Short abstract
An overview of a family of cell-surface adhesion and signaling receptors that mediate cell-cell adhesion and cell-extracellular matrix adhesion.
Abstract
The integrins are a superfamily of cell adhesion receptors that bind to extracellular matrix ligands, cell-surface ligands, and soluble ligands. They are transmembrane αβ heterodimers and at least 18 α and eight β subunits are known in humans, generating 24 heterodimers. Members of this family have been found in mammals, chicken and zebrafish, as well as lower eukaryotes, including sponges, the nematode Caenorhabditis elegans (two α and one β subunits, generating two integrins) and the fruitfly Drosophila melanogaster (five α and one β, generating five integrins). The α and β subunits have distinct domain structures, with extracellular domains from each subunit contributing to the ligand-binding site of the heterodimer. The sequence arginine-glycine-aspartic acid (RGD) was identified as a general integrin-binding motif, but individual integrins are also specific for particular protein ligands. Immunologically important integrin ligands are the intercellular adhesion molecules (ICAMs), immunoglobulin superfamily members present on inflamed endothelium and antigen-presenting cells. On ligand binding, integrins transduce signals into the cell interior; they can also receive intracellular signals that regulate their ligand-binding affinity. Here we provide a brief overview that concentrates mostly on the organization, structure and function of mammalian integrins, which have been more extensively studied than integrins in other organisms.
Gene organization and evolutionary history
The integrins are a superfamily of cell adhesion receptors that recognize mainly extracellular matrix ligands and cell-surface ligands, although some soluble ligands have been identified [1]. They are transmembrane αβ heterodimers, and at least 18 α and eight β subunits are known in humans [2] (Figure 1; lists of the integrin subunits present in mouse, chicken, zebrafish, Caenorhabditis elegans, Xenopus laevis and Drosophila melanogaster are given in Additional data file 1). Integrin α and β subunits are totally distinct, with no detectable homology between them; sequence identity among α subunits is about 30% and among β subunits 45%, indicating that both the α and the β gene families evolved by gene duplication (Figure 2). In humans, genes for both α and β subunits are located on various chromosomes. However, genes for integrins expressed in leukocytes (subunits αL, αM, αD, and αX) are clustered at 16p11, while for those expressed in platelets and endothelial cells, the αIIb and β3 genes are at 17q21.32, and the α6, α4, and αV cluster at 2q31 (Table 1). Some integrin ⟨ subunits (⟨1, ⟨2, ⟨10, ⟨11, ⟨M, ⟨L, ⟨D, and ⟨X) contain a so-called I (insertion or interaction), or A, domain, while others do not. The I-domain integrin α subunits are closely related to each other (Figure 2a). Also closely related to each other are the family of non-I-domain α subunits that recognize the RGD motif (αV, α8, α5, and αIIb) and the family of laminin-binding α subunits (α3, α6, and α7). Studies on integrin genes from lower and higher eukaryotes clearly indicate that integrin genes (both α and β) derived from a common ancestral gene by gene duplications. A genomic analysis among 24 invertebrate and vertebrate species revealed that the α and β integrin structure, along with the inserted α I domain, has been highly conserved during the evolution of vertebrates [3].
Figure 1.
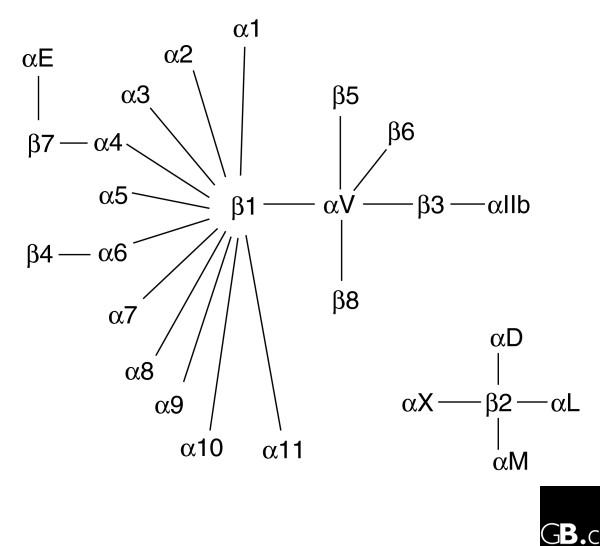
The members of the human integrin superfamily and how they combine to form heterodimeric integrins. At least 18 α subunits and eight β subunits have been identified in humans, which are able to generate 24 different integrins. Integrin subunits that bind to each other to form a heterodimer are connected by solid lines. Each integrin has distinct ligand-binding specificity and tissue and cell distribution.
Figure 2.
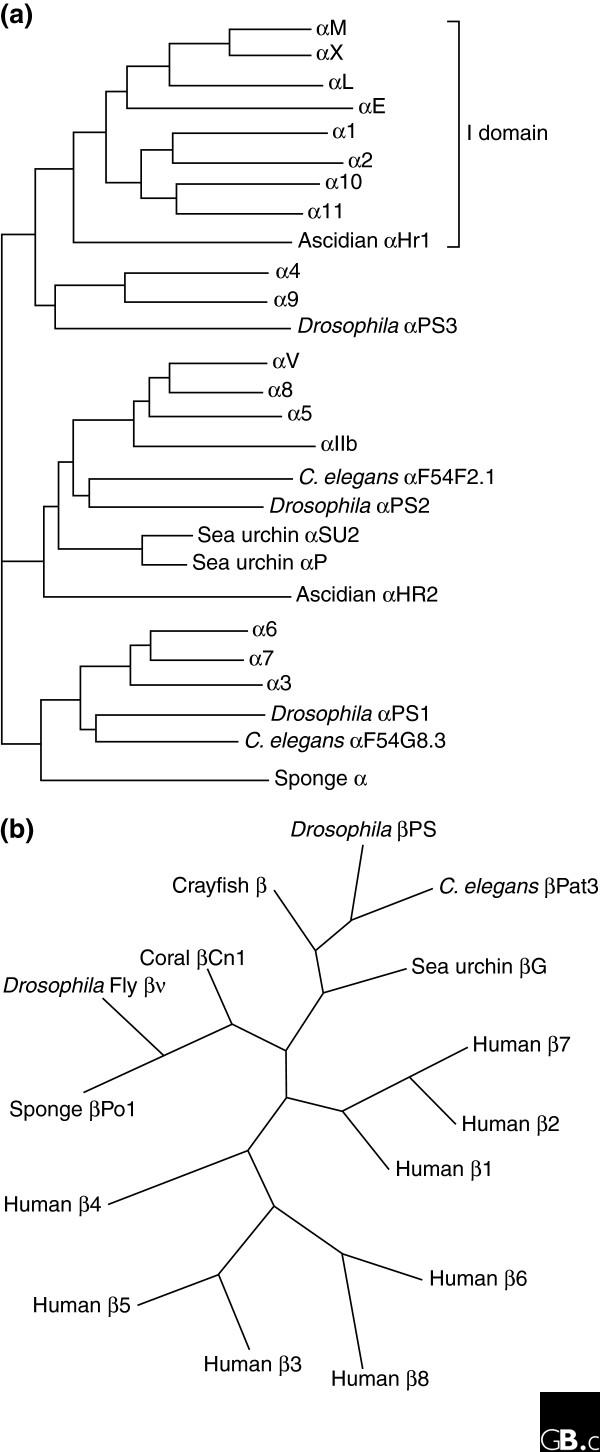
Phylogenetic trees of integrin subunits. Trees for (a) integrin α and (b) integrin β subunits are adapted from [58] and [59], respectively.
Table 1.
Human integrin subunits
| Gene symbol | Protein name | Synonyms | Gene accession number | Location (chromosome) | Protein accession number |
| ITGA1 | α1 | CD49a | NM_181501 | 5q11.2 | P56199 |
| ITGA2 | α2 | CD49b, α2 subunit of very late antigen 2 (VLA-2) | NM_002203 | 5q23-q31 | P17301 |
| ITGA2B | αIIb | GTA, CD41, GP2B, HPA3, CD41b, GPIIb | NM_000419 | 17q21.32 | P08514 |
| ITGA3 | α3 | CD49c, α3 subunit of VLA-3 | NM_002204, NM_005501 | 17q21.33 | P26006 |
| ITGA4 | α4 | CD49d, α4 subunit of VLA-4 | NM_000885 | 2q31.3 | AAB25486 |
| ITGA5 | α5 | CD49e, fibronectin receptor alpha | NM_002205 | 12q11-q13 | P08648 |
| ITGA6 | α6 | CD49f, ITGA6B | NM_000210 | 2q31.1 | P23229 |
| ITGA7 | α7 | NM_002206 | 12q13 | Q86W93 | |
| ITGA8 | α8 | NM_003638 | 10p13 | P53708 | |
| ITGA9 | α9 | NM_002207 | 3p21.3 | Q13797 | |
| ITGA10 | α10 | NM_003637 | 1q21 | O75578 | |
| ITGA11 | α11 | NM_001004439, NM_012211 | 15q23 | Q9UKX5 | |
| ITGAD | αD | NM_005353 | 16p11.2 | Q13349 | |
| ITGAE | αE | CD103, human mucosal lymphocyte antigen 1α | NM_002208 | 17p13 | P38570 |
| ITGAL | αL | CD11a (p180), lymphocyte function-associated antigen 1 (LFA-1) α subunit | NM_002209 | 16p11.2 | P20701 |
| ITGAM | αM | Mac-1, CD11b, complement receptor 3 (CR3) subunit | J03925, NM_000632 | 16p11.2 | P11215 |
| ITGAV | αV | CD51, MSK8, vitronectin receptor α (VNRα) | NM_002210 | 2q31-q32 | P06756 |
| ITGAX | αX | CD11c, CR4 subunit | NM_000887 | 16p11.2 | P20702 |
| ITGB1 | β1 | Fibronectin receptor β, CD29, MDF2, MSK12 | BC020057 | 10p11.2 | P05556 |
| ITGB2 | β2 | Leukocyte cell adhesion molecule, CD18, CR3 subunit, CR4 subunit | NM_000211 | 21q22.3 | P05107 |
| ITGB3 | β3 | CD61; GP3A; GPIIIa, platelet glycoprotein IIIa | NM_000212 | 17q21.32 | P05106 |
| ITGB4 | β4 | CD104 | NM_000213 | 17q25 | P16144 |
| ITGB5 | β5 | NM_002213 | 3q21.2 | P18084 | |
| ITGB6 | β6 | NM_000888 | 2q24.2 | P18564 | |
| ITGB7 | β7 | NM_000889 | 12q13.13 | P26010 | |
| ITGB8 | β8 | NM_002214 | 7p15.3 | P26012 |
Characteristic structural features
The crystal structures of human integrins αVβ3 [4,5] and αIIbβ3 [6] show that the extracellular portion of an integrin heterodimer consists of multiple domains (Figure 3a). The headpiece of αVβ3, which contains the ligand-binding site, consists of the β-propeller domain and the plexin-semaphorin-integrin (PSI) domain of the αV subunit, and the β I-like, or βA, domain and the hybrid domain of the β subunit. The β-propeller domain contains seven repeats of about 60 amino acids each that fold into a seven-bladed β-propeller structure similar to the β subunit of a heterotrimeric G protein. I domains contain a metal-ion-dependent adhesive site (MIDAS) and I-like domains contain a structurally similar metal-binding motif. The RGD-binding site is located at the interface between the β-propeller domain and the β I-like domain and amino-acid residues from the two domains interact directly with the RGD peptide of a ligand [5]. Mutagenesis studies have identified many other amino-acid residues that are critical for ligand binding [7,8]. These residues are discontinuous in the primary structure but are exposed on the surface of the headpiece and generate the ligand-binding surface. The crystal structure of a complete integrin-ligand complex has not yet been published, but by comparing the crystal structures of RGD-bound and unbound forms it has been found that the disulfide-linked loop structure in the β I-like domain under goes conformational changes (a movement of 1.5 Å towards the RGD peptide), and the α helix 7 in the β I-like domain moves downward on ligand binding [4,5]. Also, the hybrid domain swings outward upon integrin activation. In the I-domain integrins, the I domain can be present in either an open (active) or a closed (inactive) conformation. These are major conformational changes that affect ligand binding in the headpiece.
Figure 3.
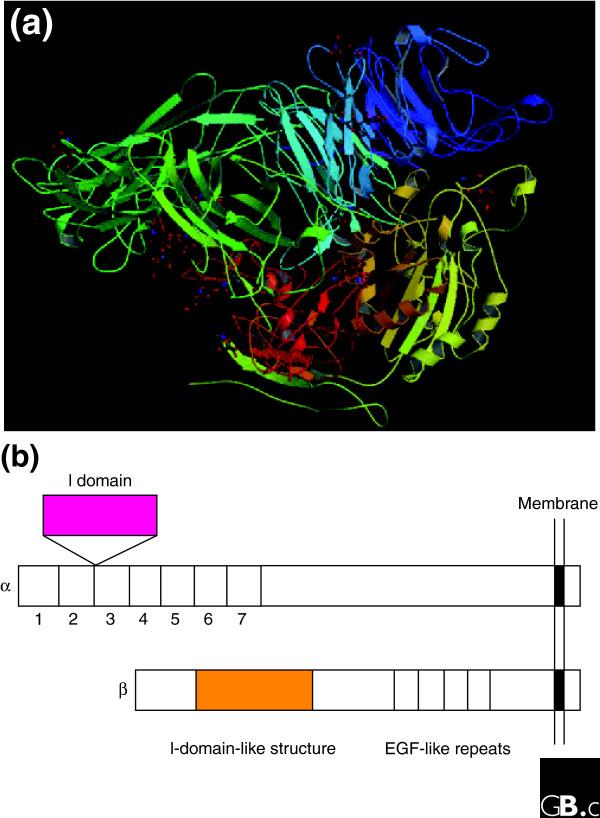
The extracellular region of a human integrin. (a) The crystal structure represents a net form of integrin αVβ3 with no bound RGD peptide (Protein Data Bank (PDB) code 1JV2) [3,4]. See PBD code 1L5G for the RGD-bound form. (b) The I (inserted or interactive) domain is present in seven human α subunits between β-propeller repeats 2 and 3, and is involved in ligand binding. An I-like domain is present in all human integrin β subunits along with four EGF-like repeats. Both the I and I-like domains have a Rossmann fold.
The cytoplasmic tails of human integrin subunits are less than 75 amino acids long (the β4 tail is an exception at a length of approximately 1,000 amino acids, which includes four fibronectin type III repeats). There is striking homology among the β-subunit cytoplasmic tails, but the α-subunit tails are highly divergent except for a conserved GFFKR motif next to the transmembrane region, which is important for association with the β tail. A large number of cytoskeletal and signaling proteins have been reported to bind to β cytoplasmic tails and some have been found to interact with specific α tails. Most integrin β tails contain one or two NPxY/F motifs (where x is any amino acid) that are part of a canonical recognition sequence for phosphotyrosine-binding (PTB) domains, which are protein modules present in a wide variety of signaling and cytoskeletal proteins. Phosphorylation of the tyrosine (Y) in the NPxY/F motif may represent a mode of regulating integrin interactions with other proteins at the cytoplasmic face of the plasma membrane. The integrin tails recruit several proteins, such as talin, that bind actin filaments, and thus form a connection to the cytoskeleton, a connection that is essential for most, if not all, integrin-mediated functions. The structural basis for talin's unique ability to activate integrins through PTBs has been defined [9]. Structural data on integrins are mostly derived from mouse and human and the structural basis for the activation of integrins through their cytoplasmic domains in other species is not yet known.
Localization and function
Integrins function as traction receptors that can both transmit and detect changes in mechanical force acting on the extracellular matrix. In mammals, some integrins are limited to certain cell types or tissues: αIIbβ3 to platelets; α6β4 to keratinocytes; αEβ7 to T cells, dendritic cells and mast cells in mucosal tissues; α4β1 to leukocytes; α4β7 to a subset of memory T cells; and the β2 integrins to leukocytes. Other integrins are widely distributed, such as αVβ3, which is expressed on endothelium. The RGD sequence in fibronectin was originally identified as an integrin-binding motif [10] and this and related sequences in extracellular matrix molecules do act as integrin-binding motifs in vivo. However, integrins also recognize many non-RGD sequences in their ligands, such as the tripeptide LDV in the immunoglobulin superfamily member vascular cell adhesion molecule 1 (VCAM-1), which is expressed on inflamed endothelium and is bound by α4β1. This pattern of integrin recognition and activation appears to be conserved among most mammals studied.
In regard to ligand specificity, the mammalian integrins can be broadly grouped into laminin-binding integrins (α1β1, α2β1, α3β1, α6β1, α7β1, and α6β4), collagen-binding integrins (α1β1, α2β1, α3β1, α10β1, and α11β1), leukocyte integrins (αLβ2, αMβ2, αXβ2, and αDβ2), and RGD-recognizing integrins (α5β1, αVβ1, αVβ3, αVβ5, αVβ6, αVβ8, and αIIbβ3). Individual integrins have unique ligand specificities (Table 2). They are further defined by those α subunits that can contain the I domain: these are α1, α2, α10, α11, αL, αM, αX, αD, and αE. Non-I-domain subunits are α3, α4, α5, α6, α7, α8, α9, αV, and αIIb. In I-domain integrins, the I domains play a central role in ligand binding and intercellular adhesion. Integrin binding among invertebrate species is less well studied; RGD sequences have been found in species as diverse as sea urchins and amoebae, however, and integrins and their biochemical functions are likely to be highly conserved in metazoa, due to the essential nature of their function.
Table 2.
Ligand-binding specificities of human integrins
| Integrins | Ligands |
| α1β1 | Laminin, collagen |
| α2β1 | Laminin, collagen, thrombospondin, E-cadherin, tenascin |
| α3β1 | Laminin, thrombospondin, uPAR |
| α4β1 | Thrombospondin, MAdCAM-1, VCAM-1, fibronectin, osteopontin, ADAM, ICAM-4 |
| α5β1 | Fibronectin, osteopontin, fibrillin, thrombospondin, ADAM, COMP, L1 |
| α6β1 | Laminin, thrombospondin, ADAM, Cyr61 |
| α7β1 | Laminin |
| α8β1 | Tenascin, fibronectin, osteopontin, vitronectin, LAP-TGF-β, nephronectin |
| α9β1 | Tenascin, VCAM-1, osteopontin, uPAR, plasmin, angiostatin, ADAM [25], VEGF-C, VEGF-D [26] |
| α10β1 | Laminin, collagen |
| α11β1 | Collagen |
| αVβ1 | LAP-TGF-β, fibronectin, osteopontin, L1 |
| αLβ2 | ICAM, ICAM-4 |
| αMβ2 | ICAM, iC3b, factor X, fibrinogen, ICAM-4, heparin |
| αXβ2 | ICAM, iC3b, fibrinogen, ICAM-4, heparin, collagen [27] |
| αDβ2 | ICAM, VCAM-1, fibrinogen, fibronectin, vitronectin, Cyr61, plasminogen |
| αIIbβ3 | Fibrinogen, thrombospondin,, fibronectin, vitronectin, vWF, Cyr61, ICAM-4, L1, CD40 ligand [28] |
| αVβ3 | Fibrinogen, vitronectin, vWF, thrombospondin, fibrillin, tenascin, PECAM-1, fibronectin, osteopontin, BSP, MFG-E8, ADAM-15, COMP, Cyr61, ICAM-4, MMP, FGF-2 [29], uPA [30], uPAR [31], L1, angiostatin [32], plasmin [33], cardiotoxin [34], LAP-TGF-β, Del-1 |
| α6β4 | Laminin |
| αVβ5 | Osteopontin, BSP, vitronectin, CCN3 [35], LAP-TGF-β |
| αVβ6 | LAP-TGF-β, fibronectin, osteopontin, ADAM |
| α4β7 | MAdCAM-1, VCAM-1, fibronectin, osteopontin |
| αEβ7 | E-cadherin |
| αVβ8 | LAP-TGF-β |
References are included for recently discovered ligands only. Abbreviations: ADAM, a disintegrin and metalloprotease; BSP, bone sialic protein; CCN3, an extracellular matrix protein; COMP, cartilage oligomeric matrix protein; Cyr61, cysteine-rich protein 61; L1, CD171; LAP-TGF-β, TGF-β latency-associated peptide; iC3b, inactivated complement component 3; PECAM-1, platelet and endothelial cell adhesion molecule 1; uPA, urokinase; uPAR, urokinase receptor; VEGF, vascular endothelial growth factor; vWF, von Willebrand Factor.
Upon binding an extracellular ligand, integrins generate an intracellular signal and, conversely, their functioning can be regulated by signals from within the cell [1]. They serve as transmembrane links between extracellular contacts (other cells or the extracellular matrix) and the actin microfilaments of the cytoskeleton, whose behavior integrins also regulate and modulate. Many different proteins on the cytoplasmic side of the membrane, such as talin, vinculin, and ERM (ezrin, radixin, moesin) actin-binding proteins, act as linker proteins to connect the cytoplasmic domains of integrins to the cytoskeleton, resulting in complex interactions [1]. Extracellular ligation of integrins triggers a large variety of signal transduction events that modulate cell behaviors such as adhesion, proliferation, survival or apoptosis, shape, polarity, motility, haptotaxis, gene expression, and differentiation, mostly through effects on the cytoskeleton.
The deletion of individual genes by gene knockout in mice shows that particular integrins play a critical role in development (the β1 integrins), vasculogenesis (αV integrins), lymphangiogenesis (α9β1), thrombus formation (αIIbβ3), the integrity of the skin (α6β4), and immune responses (the β2 integrins) (Table 3). Knockout of the gene for β3 enhanced tumorigenesis and angiogenesis [11,12], enhanced wound healing [13], and enhanced inflammation and atherosclerosis [14], suggesting that αVβ3 normally suppresses these processes.
Table 3.
Phenotypes of deletions of integrin subunits in the mouse
| Integrin subunit | Viability | Fertility | Phenotype | Reference |
| α1 | + | + | Defects in bone healing and reduced tumor angiogenesis | [36] |
| α2 | + | + | Reduced branching morphogenesis and platelet adhesion | [37] |
| α3 | Perinatal lethal | + | Kidney, lung, and skin defects | [38] |
| α4 | Embryonic lethal | - | Placental and heart defects | [39] |
| α5 | Embryonic lethal | - | Mesodermal and vascular defects | [40] |
| α6 | Perinatal lethal | + | Epidermal detachment, defect in neurogenesis | [41] |
| α7 | + | + | Muscular dystrophy | [42] |
| α8 | Perinatal lethal | + | Kidney defect | [43] |
| α9 | Perinatal lethal | + | Chylothorax (defect in lymphatic drainage) | [44] |
| αv | Embryonic and perinatal lethal | + | Cerebral hemorrhage | [45] |
| αM | + | + | Neutrophil adhesion and degranulation | [46] |
| αL | + | + | Neutrophil emigration | [47] |
| αD | + | + | Reduced T-cell response and T-cell phenotypic changes | [48] |
| αE | + | + | Skin inflammation | [49] |
| β1 | Embryonic lethal | - | Fails to gastrulate | [45,50] |
| β2 | + | + | Leukocyte adhesion deficiency | [51] |
| β3 | + | + | Platelet defect | [52] |
| β4 | Perinatal lethal | + | Epidermal detachment | [53] |
| β5 | + | + | Accelerated age-related blindness | [54] |
| β6 | + | + | Inflammation in skin and lungs | [55] |
| β7 | + | + | Gut-associated lymphocyte defects | [56] |
| β8 | Embryonic and perinatal lethal | + | Cerebral hemorrhage | [57] |
Inside-out signaling regulates integrin affinity
The affinity of individual integrins for their ligands in mammals is tightly regulated by their heterodimeric structure and by cytoplasmic signals from within the cell (inside-out signaling). Integrins can be activated intracellularly by signals from G-protein-coupled receptors that lead to phosphorylation of the cytoplasmic domain of the β subunit. The association of the α and β cytoplasmic tails appears to be required to maintain an integrin in the inactive state; the association is disrupted by treatment with agonists such as chemokines that are known to cause integrin activation and which signal via G-protein-coupled receptors [15] (Figure 4).
Figure 4.
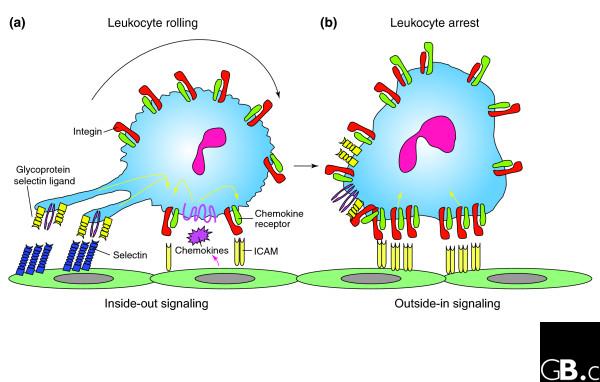
Leukocyte recruitment to the endothelial surface. (a) Binding of glycoprotein selectin ligands (yellow and purple) on the leukocyte to selectins (blue) on the endothelial surface, and weak binding of low-affinity leukocyte integrins (green) to ICAMs (pale yellow) on the endothelium facilitates cell tethering and rolling. This binding, together with signals from chemokines (pink), generates inside-out signals (yellow arrows) that shift the bound integrins to a high-affinity ligand-binding state. (b) Leukocyte arrest is mediated by clusters of high-affinity integrins (red) binding to ICAMs on the endothelial cells. These focal clusters can themselves signal outside-in to affect functions such as cell polarization and migration.
The cytoskeletal adaptor protein talin has been proposed to play a role in regulating integrin affinity. Binding of the talin head region to the integrin β cytoplasmic tail causes dissociation of the α and β tails and induces a conformational change in the extracellular region that increases its affinity for its ligand [15]. Two models have been proposed for this change in affinity. In both, the inactive integrin is in a bent conformation, with the headpiece facing the membrane. In the 'deadbolt model' the bent conformation is maintained in an activated integrin, but piston-like movements of the transmembrane regions cause sliding of the extracellular stalks of the α and β subunits, which disrupts the interaction between the headpiece and the β stalk just beyond the membrane (the deadbolt) [16]. In the 'switchblade model', dissociation of the α and β cytoplasmic and transmembrane regions leads to dislocation of an epidermal growth factor (EGF)-like repeat in the β stalk, which causes the head region to extend outwards in a switchblade-like movement [17]. In both models, these proposed events correlate within seconds with integrin 'activation', leading to conformational changes in the ligand-binding pocket of the headpiece that increase its affinity for ligand.
The affinity directly regulates the nature of the ligand binding and appears to tune the degree and kinetics of cell adhesion. In leukocytes, for instance, αLβ2 in an intermediate-affinity state will interact with its ligand on endothelium to help decelerate the leukocytes, which roll slowly along the vessel wall but do not arrest (Figure 4a). Conversion of αLβ2 to the high-affinity state by intracellular signals from other receptors mediates their complete arrest (Figure 4b) and signals cell polarization and leukocyte movement across the post-capillary venule wall into the inflamed tissue [18].
Outside-in signaling relays signals from the extracellular environment
It has been proposed that on binding extracellular ligands, mammalian integrins cluster in the membrane and transduce signals to the interior of the cell (outside-in signaling; Figure 4b). Extracellular ligand binding induces conformational changes, including the outward swing of the hybrid domain, separation of the α and β 'leg' domains (Figure 3b), and separation of the transmembrane domains, that lead to the interaction of the cytoplasmic tails with intracellular signaling molecules [16]. These include enzymes (for example, the focal adhesion kinase/c-Src, and the small GTPases Ras and Rho) and adaptors (for example, Cas/Crk and paxillin) that assemble within dynamic adhesion structures, including focal adhesions that bind cells to the extracellular matrix and podosomes (small foot-like extensions of plasma membrane) [15,19]. In this manner, the affinity of an integrin and its valence in binding ligands such as intercellular adhesion molecule-1 (ICAM-1) regulate the extent of outside-in signaling at the site of focal adhesive contacts (Figure 4). These contacts are active sites that transduce information such as the density of extracellular ligand or the magnitude and direction of extracellular forces on the cell. Integrins can also be activated from the outside by the binding of divalent cations to the metal-ion-binding sites in the I and I-like domains in the α and β subunits, respectively.
Binding of RGD-containing peptides or related compounds to a site in the headpiece of the integrin heterodimers has been shown in crystal structures of αVβ3 [4,5] and αIIbβ3 [6]. The binding site is composed of the β-propeller domain of the α subunit and the I-like domain of the β subunit. The original crystal structure of integrin αVβ3 revealed a bent conformation of the head region associated with low affinity for ligand [4,5]. It was therefore proposed that the bent form does not bind ligand or carry out outside-in signaling and that activated integrins have an extended form (see the switchblade model described above). Interestingly, it has been shown that the bent form of αVβ3 can still bind to fibronectin [20] (see the deadbolt model described above). Several intermediate forms of integrin conformation have been postulated that confer ligand-binding affinities and a different activation and cell adhesion status from either the bent or the extended forms [21].
The medical potential of antagonists to human integrins
The interaction of integrins with their ligands is a major target for the development of therapeutic drugs. A humanized anti-β3 antibody (abciximab) that blocks the binding of platelet integrin αIIbβ3 to fibrinogen has been used in the clinic to prevent thrombosis [22]. A humanized anti-α4 antibody (natalizumab) that can block the α4β1-VCAM interaction or the α4β7-mucosal addressin cell adhesion molecule (MAdCAM) interaction on mucosal endothelium has been tested in clinical trials. Natalizumab blocks leukocyte trafficking across the blood-brain barrier and thereby moderates inflammation in multiple sclerosis [23]. Anti-α4 antibody is also effective in clinical trials in ameliorating inflammatory bowel diseases, for example, Crohn's disease. Many RGD-based low-molecular-weight integrin antagonists have been developed and some of them have been approved as therapeutics (for example, eptifibatide and tirofiban as inhibitors of αIIbβ3 to reduce platelet aggregation and the formation of blood clots) [24]. As more becomes known about the relationship between integrin three-dimensional structure and how this regulates affinity for ligand and signaling into the cell, antagonists can be designed that stabilize a specific conformation, thereby promoting or blocking specific intercellular adhesion functions.
Frontiers
Integrins are transmembrane molecules that are essential for both embryonic development and immunological function by binding to a wide variety of ligands, including extra-cellular matrix molecules and members of the immunoglobulin superfamily. Their capacity to specifically recognize particular amino-acid motifs and regulate binding affinity to them lies in their heterodimeric structure. This molecular design incorporates a remarkable ability to direct conformational changes initiated at the cytoplasmic domain, and also to signal extracellular ligand binding back to the inside of the cell. Much of our current knowledge of the myriad of functions attributed to ligand binding of a particular αβ pair comes from gene knockout studies in mouse or from rare hereditary disorders in humans. Only a handful of crystal structures of integrins bound to their ligands have been solved. From these data it appears that small variations in the particular structure or charge of a ligand (that is, down to single atoms) can strongly influence the binding affinity and the capacity of the integrin to maintain a conformation that signals back into the cell. This implies that ligand binding can influence allosteric changes in the integrin, which in turn dictate how the integrin reports on the environment in which the cell finds itself. Thus, integrins serve as both sensors of their molecular surroundings and effectors that conduct motile forces exerted by the cell's cytoskeleton and from the dynamic environment (that is, shear forces within blood vessels). We are just beginning to understand the structural and chemical basis of this sensor-effector system. A particularly exciting development is the discovery of small molecules that bind tightly to the ligand-binding pocket or to other domains and allosterically stabilize integrin conformations that promote or antagonize binding. For instance, small molecules have been discovered that can allosterically tune conformations of αLβ2 that favor low, intermediate, and high-affinity binding. In this manner, it is possible to steer the adhesive response of a leukocyte in a blood vessel to promote tethering and rolling, firm arrest, or no binding at all. It may be possible to apply such small molecules as therapeutics either to promote leukocyte recruitment at sites of infection or to block their accumulation in chronic inflammatory diseases such as in rheumatoid arthritis and psoriasis. As more knowledge accumulates relating amino-acid sequence to common structural motifs associated with the allosteric control of ligand recognition and outside-in signaling to the cytoplasm, it will become possible to design small molecules that target these critical domains.
Additional data files
Additional data are available online with this article: Additional data file 1 contains tables of the integrin subunits present in the mouse, chicken, zebrafish, nematodes, Xenopus laevis, and D. melanogaster.
Supplementary Material
Tables of integrin subunits in mouse, chicken, zebrafish, C. elegans, X. laevis and D. melanogaster.
References
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov. 2003;2:703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- Huhtala M, Heino J, Casciari D, de Luise A, Johnson MS. Integrin evolution: insights from ascidian and teleost fish genomes. Matrix Biol. 2005;24:83–95. doi: 10.1016/j.matbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Andrzej J, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin αvβ3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzon-McLaughlin W, Takada Y. Critical residues for ligand binding in an I domain-like structure of the integrin beta1 subunit. J Biol Chem. 1996;271:20438–20443. doi: 10.1074/jbc.271.31.19008. [DOI] [PubMed] [Google Scholar]
- Kamata T, Tieu KK, Irie A, Springer TA, Takada Y. Amino acid residues in the alpha IIb subunit that are critical for ligand binding to integrin alpha IIbbeta 3 are clustered in the beta-propeller model. J Biol Chem. 2001;276:44275–44283. doi: 10.1074/jbc.M107021200. [DOI] [PubMed] [Google Scholar]
- Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Taverna D, Moher H, Crowley D, Borsig L, Varki A, Hynes RO. Increased primary tumor growth in mice null for beta3- or beta3/beta5-integrins or selectins. Proc Natl Acad Sci USA. 2004;101:763–768. doi: 10.1073/pnas.0307289101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna D, Crowley D, Connolly M, Bronson RT, Hynes RO. A direct test of potential roles for beta3 and beta5 integrins in growth and metastasis of murine mammary carcinomas. Cancer Res. 2005;65:10324–10329. doi: 10.1158/0008-5472.CAN-04-4098. [DOI] [PubMed] [Google Scholar]
- Reynolds LE, Conti FJ, Lucas M, Grose R, Robinson S, Stone M, Saunders G, Dickson C, Hynes RO, Lacy-Hulbert A, et al. Accelerated re-epithelialization in beta3-integrin-deficient-mice is associated with enhanced TGF-beta1 signaling. Nat Med. 2005;11:167–174. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]
- Weng S, Zemany L, Standley KN, Novack DV, La Regina M, Bernal-Mizrachi C, Coleman T, Semenkovich CF. Beta3 integrin deficiency promotes atherosclerosis and pulmonary inflammation in high-fat-fed, hyperlipidemic mice. Proc Natl Acad Sci USA. 2003;100:6730–6735. doi: 10.1073/pnas.1137612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CE, Schaff UY, Sarantos MR, Lum AF, Staunton DE, Simon SI. Dynamic shifts in LFA-1 affinity regulate neutrophil rolling, arrest, and transmigration on inflamed endothelium. Blood. 2006;107:2101–2111. doi: 10.1182/blood-2005-06-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ. Integrins and Src: dynamic duo of adhesion signaling. Trends Cell Biol. 2005;15:399–403. doi: 10.1016/j.tcb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Adair BD, Xiong JP, Maddock C, Goodman SL, Arnaout MA, Yeager M. Three-dimensional EM structure of the ectodomain of integrin {alpha}V{beta}3 in a complex with fibronectin. J Cell Biol. 2005;168:1109–1118. doi: 10.1083/jcb.200410068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantos MR, Raychaudhuri S, Lum AF, Staunton DE, Simon SI. Leukocyte function-associated antigen 1-mediated adhesion stability is dynamically regulated through affinity and valency during bond formation with intercellular adhesion molecule-1. J Biol Chem. 2005;280:28290–28298. doi: 10.1074/jbc.M501662200. [DOI] [PubMed] [Google Scholar]
- Gabriel HM, Oliveira EI. Role of abciximab in the treatment of coronary artery disease. Expert Opin Biol Ther. 2006;6:935–942. doi: 10.1517/14712598.6.9.935. [DOI] [PubMed] [Google Scholar]
- O'Connor P. Natalizumab and the role of alpha 4-integrin antagonism in the treatment of multiple sclerosis. Expert Opin Biol Ther. 2007;7:123–136. doi: 10.1517/14712598.7.1.123. [DOI] [PubMed] [Google Scholar]
- Meyer A, Auernheimer J, Modlinger A, Kessler H. Targeting RGD recognizing integrins: drug development, biomaterial research, tumor imaging and targeting. Curr Pharm Des. 2006;12:2723–2747. doi: 10.2174/138161206777947740. [DOI] [PubMed] [Google Scholar]
- Eto K, Huet C, Tarui T, Kupriyanov S, Liu HZ, Puzon-McLaughlin W, Zhang XP, Sheppard D, Engvall E, Takada Y. Functional classification of ADAMs based on a conserved motif for binding to integrin alpha 9beta 1; implications for sperm-egg binding and other cell interactions. J Biol Chem. 2002;277:17804–17810. doi: 10.1074/jbc.M200086200. [DOI] [PubMed] [Google Scholar]
- Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin {alpha}9{beta}1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnotel R, Rittie L, Poitevin S, Monboisse J-C, Nguyen P, Potron G, Maquart F-X, Randoux A, Gillery P. Human blood monocytes interact with type I collagen through {alpha}x{beta}2 integrin (CD11c-CD18, gp150-95). J Immunol. 2000;164:5928–5934. doi: 10.4049/jimmunol.164.11.5928. [DOI] [PubMed] [Google Scholar]
- Andre P, Prasad KSS, Denis CV, He M, Papalia JM, Hynes RO, Phillips DR, Wagner DD. CD40L stabilizes arterial thrombi by a [beta]3 integrin-dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- Rusnati M, Tanghetti E, Dell'Era P, Gualandris A, Presta M. alphav-beta3 integrin mediates the cell-adhesive capacity and biological activity of basic fibroblast growth factor (FGF-2) in cultured endothelial cells. Mol Biol Cell. 1997;8:2449–2461. doi: 10.1091/mbc.8.12.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarui T, Akakura N, Majumdar M, Andronicos N, Takagi J, Mazar AP, Bdeir K, Kuo A, Yarovoi SV, Cines DB, et al. Direct interaction of the kringle domain of urokinase-type plasminogen activator (uPA) and integrin alpha v beta 3 induces signal transduction and enhances plasminogen activation. Thromb Haemost. 2006;95:524–534. doi: 10.1160/TH05-06-0457. [DOI] [PubMed] [Google Scholar]
- Tarui T, Mazar AP, Cines DB, Takada Y. Urokinase-type plasminogen activator receptor (CD87) is a ligand for integrins and mediates cell-cell interaction. J Biol Chem. 2001;276:3983–3990. doi: 10.1074/jbc.M008220200. [DOI] [PubMed] [Google Scholar]
- Tarui T, Miles LA, Takada Y. Specific interaction of angiostatin with integrin {alpha}v{beta}3 in endothelial cells. J Biol Chem. 2001;276:39562–39568. doi: 10.1074/jbc.M101815200. [DOI] [PubMed] [Google Scholar]
- Tarui T, Majumdar M, Miles LA, Ruf W, Takada Y. Plasmin-induced migration of endothelial cells: A potential target for the anti-angiogenic action of angiostatin. J Biol Chem. 2002;277:33564–33570. doi: 10.1074/jbc.M205514200. [DOI] [PubMed] [Google Scholar]
- Wu PL, Lee SC, Chuang CC, Mori S, Akakura N, Wu WG, Takada Y. Non-cytotoxic cobra cardiotoxin A5 binds to integrin alpha vbeta 3 and inhibits bone resorption: Identification of cardiotoxins as non-RGD integrin-binding proteins of the Ly-6 family. J Biol Chem. 2006;281:7937–7945. doi: 10.1074/jbc.M513035200. [DOI] [PubMed] [Google Scholar]
- Lin CG, Chen C-C, Leu S-J, Grzeszkiewicz TM, Lau LF. Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J Biol Chem. 2005;280:8229–8237. doi: 10.1074/jbc.M404903200. [DOI] [PubMed] [Google Scholar]
- Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- Muller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha8beta1 is critically important for epithelialmesenchymal interactions during kidney morphogenesis. Cell. 1997;88:603–613. doi: 10.1016/S0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/MCB.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/S0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Lu H, Smith CW, Perrard J, Bullard D, Tang L, Shappell SB, Entman ML, Beaudet AL, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J Clin Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- Wu YM, Robinson DR, Kung HJ. Signal pathways in up-regulation of chemokines by tyrosine kinase MER/NYK in prostate cancer cells. Cancer Res. 2004;64:7311–7320. doi: 10.1158/0008-5472.CAN-04-0972. [DOI] [PubMed] [Google Scholar]
- Schon MP, Schon M, Warren HB, Donohue JP, Parker CM. Cutaneous inflammatory disorder in integrin alphaE (CD103)-deficient mice. J Immunol. 2000;165:6583–6589. doi: 10.4049/jimmunol.165.11.6583. [DOI] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Wilson RW, Ballantyne CM, Smith CW, Montgomery C, Bradley A, O'Brien WE, Beaudet AL. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol. 1993;151:1571–1578. [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Huang X, Griffiths M, Wu J, Farese RV, Jr, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol. 2000;20:755–759. doi: 10.1128/MCB.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N, Lohler J, Kunkel EJ, Ley K, Leung E, Krissansen G, Rajewsky K, Muller W. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S, Azumi K, Nonaka M. Cloning and characterization of integrin alpha subunits from the solitary ascidian, Halocynthia roretzi. J Immunol. 2001;166:1710–1715. doi: 10.4049/jimmunol.166.3.1710. [DOI] [PubMed] [Google Scholar]
- Brower DL, Brower SM, Hayward DC, Ball EE. Molecular evolution of integrins: genes encoding integrin beta subunits from a coral and a sponge. Proc Natl Acad Sci USA. 1997;94:9182–9187. doi: 10.1073/pnas.94.17.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables of integrin subunits in mouse, chicken, zebrafish, C. elegans, X. laevis and D. melanogaster.


