Abstract
Recent studies of several ICK ion-channel blockers suggest that lipid bilayer interactions play a prominent role in their actions. Structural similarities led to the hypothesis that bilayer interactions are important for the entire ICK family. We have tested this hypothesis by performing direct measurements of the free energy of bilayer partitioning (ΔG) of several peptide blockers using our novel quenching-enhanced fluorescence titration protocol. We show that various ICK peptides demonstrate markedly different modes of interaction with large unilamellar lipid vesicles. The mechanosensitive channel blocker, GsMTx4, and its active diastereomeric analog, D-GsMTx4, bind strongly to both anionic and zwitterionic membranes. One potassium channel gating modifier, rHpTx2gs, interacts negligibly with both types of vesicles at physiological pH, whereas another, SGTx1, interacts only with anionic lipids. The slope of ΔG dependence on surface potential is very shallow for both GsMTx4 and D-GsMTx4, indicating complex interplay of their hydrophobic and electrostatic interactions with lipid. In contrast, a cell-volume regulator, GsMTx1, and SGTx1 exhibit a very steep ΔG dependence on surface potential, resulting in a strong binding only for membranes rich in anionic lipids. The high variability of 5 kcal/mole in observed ΔG shows that bilayer partitioning is not a universal property of the ICK peptides interacting with ion channels.
A variety of peptide toxins from venomous animals are known to block different ion channels selectively and with high affinity. Two back-to-back articles that appeared in Nature, one on voltage-sensor toxin VsTx1 (1) and another on mechanosensitive channel inhibitor GsMTx4 (2), and the accompanying commentary (3), suggested a new concept for their action, which assigns a prominent role to interactions with the bilayer. Both peptides, along with a number of other channel blockers and gating modifiers (e.g., SGTx1, HpTx2), share the same ICK fold and have a well-demarcated hydrophobic face (4–9). (Sequence alignment of 17 such peptides is presented in Lee and MacKinnon (1).) These structural similarities have led to a common perception that lipid bilayer interactions are important for all ICK blockers, which we challenge here.
The hydrophobic face of ICK blockers is often dominated by aromatic residues (e.g., F-5, W-6, W-7, F-27, F-32, and F-34 in GsMTx4, or Y-1, W-5, W-7, W24, and W-31 in a cell-volume regulator GsMTx1), which are expected to contribute strongly and favorably to the free energy of bilayer partitioning, ΔG (10). Bilayer interaction of tryptophan-containing peptides often results in strong changes in intrinsic fluorescence, which, after appropriate corrections (11), can be used to determine the ΔG of bilayer partitioning using equilibrium titration (12). Surprisingly, we found that the addition of LUV to a GsMTx4 solution leads to marginal changes in tryptophan's emission (4). In contrast, there was a pronounced reduction in quenching by aqueous ions in the presence of LUV, indicating shielding of tryptophan residues by the lipid bilayer. We have taken advantage of this differential fluorescence quenching between free and membrane-bound peptide to develop a highly sensitive titration protocol that allowed us to quantify accurately their membrane interactions (4). The protocol was first verified on the well-studied peptide melittin and then applied to GsMTx4. The experiments reveal GsMTx4's substantial affinity for both zwitterionic POPC (ΔG = −6.1 kcal/mole) and anionic 25POPC:75POPG LUV (ΔG = −8.3 kcal/mole) (4). Here we apply the same methodology to other blockers, all of which have at least one tryptophan residue. All experimental details are the same as described in Posokhov et al. (4).
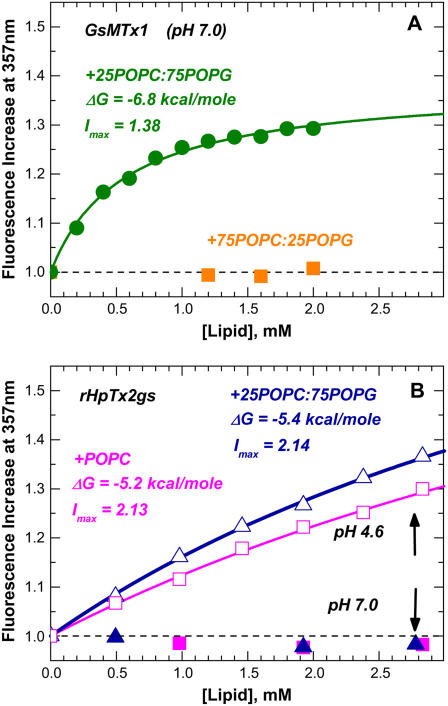
Despite their structural similarity to GsMTx4, none of the other peptides (except for the all-D enantiomer D-GsMTx4) were found to bind vesicles made of purely zwitterionic lipids or even those with a low content of anionic lipids (e.g., GsMTx1 Fig. 1 A, squares). Whereas GsMTx1 and SGTx1 bind strongly to vesicles rich in anionic lipids (Figs. 1 A and 2), no binding of rHpTx2gs was detected at pH 7.0 (Fig. 1 B, solid symbols). rHpTx2gs is unusual among the toxins in this study, in that it has six acidic amino acids. Lowering the pH to 4.6, which neutralizes the acidic amino acids, leads to a moderate level of binding. The latter appears to be virtually independent of lipid composition (Fig. 1 B, open symbols), consistent with primarily hydrophobic interactions at low pH. Given the precision of our fluorescence measurements and assuming that limiting spectroscopic response at infinite lipid concentration (Imax) is pH-independent, we estimate a negligible free energy of ΔG > −3.5 kcal/mole for membrane interactions of rHpTx2gs at the physiologically relevant pH of 7.0.
FIGURE 1.
Quenching-enhanced fluorescence titration of two ICK blockers with LUV made of lipids specified on graphs (experimental details are described in Posokhov et al. (4)). Whereas GsMTx1 (A) requires high concentration of anionic POPG lipid for binding, rHpTx2gs (B) will only interact with vesicles at acidic pH (open symbols), but not at neutral pH (solid symbols).
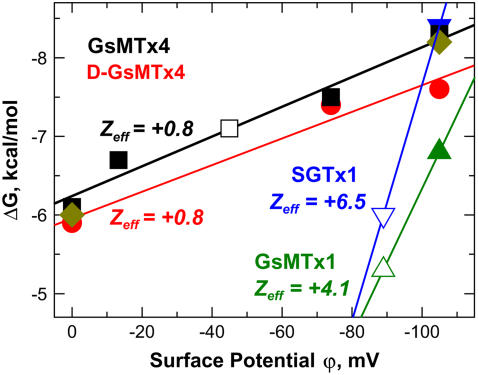
FIGURE 2.
Free energy of partitioning of four ICK blockers into membranes with various surface potential. GsMTx4 data were collected for (solid squares) POPC, 95POPC:5POPG, 75POPC:25POPG, and 25POPC:75POPG; (diamonds) PC and 25PC:75PS; and (open square) with 25POPC:75POPG in the presence of additional 450 mM KCl (all other samples contain 50 mM KI). D-GsMTx4 data (circles) were collected for POPC:POPG mixtures. SGTx1 (blue triangles) and GsMTx1 (olive triangles) data were collected for 25POPC:75POPG with (open symbols) or without (solid symbols) of additional 50 mM KCl. The effective charge for (D-)GsMTx4 peptides calculated from the slope of these dependences, Zeff, is much smaller than the net charge of the peptide. A large Zeff observed for SGTx1 and GsMTx1 is indicative of predominantly electrostatic interactions.
We have summarized our results for membrane binding properties the toxins by showing the dependence of the free energy of partitioning on the surface potential of the bilayer, ϕ (Fig. 2). The latter can be altered by changes in the content of anionic lipids (solid symbols) or by increased salt concentration (open symbols). All seven data points for GsMTx4 fall comfortably on a straight line, indicating that the ΔG doesn't depend on whether the surface potential was created by mixing POPC and POPG (solid squares), PC and PS (diamonds), or was adjusted for 25POPC:75POPG by a 10-fold increase of ionic strength (open square). Both GsMTx4 and its enantiomer D-GsMTx4 exhibit similarly strong binding with an identical slope, suggesting that chirality has a small effect on the lipid interactions of this peptide.
The slope of the ΔG versus ϕ-plot is proportional to the effective charge of the peptide Zeff, which may differ from its physical charge (13). Remarkably, (D-)GsMTx4 peptides have an effective charge of only +0.8, which is much lower than the nominal charge of +5. A reduction of Zeff is often observed for peptides that are hydrophobic and charged, and is considered a hallmark of the nonadditive contributions from electrostatic and hydrophobic interactions (13). SGTx1 and GsMTx1, on the other hand, exhibit a sharp drop in binding when ϕ of 25POPC:75POPG LUV (Fig. 2, solid blue and green symbols) is reduced by a twofold increase in ionic strength (corresponding open symbols), consistent with the total lack of interaction with POPC-rich LUV (Fig. 1 A). This behavior is indicative of the predominantly electrostatic nature of interaction of these cationic peptides with anionic membranes. The observed difference between these two peptides and GsMTx4 indicates that their mode of interaction with lipid bilayers is different and that their binding in the vicinity of an ion channel in vivo will be highly dependent on membrane potential.
The News and Views editorial (3) accompanying the initial publications suggesting the importance of bilayer partitioning of ion channel blockers VsTx1 (1) and GsMTx4 (2), ends with the suggestion that “it remains to be determined whether membrane partitioning is a common mechanism for all ion-channel gating modifiers.” The thermodynamic evidence presented here suggests that it is not. The five blockers we studied can be classified into three categories depending on their ability to interact with lipid bilayers. The mechanosensitive channel blocker GsMTx4 and its active enantiomer D-GsMTx4 belong to the first group, characterized by strong binding to both anionic and zwitterionic lipids via hydrophobic and electrostatic interactions. VsTx1 may also belong to this group, although the evidence of its ability to bind to zwitterionic membranes (1) has been recently questioned (8). The second group, composed of a cell-volume regulator GsMTx1 and the Kv2.1 channel gating modifier SGTx1, is characterized by strong electrostatic binding to anionic membranes. Finally, the Kv4 gating modifier rHpTx2gs does not bind lipid vesicles at physiological pH conditions. The structural features that determine why any peptide belongs to a given category are not obvious (e.g., the hydrophobicity pattern appears to be similar (Fig. 3)) and should be investigated with the help of site-directed mutagenesis. It is clear, however, that lipid membrane binding of tightly folded ICK peptides should be experimentally investigated in a quantitative manner before their membrane binding ability can be predicted from the sequence, as it can be done for the unfolded and helical peptides (10,13,14).
FIGURE 3.
Primary amino acid sequence alignment of the four blockers used in this study, plus VsTx1 aligned along the positions of cysteines (blue). The rest of the residues are shaded in accordance with the Wimley-White hydrophobicity scale (10), with the most hydrophobic residues (W, Y, F, L, and I) in green, and the least hydrophobic (charged) in red.
Acknowledgments
This work was supported by National Institutes of Health GM069783 (A.S.L.) and HL054887 (F.S.), and Northeast Affiliate of the American Heart Association 0235500T (M.J.M.).
Abbreviations used: ICK, inhibitor cysteine knot; LUV, large unilamellar vesicles; POPC, palmitoyloleoylphosphatidylcholine; POPG, palmitoyloleoylphosphatidylglycerol; PC, egg yolk phosphatidylcholine; PS, bovine brain phosphatidylserine; GsMTx1, YCQKWMWTCDEERKCCEGLVCRLWCKKKIEW; GsMTx4, GCLEFWWKCNPNDDKCCRPKLKCSKLFKLCNFSF-Amide; rHpTx2gs, GSDDCGKLFSGCDTNADCCEGYVCRLWCKLDW; SGTx1 (D24A), TCRYLFGGCKTTADCCKHLACRSAGKYCAWDGTF.
Editor: Anthony Watts.
References
- 1.Lee, S.-Y., and R. MacKinnon. 2004. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. 430:232–240. [DOI] [PubMed] [Google Scholar]
- 2.Suchyna, T. M., S. E. Tape, R. E. Koeppe 2nd, O. S. Anderson, F. Sachs, and P. A. Gottlieb. 2004. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 430:235–240. [DOI] [PubMed] [Google Scholar]
- 3.Garcia, M. L. 2004. Ion channels: gate expectations. Nature. 430:153–155. [DOI] [PubMed] [Google Scholar]
- 4.Posokhov, Y. O., P. A. Gottlieb, and A. S. Ladokhin. 2007. Quenching-enhanced fluorescence titration protocol for accurate determination of free energy of membrane binding. Anal. Biochem. 362:290–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarayskiy, V. V., G. Balasubramanian, V. E. Bondarenko, and M. J. Morales. 2005. Heteropoda toxin 2 is a gating modifier toxin specific for voltage-gated K+ channels of the Kv4 family. Toxicon. 45:431–442. [DOI] [PubMed] [Google Scholar]
- 6.Oswald, R. E., T. M. Suchyna, R. McFeeters, P. Gottlieb, and F. Sachs. 2002. Solution structure of peptide toxins that block mechanosensitive ion channels. J. Biol. Chem. 277:34443–34450. [DOI] [PubMed] [Google Scholar]
- 7.Lee, C. W., S. Kim, S. H. Roh, H. Endoh, Y. Kodera, T. Maeda, T. Kohno, J. M. Wang, K. J. Swartz, and J. I. Kim. 2004. Solution structure and functional characterization of SGTx1, a modifier of Kv2.1 channel gating. Biochemistry. 43:890–897. [DOI] [PubMed] [Google Scholar]
- 8.Jung, H. J., J. Y. Lee, S. H. Kim, Y.-J. Eu, S. Y. Shin, M. Milescu, K. J. Swartz, and J. I. Kim. 2005. Solution structure and lipid membrane partitioning of VSTx1, an inhibitor of the KvAP potassium channel. Biochemistry. 44:6015–6023. [DOI] [PubMed] [Google Scholar]
- 9.Bernard, C., C. Legros, G. Ferrat, U. Bischoff, A. Marquardt, O. Pongs, and H. Darbon. 2000. Solution structure of hpTX2, a toxin from Heteropoda venatoria spider that blocks Kv4.2 potassium channel. Protein Sci. 9:2059–2067. [PMC free article] [PubMed] [Google Scholar]
- 10.Wimley, W. C., and S. H. White. 1996. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 3:842–848. [DOI] [PubMed] [Google Scholar]
- 11.Ladokhin, A. S., S. Jayasinghe, and S. H. White. 2000. How to measure and analyze tryptophan fluorescence in membranes properly, and why bother? Anal. Biochem. 285:235–245. [DOI] [PubMed] [Google Scholar]
- 12.White, S. H., W. C. Wimley, A. S. Ladokhin, and K. Hristova. 1998. Protein folding in membranes: determining the energetics of peptide-bilayer interactions. Methods Enzymol. 295:62–87. [DOI] [PubMed] [Google Scholar]
- 13.Ladokhin, A. S., and S. H. White. 2001. Protein chemistry at membrane interfaces: non-additivity of electrostatic and hydrophobic interactions. J. Mol. Biol. 309:543–552. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Vidal, M., S. Jayasinghe, A. S. Ladokhin, and S. H. White. 2007. Folding amphipathic helices into membranes: amphiphilicity trumps hydrophobicity. J. Mol. Biol. 370:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]