Abstract
K+ ions seemingly permeate K-channels rapidly because channel binding sites mimic coordination of K+ ions in water. Highly selective ion discrimination should occur when binding sites form rigid cavities that match K+, but not the smaller Na+, ion size or when binding sites are composed of specific chemical groups. Although conceptually attractive, these views cannot account for critical observations: 1), K+ hydration structures differ markedly from channel binding sites; 2), channel thermal fluctuations can obscure sub-Ångström differences in ion sizes; and 3), chemically identical binding sites can exhibit diverse ion selectivities. Our quantum mechanical studies lead to a novel paradigm that reconciles these observations. We find that K-channels utilize a “phase-activated” mechanism where the local environment around the binding sites is tuned to sustain high coordination numbers (>6) around K+ ions, which otherwise are rarely observed in liquid water. When combined with the field strength of carbonyl ligands, such high coordinations create the electrical scenario necessary for rapid and selective K+ partitioning. Specific perturbations to the local binding site environment with respect to strongly selective K-channels result in altered K+/Na+ selectivities.
INTRODUCTION
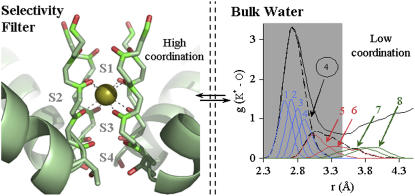
Potassium (K-)channels catalyze fast K+ ion transport across cellular membranes while simultaneously discriminating against Na+ ion permeation by more than a factor of 1000 (1). Structural studies (2–5) show bare K+ ions occupying binding sites in the narrowest channel regions in a state of high coordination by all eight surrounding oxygen ligands from the channel walls (Fig. 1).
FIGURE 1.
K+ ion coordination in selectivity filters of K-channels is markedly different from that in bulk water. KcsA, for example, offers three binding sites for K+ ions (2), S1–S3, where it coordinates with the ion using eight of its backbone carbonyl oxygen atoms (highlighted in red) at an average distance of 2.8 Å. In contrast, in a 40-ps-long AIMD (PW91) simulation of K+ ion in bulk water (generated by extending the previously reported (8) 14-ps-long trajectory), only four water molecules are seen most tightly bound to the K+ ion at an average distance of 2.8 Å. These four water molecules correspond to the maximum number that contribute to the principle maxima of the radial distribution function (g(r)); the fifth and the sixth nearest waters statistically do not contribute to the peak of the principal maxima; whereas the seventh and the eighth nearest waters are seldom seen within the canonical inner coordination shell (gray area) of the ion.
Equilibrium thermodynamics dictates that the partition coefficient of an ion between liquid water and the channel depends on the difference between the solvation free energies provided by these two environments. Transfer of an ion from water to the channel is favorable when its solvation free energy in the channel is lower than its value in the aqueous phase (aq),
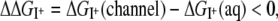 |
(1) |
where 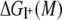 is the free energy change of the ion I+ in solvation phase M relative to the gas phase. Selective partitioning of a K+ ion into the channel is favorable when the free energy change for partitioning from water to the channel,
is the free energy change of the ion I+ in solvation phase M relative to the gas phase. Selective partitioning of a K+ ion into the channel is favorable when the free energy change for partitioning from water to the channel, 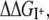 is lower for a K+ relative to a Na+ ion,
is lower for a K+ relative to a Na+ ion,
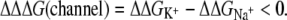 |
(2) |
In the common view of this mechanism, which emerged from a series of seminal investigations (1), fast, selective K+ ion transport in K-channels arises by two categories of “fit”. To assure rapid transport, the channel forms an environment like water for K+ ions, producing a fit in terms of both local hydration structure and hydration energy. To assure discrimination between ions, the channel maintains rigid binding sites with cavity sizes that precisely fit K+, but not the slightly smaller Na+ ions (size difference ∼0.4 Å), thus energetically destabilizing Na+ ions in the channel relative to water. Although attractive due to their conceptual simplicity and remarkably insightful in their time, today these explanations appear insufficient in light of new experimental and theoretical advances and thus undermine initiatives to engineer biomimetic channels and design new drug therapies.
The long-standing hypothesis (2,6,7) that K+ ions permeate K-channels rapidly because the binding sites mimic the coordination of K+ ions in water can be attributed in part to an earlier lack of consensus regarding ion hydration structures. A compelling body of evidence (8) now exists showing both experimentally and theoretically that smaller numbers (<8) and different arrangements of ligands coordinate K+ ions in liquid water and, more specifically, that eight water molecules seldom simultaneously coordinate a K+ ion (Fig. 1). Consequently, a new mechanism is needed to explain how K-channel binding sites match K+ hydration energies despite using higher numbers of coordinating ligands than found near K+ in liquid water.
The mechanistic view that strong ion discrimination results from a rigid cavity size that exactly fits the permeant K+ ion in K-channels has been challenged recently (9), where the authors argue that ion channel binding sites thermally fluctuate at Ångström length scales, which obscures sub-Ångström size differences between Na+ and K+ ions. Using classical force fields and molecular simulation strategies, these authors instead emphasize that chemistry, specifically the intrinsic electrostatic properties of the fluctuating carbonyl ligands in the channel, controls ion selectivity, a mechanism in line with an older ligand field strength model of ion selectivity (10,11).
Although this modified idea of ion discrimination is attractive because it allows for thermal fluctuations in the channel, it still leaves several critical observations unexplained: a), the computed selectivity (9) is not uniform across the chemically identical binding sites S1–S3 in the wild-type KcsA channel; b), recent structural studies (12) reveal that binding sites in NaK channels are composed of the same eight carbonyl ligands present in highly selective K-channels, and yet this identical ligand chemistry only weakly discriminates between Na+ and K+ ions; and c), perturbations of the proximal binding site environment with respect to strongly selective K-channels, which specifically introduce nearby hydrogen-bond (H-bond) donor groups, lead to diminished ionic selectivity as experimentally observed in the context of mutated G-protein regulated inwardly rectifying potassium (GIRK) channels (13,14), and as can be inferred from a comprehensive sequence alignment of weakly selective wild-type K-channels (15).
To resolve these issues, we apply quantum chemical methods within the framework of a statistical molecular association theory and interrogate numerous ion-binding reactions. We vary ion coordination architectures and ligand chemistries in different physical solvation environments until we arrive at a specific physiologically justified combination that reproduces both structural and thermodynamic data for selective ion partitioning into the binding sites of K-channels. Our selection and rejection criteria for different ion-binding scenarios are guided by our objective to answer three questions about K-channel mechanism. First, how and why should ion coordination numbers be different in liquid water and in K-channels, and for that matter, why should eight ligands coordinate with K+ ions and not any other “magic” number? Second, how do K+ ions permeate the channel at high diffusion rates when the energetic probability that a K+ ion forms eightfold complexes in water is negligible? And third, how do these new ideas about structural transitions in ion coordination simultaneously account for the fact that chemically identical binding sites give rise to strikingly different degrees of K+/Na+ selectivity?
Our studies lead us to a novel channel-centric paradigm for the mechanism of K+/Na+ selectivity that reconciles the critical observations neglected previously. We find that the environment surrounding the channel binding site and the binding-site coordination structure are two key components for selective ion partitioning. For K-channels, the binding-site environment is distinctly not a liquid environment and is tuned specifically to allow for local structural shifts in ion coordination architectures that ultimately enable it to differentiate energetically between Na+ and K+ ions. Perturbations of the binding-site environment with respect to strongly selective K-channels, which specifically introduce nearby H-bond donor groups, can help distort ion binding-site coordination structures and consequently lead to altered ion selectivity. We refer to this behavior as a “phase-activated” mechanism of selective ion partitioning. Although the overall mechanism of ion permeation (as opposed to partitioning) may involve kinetic effects and will require treatment of interactions between individual ion binding sites, it is important to note that interactions between binding sites can only be understood after the selective partitioning mechanisms of individual binding sites are fully elucidated.
METHODS
To understand selective ion partitioning in K-channels, we investigate the thermodynamics of coordination reactions that occur in water and in the binding sites of K-channels
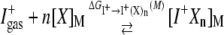 |
(3) |
for K+ and Na+ ions (I+) as a function of the number (n) and variety (X) of the coordinating ligands, concurrently with changes in the environment (M) external to a coordination complex (I+Xn). Such calculations are statistically permissible under a quasichemical organization of solution theory (16–19) as its mathematical construct formulates an ion's excess chemical potential as a sum of the independent free energies ΔG (or equilibrium constants) for reactions involving different numbers of ligands. To assess the free energies, the region around an ion is divided into inner- and outer-shell domains (see Supplementary Material for details). We define the inner-shell domain as the region containing the tighter subset of ligands directly coordinated to the ion, as opposed to more distant ligands distributed broadly within, or occupying regions beyond, the first peak of measured radial distribution functions. Since local interactions are most significant in differentiating the behavior of various ions coordinating with the same ligands, we treat the inner-shell interactions quantum mechanically using density functional theory (DFT) with the B3LYP functional. Less significant interactions with the environment outside the inner coordination shell are treated using an implicit solvent model. On multiple occasions ((8,19) see references therein) this methodology has reproduced simultaneously both the structural and the energetic properties of ion hydration.
RESULTS
The point of departure for this work derives from recent results (S. Varma and S. B. Rempe, unpublished) on ions interacting with water ligands in high and low dielectric environments. The first set of coordination reactions of interest were computed in liquid water, the reference phase for the partitioning of ions into K-channels. Despite their size difference, both K+ and Na+ ions prefer thermodynamically to coordinate directly with exactly four water hydroxyl ligands, as also statistically observed in separate ab initio molecular dynamics (AIMD) simulations (8,21–24) (see also Fig. 1). More importantly, the thermodynamic probability for these ions to coordinate simultaneously with eight hydroxyl oxygens in liquid water is negligible. Yet crystallographic data (2–5) show that K-channels utilize eightfold carbonyl-ligand geometries to partition K+ ions for fast transport, which implies that factors present in K-channels alter the structural and/or energetic properties of the ions relative to water. Viewing the problem from a different perspective, we might ask what is so special about liquid water that limits formation of higher-order ion coordination complexes.
To test whether the chemical properties of carbonyl oxygen ligands in a liquid environment result in this coordination anomaly, we determine the coordination structures and solvation free energies of both ions in liquid formamide (NH2CHO). Surprisingly, neither structural nor energetic properties of either ion changes appreciably in liquid phases even though stronger electrostatic field strengths (dipole moments) characterize the formamide carbonyl ligands compared to water hydroxyl ligands (see Supplementary Material).
Tests on the effect of the environment lead to the first clues of a new K-channel mechanism. We created an environment that contrasts with the high dielectric liquid phases described above by setting the dielectric constant of the environment to its lowest value of unity (ɛ = 1). Revisiting ion coordination reactions with water ligands, we discovered that coordination preferences are not fixed intrinsic properties of an ion. In environments characterized by a range of low dielectric constant values (ɛ < 3), both ions in fact prefer higher coordination numbers: K+ ions now favor eight water ligands, and Na+ ions six. The underlying physical mechanism that drives up ion coordination preferences, as described in detail elsewhere (S. Varma and S. B. Rempe, unpublished), is the decreased electrostatic penalty associated with extracting ligands from their low dielectric solvation phases. Conversely, in high dielectric aqueous phase, ion coordination complex formation requires overcoming these electrostatic penalties (∼8 kcal/mol for each water molecule), which increase linearly with coordination number and eventually result in substantially lowering the thermodynamic probability for formation of higher-order coordination complexes. Therefore, in the case of K-channels, if the electrostatic penalty associated with extracting carbonyl-oxygen ligands were reduced by some mechanism, then the thermodynamic probability for eightfold complex formation could be increased to values that do not obstruct K+ ion partitioning.
Even though a low macroscopic dielectric value is an unlikely descriptor of an ion channel protein, an alternative mechanism for reducing electrostatic interactions between binding-site ligands and their environment presents itself upon recognition of the local nature of polar ligand (dipole) solvation in dielectric phases (S. Varma and S. B. Rempe, unpublished; 25). Polar ligands receive the majority of their electrostatic stabilization from direct favorable interactions. As a consequence, the electrostatic penalty associated with extracting ligands can be substantially reduced without actually invoking the concept of dielectric constant but by simply eliminating direct favorable interactions of the ligands. In the case of a K-channel binding site, the specific conditions that would yield reduced electrostatic penalties for extracting carbonyl oxygen ligands for ion complex formation, regardless of its dielectric characteristics, consist of a proximal environment that is devoid of only those polar chemical groups that can directly and favorably interact with the carbonyl oxygens. Such chemical groups in this case consist of H-bond donors. Note that in the x-ray structures of four strongly selective K-channels (KcsA, MthK, KvAP, and KirBac1.1), there are no relevant side-chain H-bond donor groups within a direct coordination distance of 6 Å from the carbonyl oxygens (see Supplementary Material), and all proximal backbone H-bond donors are occupied in maintaining the integrity of the protein's three-dimensional fold.
These conditions appear to create a local environment electrostatically equivalent to the low dielectric phase found earlier that led to increased probability for formation of eightfold K+ ion hydration states. We term the particular conditions that drive up ion coordination preferences a “quasiliquid” environment. Interestingly, we observe the same behavior of increased coordination preferences when Na+ and K+ ion reaction free energies are computed instead in quasiliquid formamide (see Supplementary Material), suggesting that the chemistry of the ligands utilized by K-channels to partition K+ ions does not alter this phenomenon.
At this point, our studies suggest how the local electrostatic properties of the phase surrounding a K-channel binding site can sustain K+ ions bound in states of high eightfold coordination that contrast to the low coordination schemes preferred in liquid phase, a result that is independent of the coordinating-ligand chemistries. In alignment with previous theories, one might anticipate that K+ ions readily partition into and permeate K-channel binding sites because the eightfold binding site coordination scheme matches the preferred number of water or formamide molecules that bind to K+ ions in the same quasiliquid phase. Further calculations of ion partitioning into representative binding sites, however, indicate otherwise.
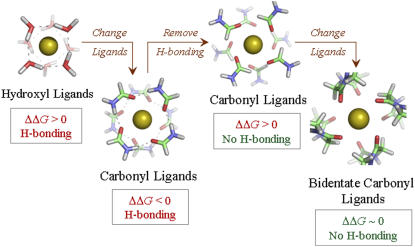
Fig. 2 illustrates the structures of eightfold complexes of K+ ions with water and formamide ligands optimized by quantum chemical methods. In both cases the eight molecules are arranged in two separate four-molecule ring-like structures around the K+ ions. This skewed cubic architecture resembles the crystallographically resolved binding site structures in the selectivity filter of K-channels (and also the NaK channel) but with one exception: The coordinating molecules in these two eightfold complexes are H-bonded to each other. In fact, we also find a certain degree of interligand H-bonding present in the five-, six-, and sevenfold complexes. In the context of K-channels, therefore, the reaction free energies obtained using these coordination complexes are physiologically irrelevant. Consequently we recompute the free energies of the five-, six-, seven- and eightfold K+ ion formamide complexes, ensuring this time that the formamide molecules avoid H-bond interactions. The quantum chemical optimized structure of the alternate non-H-bonded eightfold formamide complex of K+ ion is also illustrated in Fig. 2. This new structure is similar to K-channel binding sites as it has the same skewed cubic geometry and a similar K+-oxygen distance of 2.9 Å. We now find, however, that the free energy required to partition a K+ ion from bulk water into this new eightfold formamide geometry in a quasiliquid phase is substantially unfavorable (ΔΔG > 14 kcal/mol), which was not the case when interligand H-bonding was permitted. Fig. 3 illustrates these free energy values as part of a phase diagram.
FIGURE 2.
Different eightfold coordinated complexes of K+ ions considered in this investigation. ΔΔG refers to the free energy of partitioning a K+ ion from liquid water (reference environment) into the respective eightfold coordinated chemistries embedded in a quasiliquid phase. The interligand H-bonds are depicted using dashed lines connecting the hydrogen (white) and oxygen atoms (red). The chemistry and architecture that favors K+ ion partitioning precisely matches the binding sites S1–S3 found in the selectivity filters of K-channels.
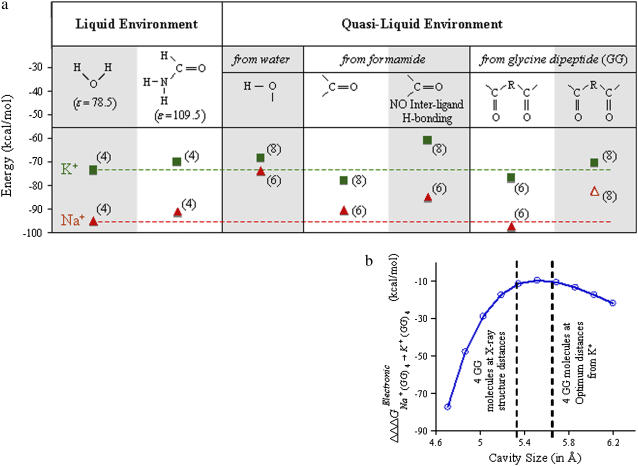
FIGURE 3.
(a) Phase diagram illustrating the structural and thermochemical effects of transferring Na+ and K+ ions from one combination of chemistry, architecture, and phase into another. The absolute free energies of solvating ions in these combinations can be inferred from the scale on the y axes of the plot, and the coordination numbers corresponding to these free energies are indicated in brackets. The hydration free energies of Na+ and K+ ions (n = 4) are also indicated as dashed lines, colored red and green, respectively. The open symbol enclosed in a dashed box denotes the only case where ligands are held rigid. This particular case represents the minimum of the cavity size-dependent K+/Na+ selectivity (∼10 kcal/mol) conferred by the chemistry of eight carbonyl ligands from four glycine dipeptide molecules arranged in a skewed cubic architecture, as estimated using the data in plot (b). From plot b, we see that irrespective of cavity size, measured as the distance between furthest oxygen atoms, the K+/Na+ ion selectivity conferred by this eightfold coordination is maintained. Since these values correspond to the lowest energy positions of the Na+ ions in the cavities, a choice of any other position for the ion will also result in K+/Na+ selectivity.
Another approach toward creating a representative K-channel binding site that avoids nonphysical interligand H-bonding in ion complex formation is to choose a different chemical ligand. We select bidentate ligands in the form of glycine dipeptide (CH3CONHCH2CONHCH3) molecules since the carbonyl oxygens in these molecules also provide a good chemical representation of the coordinating oxygen atoms present in the binding sites of K-channels. Furthermore, use of bidentate ligands improves the representation of K-channel binding sites over monodentate ligands as they capture the native bidentate character of ion-ligand association in K-channels, where each monomer of a tetrameric K-channel supplies two ligands to a binding site. Quantum chemical optimizations carried out using these bidentate ligands result in no interligand H-bonding. In fact, the optimized structure of the eightfold coordinated K+ ion complex, as illustrated in Fig. 2, matches the structures of binding sites in the selectivity filters of K-channels, with a K+-oxygen distance of 3 Å and a root mean-square deviation (RMSD) of only 0.4 Å with respect to the S2 binding site of KcsA (2). The free energy change, ΔG, associated with the formation of this eightfold bidentate K+ ion complex, plotted in the phase diagram of Fig. 3, indicates that it is energetically more stable than the eightfold complex formed from non-H-bonded formamides. An analysis of free energy contributions shows that this extra stabilization of the eightfold bidentate complex is due in part to the slightly different chemistries of the ligands as well as to the reduced loss in entropy associated with aggregation of ligands in the absence of interligand H-bonding (see Supplementary Material).
To probe the significance of the K-channel eightfold coordinated binding sites with respect to other binding site coordination possibilities, we compare the free energies for ion partitioning into eightfold and sixfold coordinated states formed by our bidentate ligands. The free energy ΔΔG required to partition a K+ ion from aqueous phase into the tetrameric eightfold bidentate complex described above is negligible. In the other set of calculations, the free energies ΔΔG for partitioning either Na+ or K+ ions from aqueous phases into their respective trimeric sixfold geometries are also negligible (see Fig. 3), which implies that in a scenario where K-channels could provide three bidentate ligands for coordination instead of four, both ions would partition into the binding site although without any apparent K+/Na+ selectivity. The thermodynamic condition essential for rapid K+ ion transport is therefore met with both sixfold and eightfold coordinated states; however, ion selectivity is lacking in the sixfold trimeric coordination scheme. Furthermore, K+ ions slightly prefer coordination by three rather than four bidentate ligands, demonstrating that K+ ions do not readily partition into K-channels to match preferred numbers of coordinating ligands.
Due to the long-range nature of electrostatic interactions, an exact estimation of the solvation energy of a K+ ion inside an entire K-channel requires further incorporation of its interactions with the remaining portion of the channel-membrane system. Nevertheless, in such a calculation we expect the solvation energy of a K+ ion to be more exothermic than the value reported here. Thermodynamic stabilization resulting from a higher dielectric constant of the protein (ɛ > 1) is expected to be larger than destabilization resulting from multi-ion repulsion in the selectivity filter, eventually resulting in a more exothermic reaction free energy value.
To understand ion selectivity in eightfold tetrameric binding sites, we calculate the interaction of a Na+ ion with four bidentate ligands, the same number and variety of ligands that allow K+ ions to partition from aqueous phase. Attempts to optimize four bidentate ligands around a Na+ ion using quantum chemical methods (DFT/B3LYP), such that all of the eight carbonyl oxygens directly coordinate the ion, fail. Instead an overall fivefold coordinated structure results, with three oxygens pushed outside the ion's inner coordination shell (see Supplementary Material). This structure can also be described as a distorted K-channel binding site structure having an RMSD of 1.8 Å with respect to the x-ray coordinates (2) of the S2 binding site of KcsA. A similar distortion of the S1 binding site of KcsA, which resulted in an overall sixfold coordinated geometry, has also been reported in a separate quantum chemical optimization study (26). Reaction free energies computed using these distorted structures present an interesting scenario. In the event that the selectivity filter were flexible enough to allow such local distortions in the number of coordinating ligands, not only would Na+ ions partition into the binding sites from aqueous phase, but also the filter would exhibit a reversed Na+/K+ selectivity. The fivefold distorted structure results in a potent reversed selectivity (ΔΔΔG) of ∼15 kcal/mol, whereas an alternate distortion corresponding to a sixfold coordination (reported elsewhere (26)) leads to a milder, yet still reversed Na+/K+ selectivity of ∼3 kcal/mol.
Next, we investigate selectivity for the possibility that the eightfold coordinated geometry formed from four glycine dipeptide ligands cannot distort other than in a manner that maintains direct coordination of the Na+ ion with all eight carbonyl oxygen atoms, yielding either a reduction or an expansion in binding site cavity size. Electronic energy differences computed for transferring a Na+ ion relative to a K+ ion (ΔΔΔGelectronic) into an eightfold bidentate complex, with coordinates that were optimized in the presence of a K+ ion, result in a K+/Na+ selectivity of −10.7 kcal/mol. Contrary to expectations that a smaller cavity size by itself would destroy selectivity by energetically stabilizing the smaller Na+ ion, we find that the same set of electronic energy calculations repeated after stepwise radial expansions and contractions of this eightfold geometry around the ions still preserve K+/Na+ selectivity, as illustrated in Fig. 3 b (see also Supplementary Material). Assuming that thermal contributions to the relative free energies approximately cancel, we find that electrostatic interactions, the dominant component of the electronic energy, arising from eight carbonyl oxygens are by themselves sufficient to achieve K+/Na+ selectivity. Moreover, thermal motions need not be identical for the two ions nor set to zero to result in ion selectivity. As computed on numerous occasions using parameterized force fields (27,28), filter selectivity appears tolerant of thermal atomic fluctuations (<1 Å), fluctuations that are in any case essential for ion translocation to take place across the narrow regions of the filter. Electrostatic interactions from this eightfold coordination result in K+/Na+ selectivity because of ligand-ligand repulsion, as elaborated in a separate molecular dynamics-based study (9). Results from our quantum chemical calculations illustrated in Fig. 3 b confirm the inferences derived from these empirical force field calculations, where atomic polarization effects were neglected.
DISCUSSION AND CONCLUSIONS
In summary, we find that direct coordination by eight carbonyl oxygens in the form of four bidentate ligands yields selective partitioning of K+ ions from an aqueous phase. Eight ligands overcoordinate both ions. In the event of structural distortions that can reduce coordination numbers to 5 or 6 during Na+ ion partitioning, ion selectivity is either nonexistent or reversed. Structural rigidity is therefore necessary for selective K+ ion partitioning, but not to maintain specific cavity sizes, as considered conventionally, but instead to maintain specific direct coordination numbers. In fact, we find a narrow window of flexibility within which the selectivity filter achieves selective K+ ion partitioning: On the one hand, if the filter were frozen solid, K+ ions could never physically partition from one binding site to another, whereas on the other hand, if it were entirely flexible like a liquid, no K+/Na+ selectivity could occur. Furthermore, it is the quasiliquid nature of the local binding-site environment that in the first place makes the direct coordination of a K+ ion with higher numbers of ligands (>6) a statistical possibility, as they are otherwise rarely observed in aqueous phase. Along with the strengths of the ligating oxygens, these two determinants, phase and coordination number, together generate the electrical scenario necessary for selective K+ ion partitioning in K-channels. To distinguish this mechanism from conventional views, we term this a “phase-activated” mechanism of selective ion partitioning.
Experiments to test this mechanism consist of creating mutations in K-channels that can facilitate reductions in binding-site coordination numbers. One way to achieve this is to disturb the quasiliquid nature of the binding-site environment by introducing proximal H-bond donor groups. Clearly this will introduce electrostatic penalties for extracting ligands from the environment for ion coordination, which will reduce the free energy for K+ ion partitioning. Nevertheless, the results from optimizing four bidentate ligands about a Na+ ion suggest a route for balancing these energetic penalties with energetic rewards to retain favorable K+ ion partitioning.
Consider a scenario where one H-bond donor group is introduced per monomer of a tetrameric K-channel. As computed earlier, the free energy to partition a Na+ ion from liquid water to a fivefold distorted structure formed from four bidentate ligands is favorable by −9.5 kcal/mol. In the event that such a distortion occurred in the selectivity filter during Na+ partitioning, only one ligand from one monomer would need to be extracted from the environment for ion coordination, whereas three carbonyl oxygen ligands on the remaining monomers could remain bonded to their respective H-bond donor groups. This implies that even when penalized electrostatically for ligand extraction, a Na+ ion will partition into the distorted selectivity filter. For example, in an extreme case of ligand extraction involving removal of one formamide molecule from liquid formamide (ɛ = 109.5), the electrostatic penalty was computed to be 10.8 kcal/mol (see section 2 of Supplementary Material). Under these conditions, a K+ ion will also partition into the selectivity filter because, as a separate calculation shows, the free energy for transferring a K+ ion from liquid water into a distorted fivefold coordination (similar to the one obtained for a Na+ ion) in a quasiliquid phase is favorable by −10.1 kcal/mol (see Supplementary Material for illustration). Clearly, introduction of one H-bond donor per monomer will not only permit K+ partitioning but will now result in a substantially diminished K+/Na+ selectivity.
Physiological characterizations of such mutations in K-channels exist, but their mechanisms could not be explained using any of the previous theories of ion selectivity. For a recent example, a mutation in a strongly selective GIRK channel that introduced an H-bond donor group in the form of a Trp side chain in the proximity of its selectivity filter resulted in a substantial reduction in its K+/Na+ selectivity (13,14). Another example comes from a comprehensive sequence alignment study of K-channels (15), which show that weakly selective K-channels, such as HYP or pacemaker channels, carry H-bond donors in the form of arginine residues proximal to their selectivity filters and that these side-chain groups are completely absent from all strongly selective K-channels.
Another way to facilitate reductions in binding site coordination numbers is to create ample structural freedom for binding site distortion along with introduction of H-bond donors. The crystal structure of the weakly selective NaK channel exemplifies this, as it shows that its ion binding sites are interspersed with water-filled cavities (12). Data from recent MD simulations (29) of the NaK channel also lend support to these original ideas (30,31). Relative proximities to bulk water perhaps also explains why the computed selectivity (9) is not uniform across the chemically identical binding sites in wild-type KcsA. The S2 site is the least exposed to water in comparison with sites S1 and S3, making it the most selective site in KcsA.
Together, these investigations introduce the interplay and individual thermochemical effects of two determinants of ion partitioning: phase and coordination number. As demonstrated, they play critical roles in selective ion-partitioning mechanisms in K-channels, suggesting their potential implications for other chemical and biological settings as well. We anticipate that future investigations that explicitly account for their effects will also prove beneficial toward developing a better understanding of the nature of ion solvation.
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Supplementary Material
Acknowledgments
The authors thank C.P. Beamis for critically reading this manuscript.
All simulations were carried out on the Intel Itanium 2 Linux cluster of the National Center for Supercomputing Applications at the University of Illinois, Urbana-Champaign. Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the U.S. Dept. of Energy.
This work was supported in part by the Dept. of Energy under contract number DE-AC04-94Al85000, and in part by the National Institutes of Health under grant No. PHS-2-PN2-EY016570B.
Editor: Peter C. Jordan.
References
- 1.Hille, B. 2001. Ionic Channels of Excitable Membranes. Sinauer Associates, Sunderland, MA.
- 2.Zhou, Y., J. H. Morais-Cabral, A. Kaufman, and R. MacKinnon. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 414:43–48. [DOI] [PubMed] [Google Scholar]
- 3.Jiang, Y., A. Lee, J. Chen, M. Cadene, B. T. Chait, and R. MacKinnon. 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. [DOI] [PubMed] [Google Scholar]
- 4.Jiang, Y., A. Lee, J. Chen, V. Ruta, M. Cadene, B. T. Chait, and R. MacKinnon. 2003. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. [DOI] [PubMed] [Google Scholar]
- 5.Kuo, A., J. M. Gulbis, J. F. Antcliff, T. Rahman, E. D. Lowe, J. Zimmer, J. Cuthbertson, F. M. Ashcroft, T. Ezaki, and D. A. Doyle. 2003. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 300:1922–1926. [DOI] [PubMed] [Google Scholar]
- 6.Bezanilla, F., and C. M. Armstrong. 1972. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J. Gen. Physiol. 60:588–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller, C. 2001. See potassium run. Nature. 414:23–24. [DOI] [PubMed] [Google Scholar]
- 8.Varma, S., and S. B. Rempe. 2006. Coordination numbers of alkali metal ions in aqueous solutions. Biophys. Chem. 124:192–199. [DOI] [PubMed] [Google Scholar]
- 9.Noskov, S. Y., S. Berneche, and B. Roux. 2004. Control of ion selectivity in potassium channels by electrostatic and dynamic properties of carbonyl ligands. Nature. 431:830–834. [DOI] [PubMed] [Google Scholar]
- 10.Eisenman, G. 1961. Symposium on Membrane Transport and Metabolism. A. Kleinzeller and A. Kotyk, editors. Academic Press, New York.
- 11.Aqvist, J., O. Alvarez, and G. Eisenman. 1992. Ion-selective properties of a small ionophore in methanol studied by free energy perturbation simulations. J. Phys. Chem. 96:10019–10025. [Google Scholar]
- 12.Shi, N., S. Ye, A. Alam, L. Chen, and Y. Jiang. 2006. Atomic structure of a Na+- and K+-conducting channel. Nature. 440:570–574. [DOI] [PubMed] [Google Scholar]
- 13.Bichet, D., Y.-F. Lin, C. A. Ibarra, C. S. Huang, B. A. Yi, Y. N. Jan, and L. Y. Jan. 2004. Evolving potassium channels by means of yeast selection reveals structural elements important for selectivity. Proc. Natl. Acad. Sci. USA. 101:4441–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bichet, D., M. Grabe, Y. N. Jan, and L. Y. Jan. 2006. Electrostatic interactions in the channel cavity as an important determinant of potassium channel selectivity. Proc. Natl. Acad. Sci. USA. 103:14355–14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shealy, R. T., A. D. Murphy, R. Ramarathnam, E. Jakobsson, and S. Subramaniam. 2003. Sequence-function analysis of the K+-selective family of ion channels using a comprehensive alignment and the KcsA channel structure. Biophys. J. 84:2929–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widom, B. 1982. Potential-distribution theory and the statistical mechanics of fluids. J. Phys. Chem. 86:869–872. [Google Scholar]
- 17.Pratt, L. R., and R. A. LaViolette. 1998. Quasi-chemical theories of associated liquids. Mol. Phys. 95:909–915. [Google Scholar]
- 18.Pratt, L. R., and S. B. Rempe. 1999. Quasi-chemical theory and implicit solvent models for simulations. In Interactions in Solution, AIP Conference Proceedings. 492:172–201. L. R. Pratt and G. Hummer, editors.
- 19.Beck, T. L., M. E. Paulaitis, and L. R. Pratt. 2006. The Potential Distribution Theorem and Models of Molecular Solutions. Cambridge University Press, New York.
- 20.Reference deleted in proof.
- 21.Rempe, S. B., and L. R. Pratt. 2001. The hydration number of Na+ in liquid water. Fluid Phase Equilibria. 183:121–132. [Google Scholar]
- 22.Rempe, S. B., D. Asthagiri, and L. R. Pratt. 2004. Inner shell definition and absolute hydration free energy of K+(aq) on the basis of quasi-chemical theory and ab initio molecular dynamics. Phys. Chem. Chem. Phys. 6:1966–1969. [Google Scholar]
- 23.Ramaniah, L., M. Bernasconi, and M. Parrinello. 1999. Ab initio molecular-dynamics simulation of K+ solvation in water. J. Chem. Phys. 111:1587–1591. [Google Scholar]
- 24.White, J. A., E. Schwegler, G. Galli, and F. Gygi. 2000. The solvation of Na+ in water: first-principles simulations. J. Chem. Phys. 113:4668–4673. [Google Scholar]
- 25.Simonson, T. 2003. Electrostatics and dynamics of proteins. Rep. Prog. Phys. 66:737–787. [Google Scholar]
- 26.Ban, F., P. Kusalik, and D. F. Weaver. 2004. Density functional theory investigations on the chemical basis of the selectivity filter in the K+ channel protein. J. Am. Chem. Soc. 126:4711–4716. [DOI] [PubMed] [Google Scholar]
- 27.Luzhkov, V. B., and J. Aqvist. 2005. Ions and blockers in potassium channels: insights from free energy simulations. Biochim. Biophys. Acta. 1747:109–120. [DOI] [PubMed] [Google Scholar]
- 28.Asthagiri, D., L. R. Pratt, and M. E. Paulaitis. 2006. Role of fluctuations in a snug-fit mechanism of KcsA channel selectivity. J. Chem. Phys. 125:24701–24706. [DOI] [PubMed] [Google Scholar]
- 29.Noskov, S. R., and B. Roux. 2007. Importance of hydration and dynamics on the selectivity of the KcSA and NaK channels. J. Gen. Physiol. 129:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varma, S., and S. B. Rempe. 2006. Selective permeation of ions across the selectivity filter of KcSA: a perspective from the coordination chemistry of ions. Biophys. J. 90:1961. (Abstr.)16361346 [Google Scholar]
- 31.Varma, S., and S. B. Rempe. 2006. Technical Report No. SAND2006-0711J, Sandia National Laboratories, Albuquerque, NM. http://arxiv.org/pdf/physics/0608180.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.