Abstract
The voltage-gated potassium channel Kv1.3 has been recently identified as a molecular target that allows for selective pharmacological suppression of effector memory T (TEM) cells without affecting the function of naïve and central memory T cells. We here investigated whether PAP-1, a small molecule Kv1.3 blocker (EC50 = 2nM), could suppress allergic contact dermatitis (ACD). In a rat model of ACD, we first confirmed that the infiltrating cells in the elicitation phase are indeed CD8+ CD45RC− memory T cells with high Kv1.3 expression. In accordance with its selective effect on TEM cells, PAP-1 did not impair sensitization, but potently suppressed oxazolone-induced inflammation by inhibiting the infiltration of CD8+ T cells and reducing the production of the inflammatory cytokines IFN- γ, IL-2, and IL-17 when administered intraperitoneally or orally during the elicitation phase. PAP-1 was equally effective when applied topically, demonstrating that it effectively penetrates skin. We further show that PAP-1 is not a sensitizer or an irritant and exhibits no toxicity in a 28-day toxicity study. Based on these results we propose that PAP-1 could potentially be developed into a drug for the topical treatment of inflammatory skin diseases such as psoriasis.
INTRODUCTION
Allergic contact dermatitis (ACD) is a CD8+ T-cell mediated cutaneous allergy (Grabbe and Schwarz, 1998; Vocanson et al., 2006) caused by haptens, which are small molecules with the ability to penetrate the skin and form complexes with proteins. Haptens can be found in plants such as poison ivy, in industrial intermediates, textile dyes, or in consumer products such as perfumes and cosmetics. ACD itself is a very common and increasing health issue but it is also a commonly used model system to evaluate potential therapies for psoriasis. For example the topical calcineurin inhibitor pimecrolimus (Elidel®), which is highly effective in psoriasis (Rappersberger et al., 2002) and atopic dermatitis, was first suggested for the treatment of inflammatory skin diseases based on its effectiveness in ACD (Meingassner et al., 1997). Similar to ACD, psoriasis is an inflammatory skin disease mediated by CD8+ T cells exhibiting the features of effector memory T cells (TEM) and producing high levels of IFN-γ (Vissers et al., 2004; Bowcock and Krueger, 2005).
The objective of this study was to investigate whether PAP-1, a small molecule blocker of the voltage-gated potassium channel Kv1.3 could suppress ACD and might therefore potentially be useful for the treatment of psoriasis. Kv1.3 is one of 76 potassium channels in the human genome (DeCoursey et al., 1984; Gutman et al., 2003) and together with the calcium-activated potassium channel KCa3.1 is expressed in human T cells (Logsdon et al., 1997; Ghanshani et al., 2000) where these two channels provide the counter-balancing potassium efflux for the calcium influx that is necessary for T-cell activation (Cahalan et al., 2001; Chandy et al., 2004). However, while naïve (CCR7+CD45RA+) and central memory (CCR7+ CD45RA−) T cells increase expression of KCa3.1 during activation and rely on this channel for their proliferation and cytokine production, CCR7− TEM (Sallusto et al., 1999) upregulate Kv1.3 and are dependent on this channel for their cytokine secretion and proliferation (Wulff et al., 2003b; Beeton et al., 2006). Based on this differential overexpression in TEM cells Kv1.3 blockers have been proposed as new TEM-cell-specific immunosuppresssants (Wulff et al., 2003a; Chandy et al., 2004) for the therapy of autoimmune diseases where autoreactive TEM cells are involved in the pathogenesis such as multiple sclerosis, type- 1 diabetes, rheumatoid arthritis, and psoriasis (Markovic- Plese et al., 2001; Viglietta et al., 2002; Wulff et al., 2003b; Fasth et al., 2004; Vissers et al., 2004). In proof of this concept, the autoreactive T cells in the blood and the infiltrating T cells in the brains from multiple sclerosis patients (Wulff et al., 2003b; Rus et al., 2005) have been found to be Kv1.3high CCR7− TEM cells and several Kv1.3- blocking peptides have been shown to treat experimental autoimmune encephalomyelitis and to effectively suppress delayed type hypersensitivity in rats and mini pigs (Koo et al., 1997, 1999; Beeton et al., 2001a, b, 2005). However, similar to the human lymphocyte function-associated antigen-3 (LFA-3)–IgG1 fusion protein Alefacept®, which targets CD2 and therefore blocks TEM-cell activation and is supposed to lead to their apoptosis in psoriatic lesions (Ellis and Krueger, 2001; Krueger, 2002; Krueger et al., 2002), the Kv1.3- blocking peptides ShK(L5) and margatoxin are peptides and require parenteral application. Through a classical medicinal chemistry approach our laboratory designed a selective small molecule blocker of Kv1.3 called PAP-1 (5-(4-phenoxybutoxy) psoralen), which blocks Kv1.3 with an EC50 of 2 nM and potently inhibits the proliferation of human CCR7− TEM cells while sparing naïve and central memory T cells (Schmitz et al., 2005). In rats, intraperitoneally (i.p.) or orally administered PAP-1 effectively suppresses delayed type hypersensitivity against ovalbumin, a CD4+ TEM-mediated inflammatory reaction. PAP-1 further exhibits an excellent selectivity for Kv1.3 over other ion channels, transporters, receptors, and P450-dependent enzymes and was found not to be phototoxic, cytotoxic, or mutagenic (Schmitz et al., 2005).
Taken together with its relatively high lipophilicity (logP = 4.03), this pharmacological profile might make PAP- 1 an ideal drug for the treatment of inflammatory skin diseases like psoriasis. Such a treatment would potentially combine the advantages of specifically targeting TEM cells, a concept that is already in clinical use with the protein Alefacept®, with the advantages of an orally available or skin permeable small molecule. As a first step we therefore tested PAP-1 in a rat model of ACD and here report that in accordance with its selective effect on TEM cells PAP-1 effectively suppresses the elicitation phase of ACD without impairing antigen presentation during the sensitization phase. We would like to point out here, that mouse models are unsuitable for testing Kv1.3 blockers because mouse T cells also express Kv1.1, Kv1.2, and Kv3.1 channels in addition of Kv1.3 and unlike human and rat T cells are not affected by Kv1.3 inhibitors (Freedman et al., 1995; Koo et al., 1997; Liu et al., 2002; Beeton and Chandy, 2005).
RESULTS
Ear infiltrating cells in rat ACD are CD8+, CD45RC−, and Kv1.3+
Contact hypersensitivity is most commonly studied in mice, however rats have been reported to develop a similar ACD reaction to dinitrofluorobenzene (Nelson et al., 1999; Elliott et al., 2006). In both cases cytotoxicity is mediated by IFN-γ-producing CD8+ effector T cells. These CD8+ cells have been postulated to be TEM cells (Saint-Mezard et al., 2004), but have never formally been shown to have this phenotype. Before testing whether PAP-1 can suppress ACD we therefore first established and characterized oxazolone-induced ACD in rats and determined whether the ear infiltrating cells in rat ACD are Kv1.3 expressing CD8+ memory T cells. Female Lewis rats were sensitized with oxazolone and then challenged 7 days later by application of either 0.5% oxazolone or vehicle onto the ear. Although vehicle-treated ears (= unchallenged ears) only displayed an occasional CD8+ T cell after 24 hours (Figure 1a), CD8+ T cells appeared in the oxazolone-treated ears within 8 hours as determined by RT-PCR and immunohistochemistry. By 24 hours the ears contained a heavy infiltrate of CD8+ T cells (Figure 1b) and CD68-positive macrophages but no CD4+ T cells (data not shown). Immunohistochemistry of consecutive sections revealed that these infiltrating CD8+ T cells were predominantly CD45RC− (Bell et al., 1998) (Figure 1c) demonstrating that they are of an effector memory phenotype. Most of the infiltrating cells also stained strongly positive with a polyclonal anti-Kv1.3 Ab (Koch et al., 1997) confirming our hypothesis that Kv1.3 potentially constitutes a target for the treatment of ACD (Figure 1e and f). In both the control ear and the challenged ear we observed some staining of the epidermis and of the follicular bulbs. This is probably nonspecific and may be due to the polyclonal origin of the antibody. Moreover we observed similar staining with other antibodies like CD3 or CD103 suggesting a tendency of the epidermis and the follicular bulbs to nonspecifically adsorb antibodies.
Figure 1. Ear infiltrating cells in ACD are CD8+, CD45RC−, and Kv1.3+.
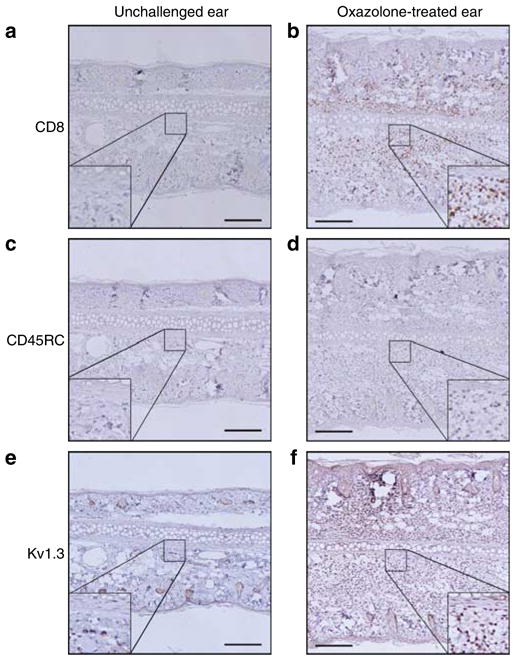
(a, c, and e) Consecutive slides from a vehicle-treated rat ear, whereas (b, d, and f) are pictures of consecutive slides from an oxazolone challenged ear. (a and b) Stained for CD8, (c and d) for CD45RC, and (e and f) for Kv1.3. Bar = 200 μm.
Pharmacokinetics of PAP-1
In order to determine if PAP-1 is sufficiently bioavailable to treat ACD we first studied its pharmacokinetics. Following intravenous administration at 6 mg/kg we measured total PAP-1 plasma concentrations at various times after application by HPLC-Mass Spectrometer (MS)/MS (Figure 2a). PAP-1 concentrations fell from a peak of 27.2 μM at 5 minutes after application with bi-exponential kinetics to 16 nM at 24 hours. The best fit of the data was obtained with a two-compartment model (Winnonlin® software), which assumes a relatively rapid distribution into tissue followed by a slower elimination with first-order kinetics. The half-life of PAP-1 was calculated from the elimination part of the plot according to the formula t1/2 = ln(2)/β and found to be 3.0±0.08 hours.
Figure 2. Pharmacokinetics of PAP-1.
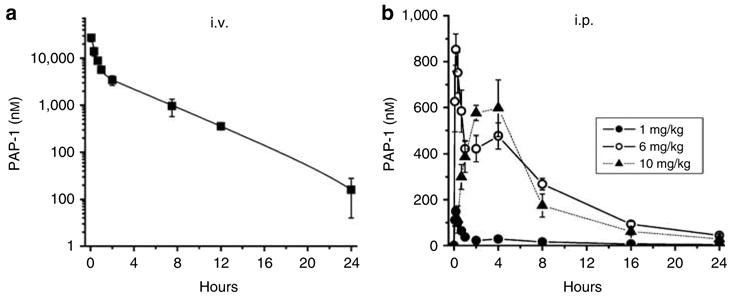
(a) Total PAP-1 plasma concentrations in Lewis rats (n = 3) following intravenous injection at 6mg/kg. The data were fitted as a second-order exponential decay for a two-compartment model with Winnonlin® software (y = A×exp−αxt+B ×exp−βxt where A = 9.58±0.72, B = 2.00±0.16, α = 3.18±0.33 hours−1 and β = 0.23±0.01 hours−1; K10 = 0.99±0.06 hours−1, K12 = 1.68±0.23 hours−1, and K21 = 0.74±0.09 hours−1; steady-state volume of distribution Vss = 1.6±0.06 l/kg. (b) Total PAP-1 plasma concentrations following i.p. injection at 1 (●), 6 (○), or 10 (▴) mg/kg (n = 3 for each concentration). All values are given as the mean±SEM of total PAP-1 concentrations.
We next injected PAP-1 i.p. at 1, 6, and 10 mg/kg and again measured total plasma concentrations at various time points (Figure 2b). At both 1 and 6 mg/kg we observed an initial spike in plasma levels followed by a plateau for roughly 5 hours and then elimination with mono-exponential kinetics. The quick increase and decrease in PAP-1 levels within the first 20 minutes after i.p. application is probably due to a rapid redistribution into tissue which is then followed by a longer phase during which absorption from the i.p. cavity, redistribution and elimination overlay, until elimination starts to dominate after 5 hours. Interestingly, after i.p. injection of 10 mg/kg we observed a much slower and steadier increase in plasma values followed by a plateau and then an exponential decrease over 24 hours. These differences in the shape of the plasma curve at different doses may be explained by slightly differing injection sites or could be caused by PAP-1 initially precipitating out of the more concentrated solution in the peritoneal cavity and thus inducing a slower release.
PAP-1’s i.p. bioavailability calculated by comparing the area under the plasma curve following intravenous and i.p. application at 6 mg/kg was found to be 16.3±0.9% (SEM). In parallel studies we also investigated PAP-1’s oral bioavailability following application by gavage in peanut oil and found it to between 10 and 20% (Beeton et al., 2006). Based on the half-life and the results of our i.p. studies, where we achieved plasma levels above 300 nM for approximately 8 hours with both 6 and 10 mg/kg, we decided to administer PAP-1 every 8 hours.
PAP-1 inhibits oxazolone-induced ear swelling during the elicitation phase when administered i.p., orally, or topically
After determining that PAP-1 is systemically available after i.p. application at 1, 6, and 10 mg/kg we used the same doses to treat the elicitation phase of ACD. The Kv1.3-blocking peptide ShK (EC50 11 pM) at a dose that has been previously shown to be effective in treating adoptive transfer experimental autoimmune encephalomyelitis (Beeton et al., 2001b) served as positive control. As shown in Figure 3a PAP-1 significantly and dose dependently reduced ACD. At 1 mg/kg the ear swelling was reduced to 71% of the control value and at 6 and 10 mg/kg to 40–43% of the control value. The fact that there was no significant difference between 6 and 10 mg/kg matches with the observation that plasma values following application of 6 and 10 mg/kg were very similar (Figure 2b). Subcutaneous treatment with ShK also reduced ACD very significantly to 32% of the control swelling. The fact that two Kv1.3 blockers of a very different chemical structure, a 4,000MW polypeptide and a small molecule, can induce the same level of inhibition demonstrates that the effect of both compounds in ACD is indeed due to blockade of Kv1.3 and not caused by an off-target effect. PAP-1 also significantly inhibited ACD when it was administered orally by gavage (Figure 3b). However, the effects were more variable probably due to differences in gastrointestinal motility leading to variations in the kinetics of absorption.
Figure 3. PAP-1 inhibits the elicitation phase of ACD when administered i.p., orally, or topically.
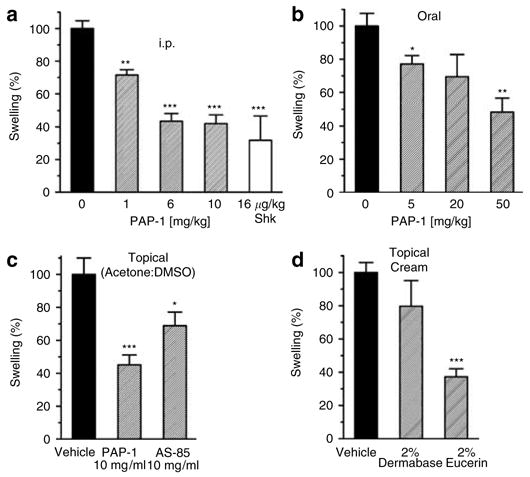
(a) Rats were treated i.p. with the vehicle Cremophor®EL/PBS (n = 30), PAP-1 at 1mg/kg (n = 9, 71.5±3.3% of control, P = 0.002), 6mg/kg (n = 20, 43.5±4.8% of control, P = 0.000001), 10 mg/kg (n = 16, 42.0±5.4% of control, P = 0.000001), or with ShK at 16 μg/kg (n = 3, 31.8±14.9% of control, P = 0.00015 vs control and P = 0.45 vs 10 mg/kg PAP-1). (b) Rats were gavaged with the vehicle peanut oil (n = 5) PAP-1 at 5mg/kg (n = 5, 77.2±5.0% of control, P = 0.037), 20 mg/kg (n = 5, 69.5±13.3% of control, P = 0.081) or 50mg/kg (n = 5, 48.4±8.2% of control, P = 0.002). (c) Rats were treated topically on the ears with 50 μl of the vehicle acetone:DMSO (n = 10), PAP-1 at 10 mg/ml (n = 10, 45.1±6.1% of control, P = 0.0002) or AS-85 at 10mg/ml (n = 9, 68.9±8.2% of control, P = 0.03). (d) PAP-1 was applied as a 2% cream in Dermabase™ (n = 7, 79.8±15.4% of control, P = 0.15) or Eucerin® (n = 8, 37.2±4.8% of control, P = 0.000001). The controls (n = 14) were treated either with “empty” Dermabase™ (n = 7) or Eucerin® (n = 7) and the results (181 μm swelling) averaged as there was no significant difference between the two vehicles. Values are given as the mean±SEM of swelling in percent. The average ear swelling in all controls from all conditions was 168.5 μm.
As the most desirable way to treat inflammatory skin diseases is obviously topically, we next tested whether PAP-1 could also reduce ACD when applied directly onto the skin. To maximize skin penetration we chose acetone:DMSO (9:1) as a first vehicle and applied 50 μl of solutions containing either PAP-1 or the related compound AS-85 at 10 mg/ml directly to the challenged ear. We included AS-85 in these experiments because it blocks Kv1.3 with similar potency (EC50 = 16 nM) but has a higher logP-value (logP 5.03 vs 4.03 for PAP-1) and had therefore previously been suggested by us for potential topical use (Schmitz et al., 2005). Although PAP- 1 reduced ear swelling to 45% of the control, AS-85 was less effective and reduced swelling only to 68% of the control (Figure 3c), demonstrating that PAP-1 is able to effectively penetrate into the skin whereas AS-85 seems be too lipophilic. A comparison of the results of the topical and the i.p. treatment with PAP-1 revealed that there was no significant difference between the two routes of application (P = 0.7 vs 10 mg/kg i.p.). Encouraged by these findings we next formulated PAP-1 in two cream bases to more closely simulate administration to humans and to determine if PAP-1 would be sufficiently liberated from a cream. With the oil-in-water base Dermabase™ we only observed a slight but not significant reduction in ear swelling, whereas PAP-1 formulated in the water-in-oil base Eucerin® significantly decreased ear swelling to 37% of the control value (Figure 3d). The poor results obtained with Dermabase™ may be explained by the fact that this formulation is water-soluble and can be wiped off very easily by the rats allowing insufficient time for PAP-1 to penetrate into the skin. In contrast, the more lipophilic Eucerin® requires a detergent to be removed and is therefore more resistant to cleaning and licking and thus gives PAP-1 more time to penetrate. These experiments do not allow us to suggest an optimal cream base for potential human therapy but they clearly prove that PAP-1 reduces ACD as effectively after topical application as after i.p. injection. Plasma samples taken after topical application further revealed that PAP-1 was achieving plasma concentrations of 30–200 nM demonstrating that it becomes systemically available and suggesting that it could even be formulated as a transdermal patch.
PAP-1 suppresses oxazolone-induced inflammation by reducing CD8+ T-cell infiltration and production of the inflammatory cytokines IFN-γ and IL-17 but not TNF-α
In vitro studies with PAP-1 and Kv1.3-blocking peptides have shown that Kv1.3 blockade potently suppresses proliferation and IFN-γ and IL-2 production by human TEM cells whereas having much less effect on the proliferation and cytokine production of naïve and central memory T cells (Beeton et al., 2005, 2006). Kv1.3 blockers have further been reported to be involved in integrin-mediated T-cell adhesion and migration (Levite et al., 2000). Based on these studies we hypothesized that PAP-1 inhibits ACD by reducing both infiltration of effector cells and production of inflammatory cytokines. We therefore immunized and challenged rats and then removed ears from vehicle- or PAP-1-treated animals 24 hours after the challenge. Immunohistochemistry revealed that PAP-1 strongly reduced the large CD8+ infiltrate observed in vehicle-treated rat ears (Figure 4a and b). We further compared messenger RNA (mRNA) expression for CD4, CD8-β, Kv1.3, IFN-γ, IL-17, IL-2, and TNF-α against unchallenged ears. CD4 mRNA was not induced in any sample (data not shown), confirming the absence of CD4+ T cells. CD8 mRNA was induced 5.9-fold in controls (Figure 4c) and PAP-1 treatment decreased CD8 mRNA induction significantly (2.4-fold, P = 0.0007) as expected from the inhibition of CD8+ T-cell recruitment observed on immunohistochemistry. Kv1.3 mRNA was induced 10.8 times in the controls (Figure 4c) confirming the involvement of Kv1.3 in the revelation phase of ACD. However, PAP-1 treatment did not decrease Kv1.3 mRNA expression as strongly (7.6-fold, P = 0.2, not significant) as we would have expected considering the CD8 mRNA reduction. This lack of effect on Kv1.3 mRNA levels could have several reasons including a lack of direct correlation between mRNA and protein expression (a phenomenon often observed with ion channels) or a masking of the PAP-1 effect by the low but clearly detectable Kv1.3 mRNA expression in the unchallenged ear which was used as a calibrator.
Figure 4. PAP-1 suppresses oxazolone-induced inflammation by reducing CD8+ T cells infiltration and production of the inflammatory cytokines IFN-γ and IL-17 but not TNF-α.
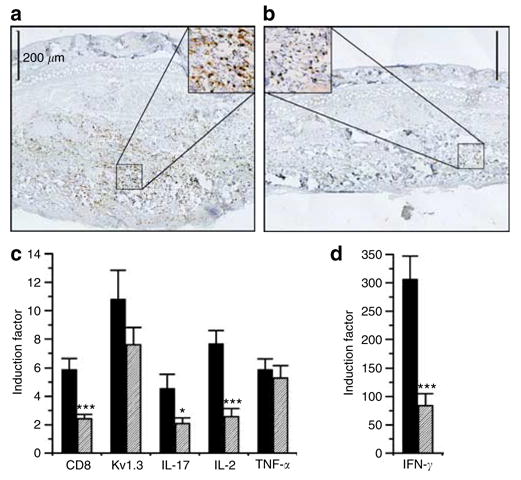
Ears were removed 24 hours after the challenge from (a) vehicle or (b) PAP-1-treated rats and stained for CD8+ T cells. Bar = 200 μm. (c and d) mRNA quantities measured for (c) CD8-β, Kv1.3, IL-17, IL-2, and TNF-α or (d) IFN-γ from vehicle (filled black bars) or PAP-1-(10mg/kg, diagonal strips) treated rats. Six unchallenged ears were used as calibrator. Results are expressed as induction factors against the calibrator±SEM (n = 10 ears).
As expected from the fact that IFN-γ is the most important proinflammatory molecule involved in ACD, oxazolone challenge induced a dramatic 300-fold increase in IFN-γ mRNA levels (Figure 4d). This increase was significantly reduced by PAP-1 treatment (84-fold, P = 0.0001) suggesting that PAP-1 effectively suppresses the effector function of the infiltrating cells. Other cytokines induced by oxazolone but to a much lower level were IL-2 (7.7-fold) and TNF-α (5.9- fold). IL-2 mRNA levels were significantly reduced by PAP-1 treatment (2.6-fold, P = 0.0002) (Figure 4c) consistent with the fact that Kv1.3 blockade suppresses nuclear factor of activated T-cell activation and thus IL-2 production (Dolmetsch et al., 1997; Chandy et al., 2004). In contrast, TNF-α mRNA levels were not reduced by PAP-1 treatment (5.3-fold, P = 0.62) (Figure 4c). Another inflammatory cytokine, that can be produced by both CD4+ (Yao et al., 1995) and CD8+ memory T cells (Shin et al., 1999) and that has been reported to be involved in the revelation phase of 2,4,6-trinitrochlorobenzene (TNCB) induced ACD in mice (Nakae et al., 2002), is IL-17. In our oxazolone-induced rat model IL-17 mRNA levels increased 4.5-fold after challenge (Figure 4c) and were reduced to 2.1-fold in PAP-1-treated rats (P = 0.039). In contrast to its effect on cytokine production in the inflamed ear, in vivo PAP-1 treatment did not inhibit ex vivo cytokine production by splenic CD8+ T cells (Figure S1).
PAP-1 does not prevent sensitization
In keeping with its mechanism of action (preferential suppression of Kv1.3high-activated TEM cells) PAP-1 should not affect antigen presentation and memory T-cell development during the sensitization phase of ACD. We therefore next treated rats with 10 mg/kg PAP-1 i.p. for 2 days before sensitization and for 3 days afterwards. The rats were then challenged 7 days after the sensitization and 4 days after the last PAP-1 application with 0.2% oxazolone. Given the fact that a drug is normally eliminated by more than 95% from the plasma within 4–5 half-lives there were no significant amounts of PAP-1 left in the plasma when the challenge occurred more than 24 half-lives after the last PAP-1 application. Following the challenge PAP-1-treated rats displayed the same ear swelling as the controls (Figure 5a; 104% of control), demonstrating that Kv1.3 blockade with PAP-1 does not prevent sensitization.
Figure 5. PAP-1 does not prevent sensitization or TPA-induced inflammation.
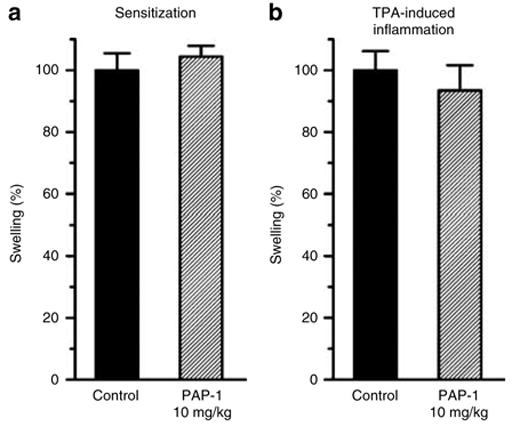
(a) PAP-1 does not prevent sensitization for ACD. Rats were injected i.p. with the vehicle Cremophor®EL/PBS (n = 8) or with PAP-1 at 10 mg/kg (n = 8) twice daily starting 2 days before sensitization with 1% oxazolone and for 3 days after sensitization. Animals were challenged with 0.2% oxazolone 7 days after sensitization. (b) PAP-1 does not suppress TPA-induced irritation. Rats were treated with 5 μg of TPA in 100 μl of AOO (4:1) on each side of both ears and injected i.p. with the vehicle Cremophor®EL/PBS (n = 4) or with PAP-1 at 10 mg/kg (n = 4) at 8-hour intervals starting 20 hours before and for 20 hours after TPA treatment. Both ears were measured. The average ear swelling in TPA controls was 110.8 μm. Values are given as the mean±SEM of swelling in percent.
PAP-1 does not prevent TPA-induced irritation
In order to confirm that PAP-1 selectively affects antigen-specific memory T-cell responses we next tested if PAP-1 suppresses nonspecific mast cell-mediated inflammation induced by the irritant 12-O-tetradecanoylphorbol 13-acetate (TPA) (Rao et al., 1993). As shown in Figure 5b, PAP-1 application did not reduce TPA-induced ear swelling (93.6% of control; P = 0.6). This result demonstrates that Kv1.3 channels are not involved in nonspecific inflammation and that PAP-1 reduces ACD by reducing the specific immune reaction.
PAP-1 displays no toxicity in a 28-day toxicity test
As PAP-1 could potentially constitute a new drug for the treatment of contact dermatitis and psoriasis we tested if it shows any toxicity after medium-term application. We treated male (n = 3) and female (n = 3) Lewis rats daily for 28 days with PAP-1 at 10 mg/kg or with vehicle. The rats were then killed and complete hematology, blood chemistry, and complete necropsy performed at the Comparative Pathology Laboratory of the University of California, Davis. As shown in Table S1 all clinical and hematological parameters were normal and there were no significant findings on necropsy of any of the major organs including gastrointestinal tract, pancreas, mesenteric lymph node, heart, thymus, lung, spleen, liver, kidney, adrenal glands, urinary bladder, genital tract, brain, and bone marrow. We also did not note any signs of distress or abnormal behavior during the trial.
PAP-1 is not a contact sensitizer in vivo or in vitro
Before proposing PAP-1 for topical or transdermal application we needed to ascertain that PAP-1 itself is not a contact sensitizer. We therefore first tested PAP-1 in the local lymph node assay (LLNA) IL-2 assay (Hariya et al., 1999; Suda et al., 2002; Azam et al., 2005), which measures the ex vivo production of IL-2 by draining lymph node cells following repeated application of a sensitizer onto the ear of Balb/c mice. This test is a non-radioactive alternative to the normal radioactive LLNA. The positive control oxazolone induced a 3.5-fold increase in IL-2 production (Figure 6), whereas the irritant Triton®X-100 did not induce any overproduction of IL-2 validating the experiments. PAP-1 at doses from 10 to 50 mg/ml, which is the maximum soluble dose, did not increase IL-2 production compared to lymph node cells from vehicle-treated mice (Figure 6). Oxazolone was also positive and PAP-1 negative when measuring IFN-γ on the same samples (data not shown).
Figure 6. PAP-1 is not a contact sensitizer according to the LLNA IL-2 method.
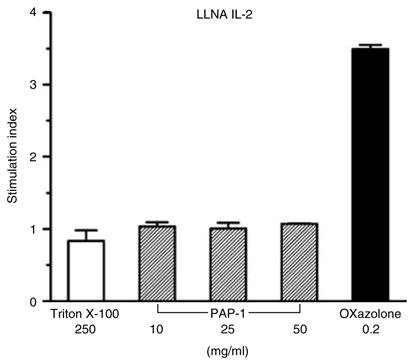
Vehicle (AOO), Triton®X-100 250 mg/ml, oxazolone 0.2mg/ml, or PAP-1 at 10, 25, or 50mg/ml were applied to mouse ears daily for 3 consecutive days. Two days later, lymph node cells from the draining lymph nodes were collected and cultured for 15 hours in the presence of 10 μg/ml PHA-P and IL-2 concentrations in culture supernatant measured by ELISA. Values are given as stimulation index, which is the ratio of IL-2 released by lymph node cells from compound-treated animals to IL-2 released by lymph node cells from vehicle-treated animals. Values are given as the average of two independent experiments with four mice per group±SEM.
In order to confirm the results obtained in the LLNA IL-2 assay we next performed an in vitro test, which is based on the induction of CD86 in the two myeloid cell lines MUTZ-3 (Azam et al., 2006) and THP-1 (Yoshida et al., 2003). Because it is necessary to perform these tests at non-toxic concentrations in order to assay the true sensitization potential of a compound (Hulette et al., 2005), we first tested the effect of PAP-1 on proliferation of the two cell lines. PAP-1 only affected proliferation at concentrations above 40 μM (data not shown) and this dose was therefore chosen as the maximum dose for the test. We next compared the effect of 10, 20, and 40 μM of PAP-1 with the negative control SDS at 200 μM and the positive control dinitrochlorobenzene at 3.5 μM on CD86 expression as measured by flow cytometry. On both the MUTZ-3 (Figure 7a) and THP-1 (Figure 7b) cells PAP-1 and SDS did not induce CD86 expression, whereas dinitrochlorobenzene induced a 2.1-fold or 7.9-fold increase, respectively. Taken together, both experiments demonstrate that PAP-1 is not a contact sensitizer.
Figure 7. PAP-1 is not a contact sensitizer in vitro.
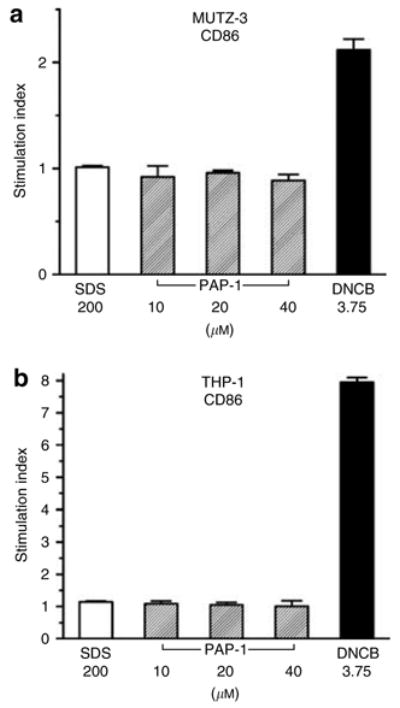
(a) MUTZ-3 and (b) THP-1 myeloid cell lines were cultured for 24 hours in the presence of vehicle (DMSO), the irritant SDS at 200 μM, the sensitizer dinitrochlorobenzene at 3.75 μM or PAP-1 at 10, 20, or 40 μM. CD86 expression was then measured by flow cytometry. Values are given as a stimulation index (SI), which is the ratio of CD86 expression of treated cells over CD86 expression of DMSO-treated cells (SI = (% of cells expressing CD86+ ×mean fluorescence intensity of CD86+ cells) of treated/(% of cells expressing CD86+ ×mean fluorescence intensity of CD86+ cells) of control). The values are the average±SEM of two independent experiments.
PAP-1 is not a skin irritant in vivo
We next checked if PAP-1 is a skin irritant and injected PAP-1 at 10 mg/kg for 15 days daily subcutaneously. For these injections great care was taken to always put the injection at the same site on the flank of the rat. Because of PAP-1’s high lipophilicity we had to use either Cremophor®EL (n = 3) or peanut oil (n = 3) as vehicles, which are both not ideal for subcutaneous injection. As expected, both vehicles were not inert and induced changes in histopathology (Table S2). Peanut oil was not completely resorbed from under the skin leading to oil deposits, which appear clear in hematoxylin and eosin stains and which are surrounded by fibroblasts, scattered lymphocytes, and macrophages. Cremophor® induced a reduction in the number of normal adipocytes at the injection site, which contained unidentified swollen cells instead. These cells are presumed to be either macrophages, that have phagocytized lipid released from the adipose tissue, or atrophic adipocytes that have lost their lipid cytoplasm owing to the detergent properties of the Cremophor®. Because of the absence of any signs of inflammation the pathologist favored the second explanation. However, with both vehicles there was no indication of any irritation associated with PAP-1. The fact that PAP-1 does not affect the normal skin further suggests that the faint staining observed with the Kv1.3 antibody in the epidermis and of the follicular bulbs (Figure 1e and f) is probably nonspecific and not owing to the presence of functional homotetrameric Kv1.3 channels in the skin.
DISCUSSION
In this study, we show that the Kv1.3 blocker PAP-1 effectively suppresses the elicitation phase of oxazolone-induced ACD in rats when administered i.p., orally, or topically without impairing the sensitization phase. PAP-1 exerts its effect by reducing the infiltration of CD8+ T cells and the production of the inflammatory cytokines IFN-γ, IL-2, and IL-17 but not of TNF-α. We further show that PAP-1 is neither a sensitizer nor an irritant and therefore propose PAP- 1 as a topical treatment for TEM cell-mediated skin diseases like ACD and psoriasis.
Similar to human and mouse ACD, the main effector cells in rat ACD are CD8+ T cells, that are negative for CD45RC (Bell et al., 1998). These cells further express high levels of the Kv1.3, which identifies them as activated TEM cells (Wulff et al., 2003b). The inflammatory cytokine profile during the revelation phase of ACD in rats is not well documented but, as expected, we found a strong type-1T-cell profile (IFN-γ, IL- 2, and TNF-α), which is very similar to the profile seen in humans and mice (for reviews see Grabbe and Schwarz, 1998; Krasteva et al., 1999; Xu et al., 2000; Dearman et al., 2003; Saint-Mezard et al., 2004). Kv1.3 blockade with PAP-1 strongly reduced infiltration of CD8+ T cells into the local site and inhibited IFN-γ and IL-2 production but had no effect on TNF-α production. This might be surprising but confirms our previous observation that Kv1.3 blockers do not suppress anti-CD3 antibody-stimulated TNF-α production by TEM cells from the synovial fluid of patients with rheumatoid arthritis whereas potently inhibiting the production of the other type-1 cytokines IL-2 and IFN-γ (Beeton et al., 2006). Moreover, in ACD TNF-α can also be produced by keratinocytes, Langerhans cells (Wang et al., 1999), and mast cells (Biedermann et al., 2000), which are probably not affected by PAP-1. This lack of effect on TNF-α production might also explain why PAP-1 does not completely suppress ACD, although it should be kept in mind that ACD against a strong sensitizer like oxazolone probably involves some unspecific reactions that are not mediated by TEM cells. We further found that PAP-1 also suppressed the production of IL-17 (Yao et al., 1995), a cytokine that is receiving increasing attention in inflammatory processes. IL-17 can be produced by both CD4+ and CD8+ memory T cells (Shin et al., 1999) and has been previously shown to be involved in the revelation phase of TNCB induced ACD in mice (Nakae et al., 2002). Our results show that IL-17 is also present in the revelation phase of rat ACD and suggest that is very likely produced by oxazolone-specific CD8+ cells. Another hypothesis would be that in addition to the dominating cytotoxic T lymphocyte 1 cells a smaller T- cell subpopulation producing IL-17 is also involved in ACD. This population could be CD4+ helper T lymphocyte 17 (Bettelli et al., 2006; Harrington et al., 2006), but based on the absence of CD4 mRNA is more likely to be their potential CD8+ equivalent which could be called “cytotoxic T lymphocyte 17”. In this case, the reduction of IL-17 production by PAP-1 would mean that these cells also rely, at least partly and at this state of development, on Kv1.3 for their effector function.
Despite effectively reducing the elicitation of ACD by inhibiting T-cell infiltration and production of inflammatory cytokines, PAP-1 does not prevent sensitization. During sensitization, naïve T cells are primed by Langerhans cells and differentiate into CCR7+ central memory T cells, which will remain in lymph nodes, and into CCR7− TEM (Sallusto et al., 1999), which will migrate to the periphery, especially to the skin if they also express cutaneous lymphocyte antigen (Saint-Mezard et al., 2004). Although naïve and central memory T cells rely on the calcium-activated potassium channel KCa3.1 for their activation, TEM cells rely on Kv1.3 (Wulff et al., 2003b). By blocking Kv1.3, PAP-1 therefore specifically targets TEM cells and in contrast to stronger immunosuppressants like cyclosporine does not affect the function of naïve and central memory T cells. The fact that PAP-1 does not impair memory development during the sensitization phase also suggests that Langerhans cells are not dependant on Kv1.3 for their activation. This is an interesting finding because the potassium channel expression in antigen-presenting cells and other cells of the myeloid lineage has not been extensively studied and the reported channels and the effect of Kv1.3 blockers vary between the cell type, the differentiation state of the cells, and the species used. For example, Kv1.3 blockers inhibit the macrophage colony-stimulating factor-induced proliferation of mouse bone marrow-derived macrophages (Vicente et al., 2003) but have no effect on Fc receptor-mediated phagocytosis and IL-1β production of human alveolar macrophages (Mackenzie et al., 2003). In addition to Kv1.3, macrophages/monocytes and myeloid cell lines have been reported to express Kv1.5, the calcium-activated channels KCa1.1 and KCa3.1 and the inward-rectifier Kir2.1 (Kubo et al., 1993; DeCoursey and Cherny, 1996; Liu et al., 2000; Blunck et al., 2001) and the interested reader is referred to a recent book chapter for a detailed discussion of potassium channel expression in macrophages (Chandy et al., 2006).
Kv1.3 blockade reduces calcium influx during TEM cell activation (Beeton et al., 2006) and thus prevents calcineurin activation and ultimately inhibits the nuclear factor of activated T-cell pathway. However, this inhibition is restricted to TEM cells and Kv1.3 blockade therefore does not prevent sensitization in ACD like the calcineurin inhibitors (Meingassner et al., 2003). Similar to PAP-1 and unlike cyclosporine A and FK-506 (Tacrolismus) which suppress both elicitation and sensitization, Pimecrolimus (Gupta and Chow, 2003) has been reported to reduce the elicitation phase of ACD with little effect on the sensitization phase (Meingassner et al., 2003). The reasons for this observation are not completely understood but the effect may be explained by a preferential tissue distribution into the skin. Based on the fact that topical or oral Pimecrolimus is a safe and efficient treatment for both atopic dermatitis (Eichenfield et al., 2002), and psoriasis (Rappersberger et al., 2002), inhibition of the nuclear factor of activated T-cell pathway certainly is a good therapeutic strategy. However, as most T cells in psoriatic lesions are of the TEM phenotype (Vissers et al., 2004) it would be desirable to selectively inhibit the function of these cells without affecting other T cells. A clinically used psoriasis drug (Kraan et al., 2002; Goedkoop et al., 2004) that is already preferentially targeting TEM cells is the LFA-3/IgG1 fusion protein Alefacept®, which is binding to CD2, a receptor that is mostly expressed on TEM and is positively involved in their activation. The Kv1.3 blocker PAP-1 inhibits the nuclear factor of activated T-cell pathway specifically in TEM cells and thus combines the advantages of these two efficient therapeutic strategies. In addition to this, PAP-1 is a small molecule, which effectively penetrates skin and is well tolerated. We therefore believe that PAP-1 constitutes a promising new drug candidate for the treatment of TEM-cell-mediated inflammatory skin diseases like ACD and psoriasis.
MATERIALS AND METHODS
Reagents
PAP-1 (5-(4-phenoxybutoxy)psoralen; formular weight = 350.37) and AS-85 (5-[4-(4-phenoxyphenoxy)butoxy]psoralen; formular weight = 442.47) were synthesized as previously described (Schmitz et al., 2005). All other reagents unless otherwise stated were purchased from Sigma-Aldrich (St Louis, MO).
Laboratory animals
Nine- to 11-week-old female Lewis rats and 10- to 12-week-old female Balb/c mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in microisolator cages with irradiated rodent chow and autoclaved water ad libitum. All experiments were in accordance with National Institutes of Health guidelines and approved by the University of California, Davis, Institutional Animal Care and Use Committee.
Pharmacokinetics
PAP-1 was dissolved in a mixture of 25% Cremophor®EL and 75% phosphate-buffered saline (PBS) at concentrations of 0.5, 3, and 5 mg/ml and then injected i.p. at 1, 6, and 10 mg/kg or intravenous at 6 mg/kg to female Lewis rats. At various time points following the injection approximately 500 μl of blood were collected from the tail into EDTA blood sample collection tubes. Plasma was separated by centrifugation and stored at −20°C pending analysis. Samples were purified using C18 solid phase extraction cartridges. Elution fractions corresponding to PAP-1 were evaporated to dryness under nitrogen and reconstituted in a mixture of acetonitrile and water (2:3). Liquid chromatography-electron spray ionization-mass spectrometry-mass spectrometry analysis was performed with a Hewlett-Packard 1100 series HPLC stack equipped with a KGaA RT 250-4 LiChrosorb RP- 18 column (Merck, Darmstadt, Germany), an HP1100 variable wavelength detector, and interfaced to a Finnigan LCQ ClassicMS. The mobile phase consisted of acetonitrile/water with 0.2% formic acid. The flow rate was 1.0 ml min−1 and the gradient was ramped from 1:1 to 9:1 acetonitrile/water over 20 min. With the column temperature maintained at 24°C, PAP-1 eluted at 14.8 min, and was detected with the variable wavelength detector and the MS in series. Quantitation was by electrospray ionization MS/MS (capillary temperature = 350°C; capillary voltage = 5V; tube lens offset = −25 V; positive ion mode; normalized collision energy = 25.7%; parent mass = 351.1 m/z; the standardized response mean = 107.0, 149.0, 251.0, 351.2) for samples between 2.5 nM (0.88 ng/ml) and 750 nM (263 ng/ml), and by the variable wavelength detector (310 nm) for samples with higher concentrations. The related compound PAP-3 (5(4-phenoxypropoxy)psoralen; FW = 336.33; room temperature = 13.5 minutes) was used as an internal standard.
The bioavailability of PAP-1 was determined by comparing the area under the plasma curve following intravenous administration at 6 mg/kg with the area under the plasma curve after i.p. administration at 6 mg/kg. The pharmacokinetics of the intravenous injection were analyzed with Winnonlin® software (Pharsight, CA).
Test on elicitation phase
Lewis rats were sensitized on the shaved abdomen with 100 μl of a freshly prepared 1% solution of oxazolone in acetone:olive oil (4:1) (AOO). Seven days later, the thickness of both ears was measured using a spring-loaded micrometer (Mitutoyo, Spokane, WA). The rats were then challenged with 25 μl of 0.2% oxazolone in AOO on the outer face of the left ear and AOO on the other ear. Both ears were measured again 24 hours later and the ear swelling calculated as the difference between ear thickness before and after challenge. The results are given as percentage of control in each experiment (% = average of ear swelling from PAP-1-treated animals/average of ear swelling from control animals × 100). The unchallenged ear was used as a negative control. Rats were excluded from the experiment if the thickness of the saline-injected ear increased by more than 10%, which occurred in less than 5% of animals.
PAP-1 was administrated i.p. or orally at 8-hour intervals starting 20 hours before the challenge. For i.p. administration PAP-1 was formulated as described in the pharmacokinetic section with Cremophor®EL/PBS. For oral administration PAP-1 was dissolved in peanut oil at concentrations of 2.5, 5, and 12.5 mg/ml and gavaged at 5, 20, and 50 mg/kg. The controls received the same volumes of the respective vehicles. A positive control group (n = 3) was treated with 16 μg/kg of the Kv1.3-blocking peptide ShK (Bachem Biosciences, King of Prussia, PA) dissolved in PBS containing 0.1% rat serum albumin.
For topical applications, PAP-1 was applied four times at 8-hour intervals starting 8 hours before the challenge. The second treatment was applied just after the challenge. When acetone:DMSO (9:1) was used as a vehicle, 25 μl of PAP-1 or AS-85 at 10mg/ml, were applied on both sides of both ears (oxazolone-challenged ear as well as AOO challenged ear). When a cream base was used as vehicle, PAP-1 was first suspended in one part of embryo tested mineral oil and then worked mechanically at 2% into nine parts of either the water-in-oil base Eucerin® (Beiersdorf, Wilton, CT) or the oil-in-water base Dermabase™ (Paddock, Minneapolis, MN). Then enough cream was applied to cover the outer surface of both ears lightly.
Test on sensitization phase
Rats were sensitized with oxazolone 1% as described in the elicitation phase tests. PAP-1 at 10mg/kg or vehicle was administrated i.p. twice a day for 2 days before the sensitization and for 3 days afterwards. The rats were then challenged 7 days after the sensitization and 4 days after the last PAP-1 application with 0.2% oxazolone.
Test on TPA-induced irritation
Rat ears were treated with 10 μg of TPA, Sigma-Aldrich, St Louis, MO) in 200 μl AOO (4:1) (100 μl on each side). Both ears were measured again 24 hours later and the ear swelling calculated as the difference between ear thickness before and after challenge. PAP-1 at 10 mg/kg was administrated i.p. at 8-hour intervals starting 20 hours before the challenge.
Immunohistochemistry
Rat ears were removed 24 hours after challenge under deep isoflurane anesthesia, fixed in formalin, embedded in paraffin, and sectioned at 5 μm. Sections were dewaxed with xylene and rehydrated through an alcohol gradient. Sections were heated with 10mM Na citrate (pH 6.5) in a microwave for 15 minutes to retrieve antigenic determinants. After treatment with 1% H2O2 to inactivate endogenous peroxidase activity and blocking with 5% goat serum in PBS, the sections were incubated overnight at 4°C with the primary antibody in PBS/2% goat serum. The following primary antibodies were used: anti-CD8 (monoclonal mouse antibody, clone OX-8, dilution 1:800), anti-CD45RC (monoclonal mouse antibody, clone OX-22, dilution 1:2,500) both from Serotec (Raleigh, NC) and anti- Kv1.3 (purified polyclonal rabbit antibody, dilution 1:5000) generously provided by H-G. Knaus (University of Innsbruck, Austria) (Koch et al., 1997). Bound primary antibodies were detected with biotinylated donkey anti-mouse IgG secondary antibodies (dilution 1:500) for CD8 and CD45RC or with biotinylated goat anti-rabbit IgG secondary antibodies (dilution 1:500) for Kv1.3 (Jackson immunoResearch, West Grove, PA) followed by a horseradish peroxidase-conjugated avidin complex (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA). After each incubation step, sections were rinsed with PBS three times for 5 minutes. Peroxidase activity was visualized with 3,3′-diaminobenzidine (Substrate Kit for Peroxidase, Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin (Fisher, Pittsburg, PA), dehydrated and mounted with Permount (Fisher).
RNA extraction and real-time PCR
Ears from control or PAP-1-(10 mg/kg i.p.) treated rats were removed 24 hours after challenge and a 1mm band cut from the middle of the ear. Tissue biopsies were collected in 500 μl of stabilization solution (nucleic acid purification lysis buffer, Applied Biosystems, Foster City, CA) and stored at −20°C. Proteinase K and two grinding beads (4mm diameter, stainless steel beads (SpexCertiprep, Metuchen, NJ) were added and the tissues homogenized in a GenoGrinder2000 (SpexCertiprep) for 2 minutes at 1,000 strokes per minutes. Protein digest was performed at 56°C for 30 minutes followed by a 30-minute period at −20°C to reduce foam and precipitate RNA. Total RNA was then extracted from the tissue lysates using a 6700 automated nucleic acid workstation (Applied Biosystems) according to the manufacturer’s instructions. Complementary DNA was synthesized from DNase digested total RNA using 100 units of SuperScript III (all Invitrogen, Carlsband, CA), 600 ng random hexadeoxyribonucleotide (pd(N)6) primers, 10U RNaseOut (RNase inhibitor), and 1mM dNTPs (all Invitrogen, Carlsbad, CA) in a final volume of 40 μl. The reverse transcription reaction proceeded for 120 minutes at 50°C. After addition of 60 μl of water, the reaction was terminated by heating for 5 minutes to 95°C and cooling on ice. Each PCR reaction contained × 20 primer and probes for the respective TaqMan system at a final concentration of 400 nM for each primer and 80 nM for the TaqMan probe and commercially available PCR mastermix (TaqMan Universal PCR Mastermix, Applied Biosystems) containing 10mM Tris-HCl (pH 8.3), 50mM KCl, 5mM MgCl2, 2.5mM deoxynucleotide triphosphates, 0.625U AmpliTaq Gold DNA polymerase, 0.25U AmpErase UNG, and 5 μl of the diluted complementary DNA sample in a final volume of 12 μl. For primer sequences see Supplementary Online Material. The samples were placed in 96-well plates and amplified in an automated fluorometer (ABI PRISM 7900 HTA FAST, ABI). ABI’s standard amplification conditions were used: 2minutes at 50°C, 10 minutes at 95°C, 40 cycles of 15 seconds at 95°C, and 60 seconds at 60°C. Fluorescent signals were collected during the annealing temperature and Ct values extracted with a threshold of 0.04 and baseline values of 3–15. For stronger signals, the baseline was adjusted manually to 3–10.
The housekeeping gene used to normalize the target gene Ct values was hypoxanthine phosphoribosyltransferase 1. This gene was the most stably transcribed during a gene validation experiment including also 18S rRNA (ssrRNA), glyceraldehyde 3-phosphate dehydrogenase, β-2 microglobulin and (transferrin receptor 2; CD71). Final quantitation was performed with the comparative Ct method (User Bulletin No. 2, Applied Biosystems) and results are reported as relative transcription or the n-fold difference relative to a calibrator complementary DNA (i.e. lowest target gene transcription). In brief, the housekeeping gene, hypoxanthine phosphoribosyltransferase 1 was used to normalize the Ct values of the target genes (ΔCt). The ΔCt was calibrated against the weakest signal within each target gene. The relative linear amount of target molecules relative to the calibrator, was calculated by 2-ΔΔCt. All gene transcription is expressed as an n-fold difference relative to the mRNA amount in unchallenged ears.
Toxicity studies
Cutaneous sensitization
PAP-1 was tested for contact sensitization both in vivo and in vitro. The in vivo test used was an alternative to the classical LLNA called the non-radioactive LLNA-IL-2, which is based on the ex vivo IL-2 production by draining lymph node cells during the sensitization phase. The test was performed as previously described (Azam et al., 2005) with PAP-1 doses of 10, 25, and 50 mg/ml in a 4:1 mixture of AOO (50 mg/ml was the maximum soluble dose). The irritant Triton®X-100 was used at a dose of 250 mg/ml as a negative control and oxazolone at 0.02 mg/ml as a positive control.
For the in vitro test CD86 induction in the two myeloid cell lines MUTZ-3 (DSMZ, Braunschweig, Germany) and THP-1 (ATCC, Manassas, VA) was measured by flow cytometry as described previously (Azam et al., 2006). PAP-1 was used at concentrations of 10, 20, and 40 μM. The latest was the highest non-toxic dose on the cell lines measured by the Alamar Blue proliferation test (Invitrogen, Carlsbad, CA). The irritant SDS at 200 μM was used as a negative control and the strong sensitizer 1-chloro-2,4-dinitrobenzene at 3.75 μM as a positive control.
See Supplementary Material for 28-day toxicity study and cutaneous irritation test.
Statistical analysis
Statistical analyses were performed using one-way analysis of variance (Origin software). P<0.05 was used as the level of significance. *P<0.05, **P<0.01, ***P<0.001.
Supplementary Material
Primers for real-time PCR
Figure S1. In vivo PAP-1 treatment does not affect ex vivo cytokine production by splenic CD8+ T cells.
Table S1. 28-Day toxicity test of PAP-1.
Table S2. 15-Day in vivo cutaneous irritation test of PAP-1.
Acknowledgments
We thank Dr Christian M Leutenegger from the Lucy Whittier Molecular and Diagnostic Core Facility at UC Davis for expert analysis of mRNA levels, Maggie Chiu for paraffin embedding and expert preparation of sections, Girija Raman for help with the ELISA and the Laboratory of Cellular and Molecular Biology from the Afssaps (French Health Products Safety Agency) for performing the in vitro sensitization assay on MUTZ-3 and THP1 cells. This work was supported by a UC Davis Health Science Research Award and NIH (GM076063).
Abbreviations
- ACD
allergic contact dermatitis
- i.p
intraperitoneally
- Kv
voltage-gated, potassium channel
- LFA-3
lymphocyte function-associated antigen-3
- LLNA
local lymph node assay
- mRNA
messenger RNA
- PAP-1
phenoxyalkoxypsoralen-1(5-(4-phenoxybutoxy)psoralen
- PBS
phosphate-buffered saline
- TEM
effector memory T cell
- TNCB
2,4,6- trinitrochlorobenzene
Footnotes
CONFLICT OF INTEREST
HW is co-founder of the company, AIRMID, which has negotiated a 1-year option agreement with the University of California to license the patent for PAP-1 and related compounds. The company is intending to develop Kv1.3 blockers for the treatment of autoimmune diseases.
References
- Azam P, Peiffer JL, Chamousset D, Tissier MH, Bonnet PA, Vian L, et al. The cytokine-dependent MUTZ-3 cell line as an in vitro model for the screening of contact sensitizers. Toxicol Appl Pharmacol. 2006;212:14–23. doi: 10.1016/j.taap.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Azam P, Peiffer JL, Ourlin JC, Bonnet PA, Tissier MH, Vian L, et al. Qualitative and quantitative evaluation of a local lymph node assay based on ex vivo interleukin-2 production. Toxicology. 2005;206:285–98. doi: 10.1016/j.tox.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Beeton C, Barbaria J, Giraud P, Devaux J, Benoliel AM, Gola M, et al. Selective blocking of voltage-gated K+ channels improves experimental autoimmune encephalomyelitis and inhibits T cell activation. J Immunol. 2001a;166:936–44. doi: 10.4049/jimmunol.166.2.936. [DOI] [PubMed] [Google Scholar]
- Beeton C, Chandy KG. Potassium channels, memory T cells, and multiple sclerosis. Neuroscientist. 2005;11:550–62. doi: 10.1177/1073858405278016. [DOI] [PubMed] [Google Scholar]
- Beeton C, Pennington MW, Wulff H, Singh S, Nugent D, Crossley G, et al. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol Pharmacol. 2005;67:1369–81. doi: 10.1124/mol.104.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, Bernard D, et al. Selective blockade of T lymphocyte K+ channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci USA. 2001b;98:13942–7. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA. 2006;103:17414–9. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EB, Sparshott SM, Bunce C. CD4+ T-cell memory, CD45R subsets and the persistence of antigen – a unifying concept. Immunol Today. 1998;19:60–4. doi: 10.1016/s0167-5699(97)01211-5. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–52. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunck R, Scheel O, Muller M, Brandenburg K, Seitzer U, Seydel U. New insights into endotoxin-induced activation of macrophages: involvement of a K+ channel in transmembrane signaling. J Immunol. 2001;166:1009–15. doi: 10.4049/jimmunol.166.2.1009. [DOI] [PubMed] [Google Scholar]
- Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Wulff H, Chandy KG. Molecular properties and physiological roles of ion channels in the immune system. J Clin Immunol. 2001;21:235–52. doi: 10.1023/a:1010958907271. [DOI] [PubMed] [Google Scholar]
- Chandy KG, Wulff H, Beeton C, Calabresi PA, Gutman GA, Pennington M. Kv1.3 potassium channel: physiology, pharmacology and therapeutic indications. In: Triggle DJ, Gopalakrishnan M, Rampe D, Zheng W, editors. Voltage-gated ion channels as drug targets. Wiley-VCH; 2006. pp. 214–74. [Google Scholar]
- Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. Potassium channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–9. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearman RJ, Betts CJ, Humphreys N, Flanagan BF, Gilmour NJ, Basketter DA, et al. Chemical allergy: considerations for the practical application of cytokine profiling. Toxicol Sci. 2003;71:137–45. doi: 10.1093/toxsci/71.2.137. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465–8. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV. Voltage-activated proton currents in human THP-1 monocytes. J Membr Biol. 1996;152:131–40. doi: 10.1007/s002329900092. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–8. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Eichenfield LF, Lucky AW, Boguniewicz M, Langley RG, Cherill R, Marshall K, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002;46:495–504. doi: 10.1067/mjd.2002.122187. [DOI] [PubMed] [Google Scholar]
- Elliott JC, Picker MJ, Sparrow AJ, Lysle DT. Dissociation between sex differences in the immunological, behavioral, and physiological effects of kappa- and delta-opioids in Fischer rats. Psychopharmacology (Berlin) 2006;185:66–75. doi: 10.1007/s00213-005-0267-1. [DOI] [PubMed] [Google Scholar]
- Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345:248–55. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- Fasth AE, Cao D, van Vollenhoven R, Trollmo C, Malmstrom V. CD28nullCD4+ T cells – characterization of an effector memory T-cell population in patients with rheumatoid arthritis. Scand J Immunol. 2004;60:199–208. doi: 10.1111/j.0300-9475.2004.01464.x. [DOI] [PubMed] [Google Scholar]
- Freedman BD, Fleischmann BK, Punt JA, Gaulton G, Hashimoto Y, Kotlikoff MI. Identification of Kv1.1 expression by murine CD4−CD8− thymocytes. A role for voltage-dependent K+ channels in murine thymocyte development. J Biol Chem. 1995;270:22406–11. doi: 10.1074/jbc.270.38.22406. [DOI] [PubMed] [Google Scholar]
- Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation: molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137–49. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- Goedkoop AY, de Rie MA, Picavet DI, Kraan MC, Dinant HJ, van Kuijk AW, et al. Alefacept therapy reduces the effector T-cell population in lesional psoriatic epidermis. Arch Dermatol Res. 2004;295:465–73. doi: 10.1007/s00403-004-0450-y. [DOI] [PubMed] [Google Scholar]
- Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Chow M. Pimecrolimus: a review. J Eur Acad Dermatol Venereol. 2003;17:493–503. doi: 10.1046/j.1468-3083.2003.00692.x. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, et al. International union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev. 2003;55:583–6. doi: 10.1124/pr.55.4.9. [DOI] [PubMed] [Google Scholar]
- Hariya T, Hatao M, Ichikawa H. Development of a non-radioactive endpoint in a modified local lymph node assay. Food Chem Toxicol. 1999;37:87–93. doi: 10.1016/s0278-6915(98)00102-1. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–56. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Hulette BC, Ryan CA, Gildea LA, Gerberick GF. Relationship of CD86 surface marker expression and cytotoxicity on dendritic cells exposed to chemical allergen. Toxicol Appl Pharmacol. 2005;209:159–66. doi: 10.1016/j.taap.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Koch RO, Wanner SG, Koschak A, Hanner M, Schwarzer C, Kaczorowski GJ, et al. Complex subunit assembly of neuronal voltage-gated K+ channels. Basis for high-affinity toxin interactions and pharmacology. J Biol Chem. 1997;272:27577–81. doi: 10.1074/jbc.272.44.27577. [DOI] [PubMed] [Google Scholar]
- Koo GC, Blake JT, Shah K, Staruch MJ, Dumont F, Wunderler D, et al. Correolide and derivatives are novel immunosuppressants blocking the lymphocyte Kv1.3 potassium channels. Cell Immunol. 1999;197:99–107. doi: 10.1006/cimm.1999.1569. [DOI] [PubMed] [Google Scholar]
- Koo GC, Blake JT, Talento A, Nguyen M, Lin S, Sirotina A, et al. Blockade of the voltage-gated potassium channel Kv1.3 inhibits immune responses in vivo. J Immunol. 1997;158:5120–8. [PubMed] [Google Scholar]
- Kraan MC, Van Kuijk AW, Dinant HJ, Goedkoop AY, Smeets TJ, De Rie MA, et al. Alefacept treatment in psoriatic arthritis: reduction of the effector T cell population in peripheral blood and synovial tissue is associated with improvement of clinical signs of arthritis. Arthritis Rheum. 2002;46:2776–84. doi: 10.1002/art.10543. [DOI] [PubMed] [Google Scholar]
- Krasteva M, Kehren J, Ducluzeau MT, Sayag M, Cacciapuoti M, Akiba H, et al. Contact dermatitis I. Pathophysiology of contact sensitivity. Eur J Dermatol. 1999;9:65–77. [PubMed] [Google Scholar]
- Krueger GG. Selective targeting of T cell subsets: focus on alefacept – a remittive therapy for psoriasis. Expert Opin Biol Ther. 2002;2:431–41. doi: 10.1517/14712598.2.4.431. [DOI] [PubMed] [Google Scholar]
- Krueger GG, Papp KA, Stough DB, Loven KH, Gulliver WP, Ellis CN. A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2002;47:821–33. doi: 10.1067/mjd.2002.127247. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel (see comments) Nature. 1993;362:127–33. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Levite M, Cahalon L, Peretz A, Hershkoviz R, Sobko A, Ariel A, et al. Extracellular K+ and opening of voltage-gated potassium channels activate T cell integrin function: physical and functional association between Kv1.3 channels and beta1 integrins. J Exp Med. 2000;191:1167–76. doi: 10.1084/jem.191.7.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QH, Fleischmann BK, Hondowicz B, Maier CC, Turka LA, Yui K, et al. Modulation of Kv channel expression and function by TCR and costimulatory signals during peripheral CD4+ lymphocyte differentiation. J Exp Med. 2002;196:897–909. doi: 10.1084/jem.20020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QH, Williams DA, McManus C, Baribaud F, Doms RW, Schols D, et al. HIV-1 gp120 and chemokines activate ion channels in primary macrophages through CCR5 and CXCR4 stimulation. Proc Natl Acad Sci USA. 2000;97:4832–7. doi: 10.1073/pnas.090521697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon NJ, Kang J, Togo JA, Christian EP, Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J Biol Chem. 1997;272:32723–6. doi: 10.1074/jbc.272.52.32723. [DOI] [PubMed] [Google Scholar]
- Mackenzie AB, Chirakkal H, North RA. Kv1.3 potassium channels in human alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2003;285:L862–8. doi: 10.1152/ajplung.00095.2003. [DOI] [PubMed] [Google Scholar]
- Markovic-Plese S, Cortese I, Wandinger KP, McFarland HF, Martin R. CD4+CD28− costimulation-independent T cells in multiple sclerosis. J Clin Invest. 2001;108:1185–94. doi: 10.1172/JCI12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meingassner JG, Fahrngruber H, Bavandi A. Pimecrolimus inhibits the elicitation phase but does not suppress the sensitization phase in murine contact hypersensitivity, in contrast to tacrolimus and cyclosporine A. J Invest Dermatol. 2003;121:77–80. doi: 10.1046/j.1523-1747.2003.12331.x. [DOI] [PubMed] [Google Scholar]
- Meingassner JG, Grassberger M, Fahrngruber H, Moore HD, Schuurman H, Stutz A. A novel anti-inflammatory drug, SDZ ASM 981, for the topical and oral treatment of skin diseases: in vivo pharmacology. Br J Dermatol. 1997;137:568–76. doi: 10.1111/j.1365-2133.1997.tb03788.x. [DOI] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Nelson CJ, How T, Lysle DT. Enhancement of the contact hypersensitivity reaction by acute morphine administration at the elicitation phase. Clin Immunol. 1999;93:176–83. doi: 10.1006/clim.1999.4783. [DOI] [PubMed] [Google Scholar]
- Rao TS, Currie JL, Shaffer AF, Isakson PC. Comparative evaluation of arachidonic acid (AA)- and tetradecanoylphorbol acetate (TPA)-induced dermal inflammation. Inflammation. 1993;17:723–41. doi: 10.1007/BF00920477. [DOI] [PubMed] [Google Scholar]
- Rappersberger K, Komar M, Ebelin ME, Scott G, Burtin P, Greig G, et al. Pimecrolimus identifies a common genomic anti-inflammatory profile, is clinically highly effective in psoriasis and is well tolerated. J Invest Dermatol. 2002;119:876–87. doi: 10.1046/j.1523-1747.2002.00694.x. [DOI] [PubMed] [Google Scholar]
- Rus H, Pardo CA, Hu L, Darrah E, Cudrici C, Niculescu T, et al. The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc Natl Acad Sci USA. 2005;102:11094–9. doi: 10.1073/pnas.0501770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Mezard P, Rosieres A, Krasteva M, Berard F, Dubois B, Kaiserlian D, et al. Allergic contact dermatitis. Eur J Dermatol. 2004;14:284–95. [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Sankaranarayanan A, Azam P, Schmidt-Lassen K, Homerick D, Hansel W, et al. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol Pharmacol. 2005;68:1254–70. doi: 10.1124/mol.105.015669. [DOI] [PubMed] [Google Scholar]
- Shin HC, Benbernou N, Esnault S, Guenounou M. Expression of IL-17 in human memory CD45RO+ T lymphocytes and its regulation by protein kinase A pathway. Cytokine. 1999;11:257–66. doi: 10.1006/cyto.1998.0433. [DOI] [PubMed] [Google Scholar]
- Suda A, Yamashita M, Tabei M, Taguchi K, Vohr HW, Tsutsui N, et al. Local lymph node assay with non-radioisotope alternative endpoints. J Toxicol Sci. 2002;27:205–18. doi: 10.2131/jts.27.205. [DOI] [PubMed] [Google Scholar]
- Vicente R, Escalada A, Coma M, Fuster G, Sanchez-Tillo E, Lopez-Iglesias C, et al. Differential voltage-dependent K+ channel responses during proliferation and activation in macrophages. J Biol Chem. 2003;278:46307–20. doi: 10.1074/jbc.M304388200. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Kent SC, Orban T, Hafler DA. GAD65-reactive T cells are activated in patients with autoimmune type 1a diabetes. J Clin Invest. 2002;109:895–903. doi: 10.1172/JCI14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers WH, Arndtz CH, Muys L, van Erp PE, de Jong EM, van de Kerkhof PC. Memory effector (CD45RO+) and cytotoxic (CD8+) T cells appear early in the margin zone of spreading psoriatic lesions in contrast to cells expressing natural killer receptors, which appear late. Br J Dermatol. 2004;150:852–9. doi: 10.1111/j.1365-2133.2004.05863.x. [DOI] [PubMed] [Google Scholar]
- Vocanson M, Hennino A, Cluzel-Tailhardat M, Saint-Mezard P, Benetiere J, Chavagnac C, et al. CD8+ T cells are effector cells of contact dermatitis to common skin allergens in mice. J Invest Dermatol. 2006;126:815–20. doi: 10.1038/sj.jid.5700174. [DOI] [PubMed] [Google Scholar]
- Wang B, Amerio P, Sauder DN. Role of cytokines in epidermal Langerhans cell migration. J Leukoc Biol. 1999;66:33–9. doi: 10.1002/jlb.66.1.33. [DOI] [PubMed] [Google Scholar]
- Wulff H, Beeton C, Chandy KG. Potassium channels as therapeutic targets for autoimmune disorders. Curr Opin Drug Discov Devel. 2003a;6:640–7. [PubMed] [Google Scholar]
- Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Beeton C, et al. The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. J Clin Invest. 2003b;111:1703–13. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Bjarnason B, Elmets CA. Sensitization versus elicitation in allergic contact dermatitis: potential differences at cellular and molecular levels. Am J Contact Dermatol. 2000;11:228–34. doi: 10.1053/ajcd.2000.8009. [DOI] [PubMed] [Google Scholar]
- Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–6. [PubMed] [Google Scholar]
- Yoshida Y, Sakaguchi H, Ito Y, Okuda M, Suzuki H. Evaluation of the skin sensitization potential of chemicals using expression of co-stimulatory molecules, CD54 and CD86, on the naive THP-1 cell line. Toxicol In Vitro. 2003;17:221–8. doi: 10.1016/s0887-2333(03)00006-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers for real-time PCR
Figure S1. In vivo PAP-1 treatment does not affect ex vivo cytokine production by splenic CD8+ T cells.
Table S1. 28-Day toxicity test of PAP-1.
Table S2. 15-Day in vivo cutaneous irritation test of PAP-1.


