The presence of 20% DGDG in POPC membranes also led to a downfield shift in νP=Oas peak position (Fig. 4 B), as shown before for egg PC/DGDG membranes (30). In both pure POPC and 80% POPC/20% DGDG membranes, increasing concentrations of Chol gradually shifted νP=Oas to lower wavenumbers (Fig. 4 B). Since the two curves run almost in parallel, it can be assumed that Chol and the sugars from the DGDG headgroups show an additive H-bonding behavior.
Distinguishing the effects of fatty acyl chain unsaturation from the effects of sugar headgroups
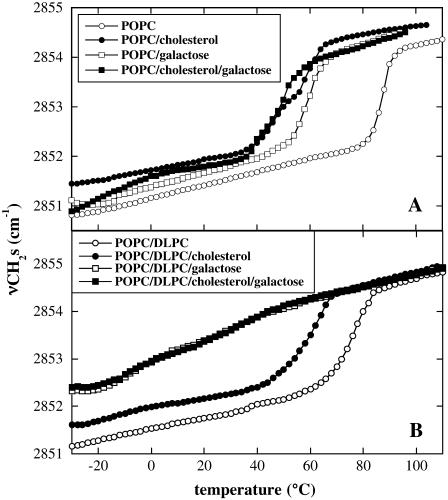
The combination of POPC, DGDG, and Chol resulted in a much lower Tm than the combination of POPC and Chol. In addition, in the presence of DGDG, Chol had a bigger effect on Tm than in pure POPC membranes (e.g., 10 mol % Chol-depressed Tm by 36°C in pure POPC membranes and by 50°C in 80% POPC/20% DGDG membranes (Figs. 1 and 2). Since DGDG is a highly unsaturated lipid that contains predominantly 18:3 fatty acyl chains (39,40), these differences may be due to the differences in unsaturation between POPC and DGDG or to the different headgroups. We have recently shown (24) that the thermotropic phase behavior of dry POPC liposomes containing 20% DGDG is mainly determined by the interactions between the headgroups of POPC and DGDG and only to a small degree by the unsaturation of the fatty acyl chains in DGDG. The effect of the DGDG headgroup could be mimicked by the addition of soluble galacose (24). Therefore, we compared the effects of Chol on Tm in dry membranes containing either POPC, or 80% POPC and 20% of the highly unsaturated (18:3/18:3) PC DLPC in the presence or absence of the amount of Gal corresponding to the amount of this sugar in the headgroups of membranes containing 20% DGDG (Fig. 5).
FIGURE 5.
Temperature dependence of νCH2s of dry liposomes prepared from POPC (A) or 80% POPC/20% DLPC (B). Where indicated, the membranes contained 10 mol % Chol in addition to the phospholipids. Liposomes were either prepared in water or in 22.2 mM Gal, corresponding to the amount of sugar present in liposomes containing 20% DGDG.
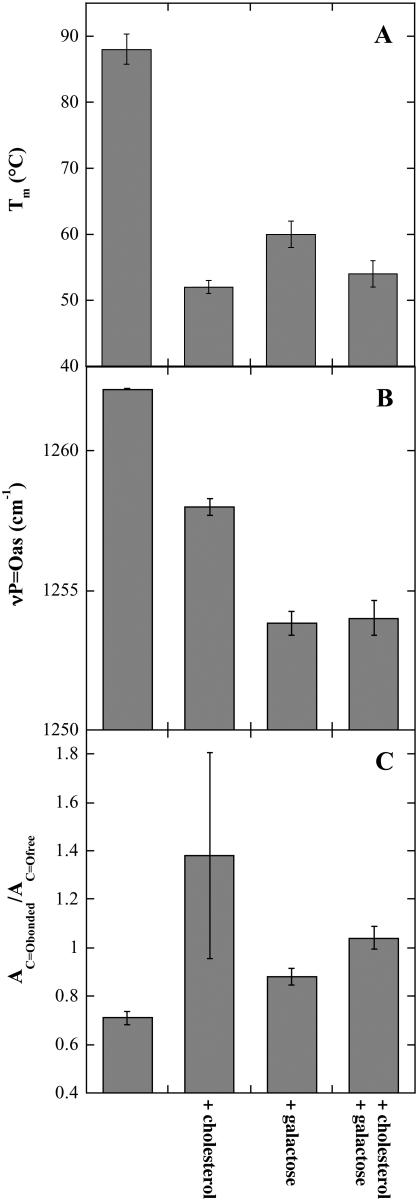
At this low concentration, Gal decreased Tm of dry POPC liposomes from 88°C to 60°C (Fig. 5 A), whereas 10 mol % Chol decreased Tm to 54°C. The combination of Chol and Gal resulted in the same phase behavior of POPC membranes as the addition of Chol alone (Fig. 5 A).
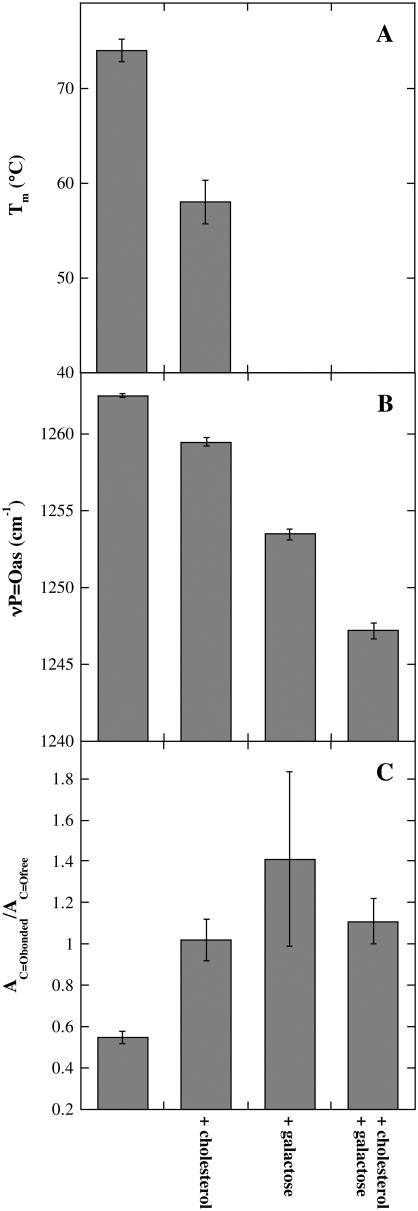
Addition of 20% DLPC to POPC decreased Tm of the bilayers to 74°C, and Chol induced an additional reduction to 58°C (Fig. 5 B). When Gal corresponding to 20% DGDG headgroups was added to either of these membranes, no clear phase transition could be determined anymore. This indicates that in liposomes containing DLPC, the effects of the sugar on Tm were much stronger than the effects of Chol.
An analysis of the H-bonding interactions between the phospholipids, Gal, and Chol is shown in Figs. 6 and 7, together with the Tm values for comparison. In POPC membranes (Fig. 6), νP=Oas was shifted downfield more strongly by Gal than by Chol and the presence of Chol had no influence on the effect of Gal. In membranes containing 80% POPC and 20% DLPC (Fig. 7), on the other hand, Chol only had a minor influence on the νP=Oas peak position, Gal had a bigger effect, and the two substances together showed an additive effect.
FIGURE 6.
Lipid phase transition temperature (Tm; A), the νP=Oas peak position (B), and AC=Obonded/AC=Ofree ratio (C) determined from dry POPC liposomes. νP=Oas and νC=O spectra were recorded at 90°C. The samples contained additional Chol and Gal as indicated (compare Fig. 5). The error bars indicate mean ± SE from measurements on three samples.
FIGURE 7.
Lipid phase transition temperature (Tm; A), the νP=Oas peak position (B), and AC=Obonded/AC=Ofree ratio (C) determined from dry liposomes composed of 80% POPC/20% DLPC. νP=Oas and νC=O spectra were recorded at 90°C. The samples contained additional Chol and Gal as indicated (compare Fig. 5). The error bars indicate mean ± SE from measurements on three samples.
The ratios of the fitted areas of the C=Obonded and C=Ofree conformers show that in POPC membranes the population of C=O groups involved in H-bonding in the presence of Chol was higher than in the presence of Gal, whereas in the presence of both additives the ratio was slightly higher than in the presence of only the sugar (Fig. 6). Again, POPC/DLPC membranes showed a different behavior, with Chol showing less H-bonding to the C=O groups than Gal (Fig. 7). In these membranes, AC=Obonded/AC=Ofree in the presence of Gal was independent of the presence of Chol.
The degree of fatty acyl chain unsaturation determines the effects of Chol and sugar on dry phosphatidylcholines
We used liposomes composed of the completely saturated phospholipid DSPC (18:0/18:0) and mixtures of DSPC with 20% of the unsaturated lipids DOPC (18:1/18:1) or DLPC (18:3/18:3) to systematically investigate the contribution of phospholipid unsaturation to the effect of Chol and Gal on lipid phase behavior in mixed membranes. Fig. 8 shows the corresponding melting curves of the dry liposomes. In addition, samples containing liposomes made from pure DOPC or 80% DOPC/20% DLPC are shown.
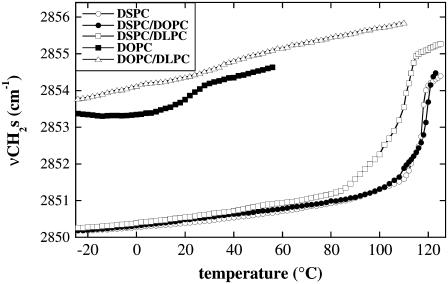
FIGURE 8.
Temperature-dependent increase in νCH2s of dry liposomes composed of the following: 100% DSPC, 80% DSPC/20% DOPC, 80% DSPC/20% DLPC, 100% DOPC, or 80% DOPC/20% DLPC.
For the fully saturated lipid (100% DSPC), Tm in the dry state was 125°C and addition of 20% DOPC, i.e., two double bonds per additional lipid molecule, did not alter the phase behavior of the membranes measurably. Including 20% of a lipid containing six additional double bonds (DLPC) led to a decrease of Tm to 108°C. νCH2s was increased in the liquid-crystalline state in these membranes compared to those composed of 100% DSPC or 80% DSPC/20% DOPC, whereas νCH2s in the gel phase was indistinguishable. For comparison, Fig. 8 also shows the melting curves of vesicles composed of pure DOPC or 80% DOPC/20% DLPC. It can be seen that the substitution of DSPC by DOPC led to dramatic changes in phase behavior and to a general increase in νCH2s, especially in the gel phase of pure DOPC.
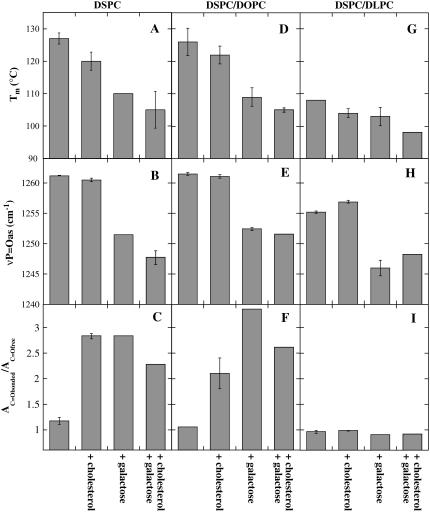
The effect of Chol (10 mol %) and externally added Gal on the phase behavior of liposomes containing PCs with different degrees of unsaturation is shown in Fig. 9 A for pure DSPC, in Fig. 9 D for 80% DSPC/20% DOPC, and in Fig. 9 G for 80% DSPC/20% DLPC. Every panel shows results from samples containing only PC liposomes or in addition Chol, Gal, or both additives. Both Chol and Gal decreased the Tm of all PC liposomes, and the combination of Chol and Gal showed an additional small decrease in Tm.
FIGURE 9.
Lipid phase transition temperature (Tm; A, D, and G), the νP=Oas peak position (B, E, and H), and AC=Obonded/AC=Ofree ratio (C, F, and I) determined from dry liposomes composed of the following: 100% DSPC, 80% DSPC/20% DOPC, or 80% DSPC/20% DLPC. νP=Oas and νC=O spectra were recorded at 140°C. The samples contained additional Chol and Gal as indicated (compare Fig. 5). The error bars indicate mean ± SE from measurements on three samples.
Fig. 9 also shows data concerning the H-bonding between the PCs and Chol or Gal, or a combination of both additives. The position of the νP=Oas vibration (Fig. 9, B, E, and H) was not significantly influenced by the presence of Chol, whereas the presence of Gal, alone or in combination with Chol, led to a clear downfield shift in νP=Oas.
Deconvolution of the νC=O peaks of the PCs revealed the degree of H-bonding between the C=O groups of the lipids and the additives through the calculation of the AC=Obonded/AC=Ofree ratio (Fig. 9, C, F, and I; compare Fig. 3). It can be seen that in both pure DSPC and in 80% DSPC/20% DOPC membranes, extensive H-bonding between the phospholipids and Chol and Gal took place. Although in the case of the mixed membranes, H-bonding was more extensive with the sugar than with Chol (Fig. 9 F), both were equally effective in the pure saturated lipid DSPC (Fig. 9 C). The samples containing a combination of sugar and sterol showed a lower degree of H-bonding to the lipid C=O groups than the samples containing only Gal. Quite strikingly, membranes containing 80% DSPC/20% DLPC showed only a comparatively small extent of H-bonding interactions between the PCs and either additive (Fig. 9 I). This is similar to the small effects seen in pure POPC membranes (Fig. 6; note the different scales of the axes in Figs. 6 and 9).
DISCUSSION
Sterols are an important part of most biological membranes (see Ohvo-Reikila et al. (1) and Yeagle (2) for reviews) and play an important role in the formation of liquid-ordered domains, or rafts (3–5). Although Chol is the predominant sterol in the membranes of higher animals, including humans, other organisms, such as plants, contain different sterols that vary in their structure and ability to induce a liquid-ordered phase. In particular, the major plant sterol stigmasterol has also been shown to induce raft formation in liposomes (41–44). Since DGDG is a predominant plant lipid that is not only present in chloroplast membranes but under some growth conditions also in the plasma membrane (45), we were interested in possible interactions between this glycolipid and sterols. Since we found that the effects of Chol and stigmasterol on the phase behavior of dry membranes composed of either POPC or 20% DGDG/80% POPC were indistinguishable (data not shown), we decided to use only Chol in all subsequent experiments, as this is the best investigated sterol so far and the only one for which data for membranes in the dry state have been published (see Introduction).
Effect of Chol on the phase behavior of dry bilayers
In fully hydrated DPPC, Chol only marginally reduces Tm, whereas the enthalpy of the transition is strongly decreased (6,13,28). On the other hand, in dry DPPC, Tm is progressively decreased as a function of Chol content (10). This indicates that Chol may have significantly different effects on dry and hydrated lipid bilayers, justifying a systematic study of the effects of Chol on dry membranes of different composition.
In dry POPC bilayers, increasing concentrations of Chol led to a gradual depression of Tm (Figs. 1 A and 2) as determined by FTIR spectroscopy, similar to the effect observed by DSC in dry DPPC (10). It should, however, be noted that the effect of Chol on Tm was much smaller in DPPC than in POPC liposomes in the dry state (13°C vs. 36°C at 9 or 10 mol % Chol, respectively; Table 1). In addition, FTIR indicated increased mobility of the fatty acyl chains in the gel as well as in the liquid-crystalline phase, in contrast to the fully hydrated state where mobility is increased in the gel and decreased in the liquid-crystalline phase (2).
TABLE 1.
Effects of lipid composition, Chol and Gal on the gel to liquid-crystalline phase transitions of dry membranes
| Lipid composition | ΔTm ± Chol (°C) | ΔTm ± Gal (°C) | ΔTm ± (Chol + Gal) (°C) |
|---|---|---|---|
| 100% POPC | 36 | 28 | 36 |
| 20% DGDG/ 80% POPC | 52 | n.d. | n.d. |
| 20% DLPC/ 80% POPC | 16 | n.t. | n.t. |
| 100% DSPC | 7 | 17 | 22 |
| 20% DOPC/ 80% DSPC | 4 | 17 | 21 |
| 20% DLPC/ 80% DSPC | 4 | 5 | 10 |
| 100% DPPC* | 13 | n.d. | n.d. |
ΔTm denotes the difference between the phase transition temperature of the pure diacyl lipid bilayers in the absence of additives and the same bilayers in the presence of either 10 mol % Chol (±Chol) or Gal corresponding to the sugar content in samples containing 20% DGDG (±Gal) or a combination of both additives (±(Chol + Gal)). n.d., not determined; n.t., no transition temperature determined in the presence of additives (compare Fig. 5 B).
Data from Ohtake et al. (10) for freeze-dried DPPC liposomes containing 9 mol % Chol.
The effect of Chol on the thermotropic phase behavior of dry liposomes containing 20% DGDG in 80% POPC was strikingly different from the effect on pure POPC (Figs. 1 and 2). The decrease in Tm was not gradual, as in pure POPC, but rather happened over a narrow concentration range (1–2.5 mol % Chol). In addition, Tm was decreased considerably more in the mixed membranes than in pure POPC liposomes (Table 1). These differences in the effect of Chol could be due either to the presence of the diGal headgroups of DGDG or to the higher degree of unsaturation of fatty acyl chains, as DGDG contains predominantly 18:3 fatty acids (39,40). To distinguish between these possibilities, we discuss first the influence of fatty acid unsaturation on the effects of Chol.
For the most direct comparison, we included 20% DLPC (di 18:3) into POPC liposomes. In these membranes, however, 10 mol % Chol decreased Tm only by 16°C (Table 1), or 20°C less than in pure POPC and 36°C less than in 20% DGDG/80% POPC membranes. The fact that increasing the average degree of unsaturation by adding 20% DLPC to POPC in a membrane reduced the influence of Chol on the thermotropic behavior of the dry liposomes seems to be in accord with the behavior of fully hydrated membranes, where increased unsaturation also reduces the effects of Chol. On the other hand, published data for fully saturated DPPC (10) indicate that here the effect of Chol on the dry bilayer is even less pronounced (Table 1).
Since in this case freeze-dried liposomes were investigated by DSC whereas air-dried liposomes were measured by FTIR, these technical differences may be responsible for this apparent contradiction. However, our data on another fully saturated lipid (DSPC) corroborate the finding by Ohtake et al. (10). In pure dry DSPC, 10 mol % Chol only reduced Tm by 7°C (Table 1), indicating that in the dry state, in contrast to the fully hydrated state, saturated lipids are less affected by the presence of Chol than a monounsaturated lipid. This small effect of Chol on DSPC membranes is further reduced by the addition of diunsaturated lipids (20% DOPC or DLPC), similar to the situation in POPC after addition of DLPC (Table 1).
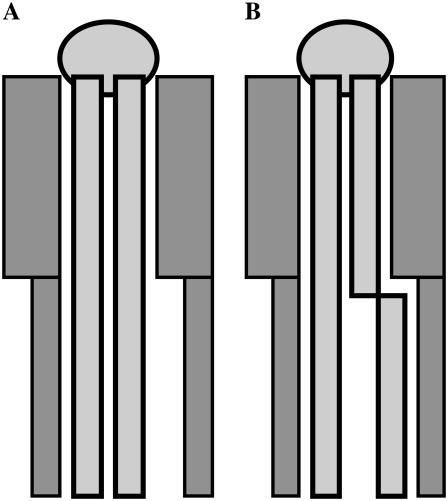
This outstanding effect of Chol on dry POPC bilayers may be interpreted following the structural arguments proposed by Huang (46). He suggested that lipids containing one saturated and one unsaturated fatty acyl chain could interact through the fully saturated straight chain with the planar α-side of Chol, whereas the kink in the monounsaturated chain would predispose it to interact with the β-side due to the two protruding methyl groups. We suggest that the strong effect of Chol on Tm in POPC can be explained following the same logic. In the gel phase, when both fatty acyl chains of POPC are straight, free space is created by the presence of Chol (Fig. 10 A), which can be filled easily by trans-gauche transitions in the unsaturated fatty acyl chain (Fig. 10 B), resulting in a strongly reduced Tm. This hypothesis is in agreement with recent neutron scattering and molecular dynamics simulation results showing that the lower part of phospholipid fatty acyl chains shows increased mobility at temperatures significantly below Tm (47).
FIGURE 10.
Schematic representation of the packing of Chol and POPC molecules in dry membranes. In the gel phase (A) POPC (light shaded) and Chol (dark shaded) pack only loosely, because the β-side of Chol has two protruding methyl groups in the upper part of the molecule. The resulting free space in the hydrophobic region of the bilayer is easily filled by trans-gauche isomerization at the point of the double bond in the monounsaturated fatty acyl chain (B).
Although the data discussed above clearly show that unsaturation has a strong influence on the effect of Chol on the phase behavior of dry membranes, it is also obvious that the difference in unsaturation is not responsible for the large difference between the Chol effects in pure POPC and DGDG/POPC mixed membranes. We therefore investigated the possible contribution of the sugar headgroup of DGDG by adding Gal at a concentration that corresponds to 20% DGDG to samples containing pure PC liposomes. As noted previously (24), this low concentration of Gal had a moderate influence on Tm in POPC membranes but a much stronger effect in membranes containing 20% DLPC and 80% POPC (Fig. 5; Table 1). The effect of Gal was considerably smaller in DSPC membranes, independent of the addition of unsaturated lipids (Fig. 9; Table 1). Since these membranes are in the gel state during drying, their tight packing prohibits interaction of the sugar with the headgroups and the resulting depression of Tm, which is observed in more unsaturated lipids (48).
When Gal was added to liposomes containing 10 mol % Chol, the strongest effect was observed in samples composed of 20% DLPC/80% POPC, mimicking the situation in membranes containing 20% DGDG/80% POPC (Table 1). In these samples, no clear phase transition was detectable anymore (compare Fig. 5 B), indicating that it was the particular combination of fatty acid unsaturation and Gal headgroup that resulted in the strong effect of Chol on DGDG-containing POPC membranes. Interestingly, in DSPC-based membranes the effects of Chol and Gal were strictly additive but always lower than in POPC or DLPC/POPC membranes (Table 1).
The contribution of H-bonding interactions to the effects of Chol on the phase behavior of dry membranes
Chol is located in the bilayer parallel to the acyl chains with its OH group in the interfacial region (see Yeagle (2) for a review), mediating its H-bonding with water (in hydrated membranes) and with the carbonyl and phosphate groups of phospholipids (29). Integration of Chol between the acyl chains of dry POPC liposomes resulted in a concentration-dependent decrease in the position of νP=Oas (Fig. 4 B) due to H-bonding of Chol to the P=O group. In parallel, the population of C=O conformers involved in H-bonding to Chol was also increased (Figs. 3 and 4 A). These results indicate that Chol can interact via H-bonding with POPC in dry liposomes.
In dry 20% DGDG/80% POPC liposomes, there was already extensive H-bonding between the P=O and C=O groups of the lipids and the OH moieties of the diGal headgroups of DGDG (Fig. 4; (30)). With increasing Chol concentrations more H-bonds were formed with the POPC P=O groups (Fig. 4 B), in addition to the H-bonds between DGDG and POPC. The population of C=O groups involved in interactions with the OH groups of Chol also increased with increasing Chol concentrations (Fig. 4 A), but AC=Obonded/AC=Ofree was identical in POPC and DGDG/POPC membranes at high Chol concentrations, indicating that the OH groups from Chol replaced the OH groups from DGDG diGal at the C=O sites.
Analysis of the interactions of Chol and Gal with the C=O and P=O groups of all investigated PC bilayers showed that the degree of H-bonding to P=O groups was higher for Gal than for Chol, whereas more H-bonding occurred between the C=O groups and Chol than Gal (Figs. 6, 7, and 9). Since Chol is expected to be situated deeper in the membrane than the externally added Gal, this finding is in accordance with the relative positioning of the two molecules. It is also in agreement with molecular dynamics simulations of fully hydrated PC bilayers, showing more interactions of Chol with C=O than P=O groups (15) and preferential interactions of Chol with C=O and trehalose with P=O groups (17). Also, in the dry state, sucose and various oligosaccharides interact more strongly with P=O than C=O groups of egg PC (20,22). Obviously, in the dry state more interactions of Chol with P=O and of sugars with C=O are possible than in the fully hydrated state, as no competition with water molecules for H-bonding sites takes place, explaining the stronger H-bonding we found in the dry state compared to the molecular dynamics simulations performed under fully hydrated conditions.
From Fig. 9 it can be seen that the addition of diunsaturated lipids to DSPC bilayers decreased the interactions of Chol with the C=O and increased the interactions of Gal with the P=O groups in the liquid-crystalline phase. We interpret this as the effect of increased spacing between the lipids due to fatty acyl chain unsaturation. This would move the C=O groups farther apart and could therefore reduce binding to Chol OH groups. On the other hand, it has been shown previously that increased lipid spacing increases the interactions of various sugars with dry lipid bilayers, thereby enhancing H-bonding to the P=O groups (see Hincha et al. (38) for a review).
The relative importance of H-bonding interactions for the depression of Tm by Chol can be estimated by comparing the differences in Tm and AC=Obonded/AC=Ofree in all PC membranes in the absence and presence of Chol (Figs. 6, 7, and 9). This comparison strongly suggests that H-bonding is only of minor importance for the effect of Chol on the phase behavior of dry membranes. For instance, in dry POPC membranes, addition of 10 mol % Chol depressed Tm by 36°C and increased AC=Obonded/AC=Ofree by 0.67, whereas in dry DSPC Tm was depressed by 7°C but AC=Obonded/AC=Ofree increased by 1.66. We conclude from these data that hydrophobic interactions and packing constraints in the fatty acyl chain region of the bilayers dominate the effects of Chol on the phase behavior of dry membranes.
Acknowledgments
We thank Anja Braig for her excellent help with the FTIR experiments.
We thank the Deutsche Forschungsgemeinschaft and the Max-Planck-Society for financial support.
Antoaneta V. Popova's permanent address is Institute of Biophysics, Bulgarian Academy of Sciences, 1113 Sofia, Bulgaria.
Editor: Antoinette Killian.
References
- 1.Ohvo-Reikila, H., B. Ramstedt, P. Leppimaki, and J. P. Slotte. 2002. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41:66–97. [DOI] [PubMed] [Google Scholar]
- 2.Yeagle, P. L. 1985. Cholesterol and cell membranes. Biochim. Biophys. Acta. 822:267–287. [DOI] [PubMed] [Google Scholar]
- 3.Barenholz, Y. 2002. Cholesterol and other membrane active sterols: from membrane evolution to “rafts”. Prog. Lipid Res. 41:1–5. [DOI] [PubMed] [Google Scholar]
- 4.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136. [DOI] [PubMed] [Google Scholar]
- 5.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- 6.Vist, M. R., and J. H. Davis. 1990. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 29:451–464. [DOI] [PubMed] [Google Scholar]
- 7.Brzustowicz, M. R., W. Stillwell, and S. R. Wassall. 1999. Molecular organization of cholesterol in polyunsaturated phospholipid membranes: a solid state 2H NMR investigation. FEBS Lett. 451:197–202. [DOI] [PubMed] [Google Scholar]
- 8.Davis, P. J., and K. M. W. Keough. 1983. Differential scanning calorimetric studies of aqueous dispersions of mixtures of cholesterol with some mixed-acid and single-acid phosphatidylcholines. Biochemistry. 22:6334–6340. [Google Scholar]
- 9.Kariel, N., E. Davidson, and K. M. W. Keough. 1991. Cholesterol does not remove the gel-liquid crystalline phase transition of phosphatidylcholines containing two polyenoic acyl chains. Biochim. Biophys. Acta. 1062:70–76. [DOI] [PubMed] [Google Scholar]
- 10.Ohtake, S., C. Schebor, S. P. Palecek, and J. J. de Pablo. 2005. Phase behavior of freeze-dried phospholipid-cholesterol mixtures stabilized with trehalose. Biochim. Biophys. Acta. 1713:57–64. [DOI] [PubMed] [Google Scholar]
- 11.Harrigan, P. R., T. D. Madden, and P. R. Cullis. 1990. Protection of liposomes during dehydration or freezing. Chem. Phys. Lipids. 52:139–149. [DOI] [PubMed] [Google Scholar]
- 12.van Winden, E. C. A., and D. J. A. Crommelin. 1999. Short term stability of freeze-dried, lyoprotected liposomes. J. Controlled Release. 58:69–86. [DOI] [PubMed] [Google Scholar]
- 13.Ohtake, S., C. Schebor, and J. J. de Pablo. 2006. Effects of trehalose on the phase behavior of DPPC-cholesterol unilamellar vesicles. Biochim. Biophys. Acta. 1758:65–73. [DOI] [PubMed] [Google Scholar]
- 14.Yeagle, P. L., W. C. Hutton, C.-H. Huang, and R. B. Martin. 1977. Phospholipid head-group conformations; intermolecular interactions and cholesterol effects. Biochemistry. 16:4344–4349. [DOI] [PubMed] [Google Scholar]
- 15.Pasenkiewicz-Gierula, M., T. Rog, K. Kitamura, and A. Kusumi. 2000. Cholesterol effects on the phosphatidylcholine bilayer polar region: a molecular dynamics study. Biophys. J. 78:1376–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeagle, P. L., W. C. Hutton, C.-H. Huang, and R. B. Martin. 1975. Headgroup conformation and lipid-cholesterol association in phosphatidylcholine vesicles: a 31P(1H) nuclear Overhauser effect study. Proc. Natl. Acad. Sci. USA. 72:3477–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doxastakis, M., A. K. Sum, and J. J. de Pablo. 2005. Modulating membrane properties: the effect of trehalose and cholesterol on a phospholipid bilayer. J. Phys. Chem. B. 109:24173–24181. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald, R. C., R. I. MacDonald, B. P. M. Menco, K. Takeshita, N. K. Subbarao, and L. Hu. 1991. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta. 1061:297–303. [DOI] [PubMed] [Google Scholar]
- 19.Hincha, D. K., E. Zuther, E. M. Hellwege, and A. G. Heyer. 2002. Specific effects of fructo- and gluco-oligosaccharides in the preservation of liposomes during drying. Glycobiology. 12:103–110. [DOI] [PubMed] [Google Scholar]
- 20.Cacela, C., and D. K. Hincha. 2006. Low amounts of sucrose are sufficient to depress the phase transition temperature of dry phosphatidylcholine, but not for lyoprotection of liposomes. Biophys. J. 90:2831–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowe, J. H., A. E. Oliver, F. A. Hoekstra, and L. M. Crowe. 1997. Stabilization of dry membranes by mixtures of hydroxyethyl starch and glucose: the role of vitrification. Cryobiology. 35:20–30. [DOI] [PubMed] [Google Scholar]
- 22.Cacela, C., and D. K. Hincha. 2006. Monosaccharide composition, chain length and linkage type influence the interactions of oligosaccharides with dry phosphatidylcholine membranes. Biochim. Biophys. Acta. 1758:680–691. [DOI] [PubMed] [Google Scholar]
- 23.Mantsch, H. H., and R. N. McElhaney. 1991. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipids. 57:213–226. [DOI] [PubMed] [Google Scholar]
- 24.Popova, A. V., and D. K. Hincha. 2005. Effects of the sugar headgroup of a glycoglycerolipid on the phase behavior of phospholipid model membranes in the dry state. Glycobiology. 15:1150–1155. [DOI] [PubMed] [Google Scholar]
- 25.Harris, J. S., D. E. Epps, S. R. Davio, and F. J. Kezdy. 1995. Evidence for transbilayer, tail-to-tail cholesterol dimers in dipalmitoylglycerophosphocholine liposomes. Biochemistry. 32:516–522. [DOI] [PubMed] [Google Scholar]
- 26.Huang, J., J. T. Buboltz, and G. W. Feigenson. 1999. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim. Biophys. Acta. 1417:89–100. [DOI] [PubMed] [Google Scholar]
- 27.McMullen, T. P. W., R. N. A. H. Lewis, and R. N. McElhaney. 1994. Comparative differential scanning calorimetric and FTIR and 31P-NMR spectroscopic studies of the effects of cholesterol and androstenol on the thermotropic phase behavior and organization of phosphatidylcholine bilayers. Biophys. J. 66:741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMullen, T. P. W., R. N. A. H. Lewis, and R. N. McElhaney. 1993. Differential scanning calorimetric study of the effects of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 32:516–522. [DOI] [PubMed] [Google Scholar]
- 29.Boggs, J. M. 1987. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim. Biophys. Acta. 906:353–404. [DOI] [PubMed] [Google Scholar]
- 30.Popova, A. V., and D. K. Hincha. 2003. Intermolecular interactions in dry and rehydrated pure and mixed bilayers of phosphatidylcholine and digalactosyldiacylglycerol: a Fourier-transform infrared spectroscopy study. Biophys. J. 85:1682–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selle, C., and W. Pohle. 1998. Fourier transform infrared spectroscopy as a probe for the study of the hydration of lipid self-assemblies. II. Water binding versus phase transitions. Biospectroscopy. 4:281–294. [DOI] [PubMed] [Google Scholar]
- 32.Wong, P. T. T., and H. H. Mantsch. 1988. High-pressure infrared spectroscopic evidence of water binding sites in 1,2-diacyl phospholipids. Chem. Phys. Lipids. 46:213–224. [Google Scholar]
- 33.Arrondo, J. L. R., and F. M. Goni. 1998. Infrared studies of protein-induced perturbations of lipids in lipoproteins and membranes. Chem. Phys. Lipids. 96:53–68. [DOI] [PubMed] [Google Scholar]
- 34.Blume, A., W. Hübner, and G. Messner. 1988. Fourier transform infrared spectroscopy of 13C=O-labeled phospholipids. Hydrogen bonding to carbonyl groups. Biochemistry. 27:8239–8249. [DOI] [PubMed] [Google Scholar]
- 35.Pasenkiewicz-Gierula, M., Y. Takaoka, H. Miyagawa, K. Kitamura, and A. Kusumi. 1999. Charge pairing of headgroups in phosphatidylcholine membranes: a molecular dynamics simulation study. Biophys. J. 76:1228–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popova, A. V., and D. K. Hincha. 2004. Specific interactions of tryptophan with phosphatidylcholine and digalactosyldiacylglycerol in pure and mixed bilayers in the dry and hydrated state. Chem. Phys. Lipids. 132:171–184. [DOI] [PubMed] [Google Scholar]
- 37.Lewis, R. N. A. H., and R. N. McElhaney. 1998. The structure and organization of phospholipid bilayers as revealed by infrared spectroscopy. Chem. Phys. Lipids. 96:9–21. [Google Scholar]
- 38.Hincha, D. K., A. V. Popova, and C. Cacela. 2006. Effects of sugars on the stability of lipid membranes during drying. In Advances in Planar Lipid Bilayers and Liposomes. A. Leitmannova Liu, editor. Elsevier, Amsterdam. 189–217.
- 39.Klaus, D., H. Härtel, L. M. Fitzpatrick, J. E. Froehlich, J. Hubert, C. Benning, and P. Dörmann. 2002. Digalactosyldiacylglycerol synthesis in chloroplasts of the Arabidopsis dgd1 mutant. Plant Physiol. 128:885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn, P. J., and W. P. Williams. 1983. The structural role of lipids in photosynthetic membranes. Biochim. Biophys. Acta. 737:223–266. [Google Scholar]
- 41.Hallig, K. K., and J. P. Slotte. 2004. Membrane properties of plant sterols in phospholipid bilayers as determined by differential scanning calorimetry, resonance energy transfer and detergent-induced solubilization. Biochim. Biophys. Acta. 1664:161–171. [DOI] [PubMed] [Google Scholar]
- 42.Shahedi, V., G. Oradd, and G. Lindblom. 2006. Domain-formation in DOPC/SM bilayers studied by pfg-NMR: effect of sterol structure. Biophys. J. 91:2501–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu, R., L. Chen, Z. Yu, and P. J. Quinn. 2006. Phase diagram of stigmasterol-dipalmitoylphosphatidylcholine mixtures dispersed in excess water. Biochim. Biophys. Acta. 1758:764–771. [DOI] [PubMed] [Google Scholar]
- 44.Xu, X., R. Bittman, G. Duportail, D. Heissler, C. Vilcheze, and E. London. 2001. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). J. Biol. Chem. 276:33540–33546. [DOI] [PubMed] [Google Scholar]
- 45.Andersson, M. X., M. H. Stridh, K. E. Larsson, C. Liljenberg, and A. S. Sandelius. 2003. Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett. 537:128–132. [DOI] [PubMed] [Google Scholar]
- 46.Huang, C.-H. 1977. A structural model for the cholesterol-phosphatidylcholine complexes in bilayer membranes. Lipids. 12:348–356. [DOI] [PubMed] [Google Scholar]
- 47.Doxastakis, M., V. Garcia Sakai, S. Ohtake, J. K. Maranas, and J. J. de Pablo. 2007. A molecular view of melting in anhydrous phospholipidic membranes. Biophys. J. 92:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crowe, J. H., F. A. Hoekstra, K. H. N. Nguyen, and L. M. Crowe. 1996. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim. Biophys. Acta. 1280:187–196. [DOI] [PubMed] [Google Scholar]