Abstract
The fourth edition of the World Health Organization (WHO) classification of tumours of the central nervous system, published in 2007, lists several new entities, including angiocentric glioma, papillary glioneuronal tumour, rosette-forming glioneuronal tumour of the fourth ventricle, papillary tumour of the pineal region, pituicytoma and spindle cell oncocytoma of the adenohypophysis. Histological variants were added if there was evidence of a different age distribution, location, genetic profile or clinical behaviour; these included pilomyxoid astrocytoma, anaplastic medulloblastoma and medulloblastoma with extensive nodularity. The WHO grading scheme and the sections on genetic profiles were updated and the rhabdoid tumour predisposition syndrome was added to the list of familial tumour syndromes typically involving the nervous system. As in the previous, 2000 edition of the WHO ‘Blue Book’, the classification is accompanied by a concise commentary on clinico-pathological characteristics of each tumour type. The 2007 WHO classification is based on the consensus of an international Working Group of 25 pathologists and geneticists, as well as contributions from more than 70 international experts overall, and is presented as the standard for the definition of brain tumours to the clinical oncology and cancer research communities world-wide.
Introduction and historical annotation
The international classification of human tumours published by the World Health Organization (WHO) was initiated through a resolution of the WHO Executive Board in 1956 and the World Health Assembly in 1957. Its objectives have remained the same until today: to establish a classification and grading of human tumours that is accepted and used worldwide. Without clearly defined histopathological and clinical diagnostic criteria, epidemiological studies and clinical trials could not be conducted beyond institutional and national boundaries.
The first edition on the histological typing of tumours of the nervous system was edited by Zülch and published in 1979 [52]. The second edition reflected the advances brought about by the introduction of immunohistochemistry into diagnostic pathology; it was edited by Kleihues et al. [24]. The third edition, edited by Kleihues and Cavenee and published in 2000 [26], incorporated genetic profiles as additional aids to the definition of brain tumours. In contrast to the previous ‘WHO Blue Books’ (as the series is commonly termed), the third edition included concise sections on epidemiology, clinical signs and symptoms, imaging, prognosis and predictive factors. Throughout the series, the classification was based on the consensus of an international Working Group. This also applies to the fourth edition; a group of 25 pathologists and geneticists convened at the German Cancer Research Center in Heidelberg in November 2006 and the results of their deliberations and those of an additional 50 contributors are contained in the 2007 WHO classification of tumours of the central nervous system [35]. As the book title indicates, the focus is on tumours of the central nervous system, including tumours of cranial and paraspinal nerves. Tumours of the peripheral nervous system, e.g. neuroblastomas of the sympathetic nervous system and aesthesioneuroblastoma, are covered in other volumes of the WHO Blue Book series.
ICD-O Coding
The international classification of diseases for oncology (ICD-O) was established more than 30 years ago and serves as an indispensable interface between pathologists and cancer registries. It assures that histopathologically stratified population-based incidence and mortality data become available for epidemiological and oncological studies. The histology (morphology) code is increasingly complemented by genetic characterization of human neoplasms. The ICD-O histology codes have been adopted by the systematized nomenclature of medicine (SNOMED), issued by the College of American Pathologists (CAP). The ICD-O topography codes largely correspond to those of the tenth edition of the International statistical classification of diseases, injuries and causes of death (ICD-10) of the WHO.
The third edition of ICD-O (ICD-O-3) was published in 2000 [8] and contains the codes proposed in the previous edition of the WHO Blue Books [26]. For the fourth edition, published in the summer of 2007 [35], preliminary codes were introduced for several new entities and variants (Tables 1, 2 ).
Table 1.
The 2007 WHO Classification of Tumours of the Central Nervous System. Reprinted from Ref. 35
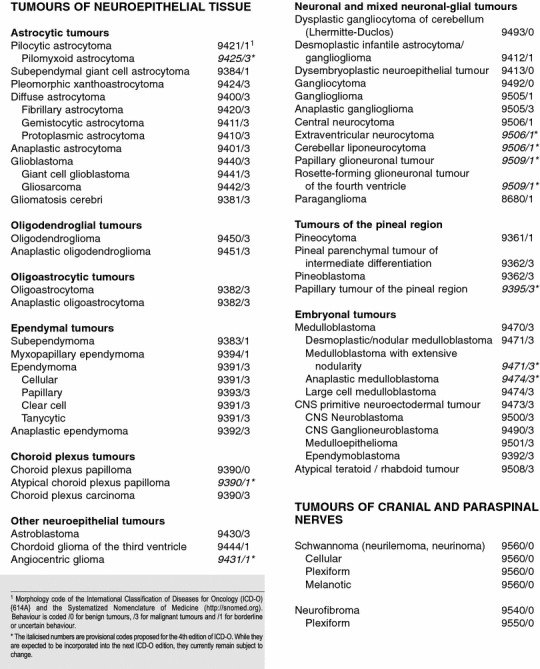

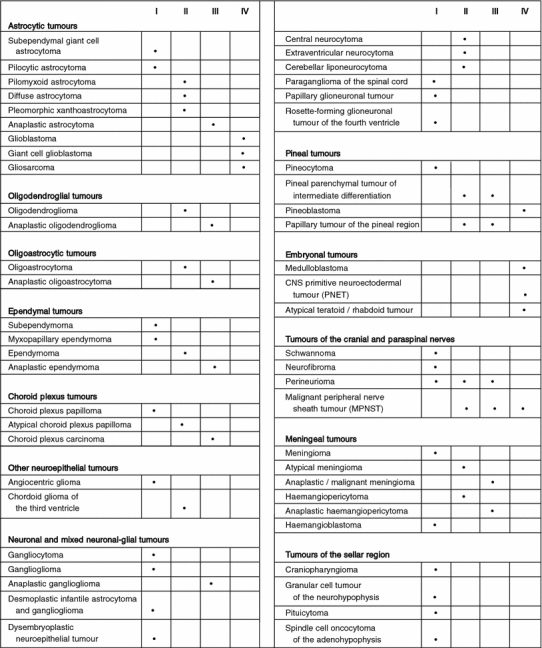
Table 2.
WHO Grading of Tumours of the Central Nervous System. Reprinted from Ref. 35
Entities, variants and patterns
The Working Group distinguished between clinico-pathological entities, variants of entities and histological patterns. To be included in the WHO classification, two or more reports from different institutions were considered mandatory. In addition, a new entity had to be characterized by distinctive morphology, location, age distribution and biologic behaviour, and not simply by an unusual histopathological pattern. Variants were defined as being reliably identified histologically and having some relevance for clinical outcome, but as still being part of a previously defined, overarching entity. Finally, patterns of differentiation were considered identifiable histological appearances, but that did not have a distinct clinical or pathological significance.
New entities
The Working Group for the fourth edition proposed to add eight new entities.
Angiocentric glioma
ICD-O 9431/1, WHO grade I
This newly identified tumour (Fig. 1) occurs predominantly in children and young adults (mean age at surgery, 17 years), with refractory epilepsy as the leading clinical symptom. A total of 28 cases has been reported from the United States [51], France [33], and Austria/Germany [41]. Angiocentric gliomas are located superficially, the most common sites being the fronto-parietal cortex and the temporal lobe as well as the hippocampal region. FLAIR images show well delineated, hyperintense, non-enhancing cortical lesions, often with a stalk-like extension to the subjacent ventricle [41]. The tumours are stable or slowly growing and histopathologically characterized by monomorphous bipolar cells, an angiocentric growth pattern and immunoreactivity for EMA, GFAP, S-100 protein and vimentin, but not for neuronal antigens. Despite frequent extension of angiocentric glioma to the ventricular wall and the presence of microscopic features suggestive of ependymal differentiation, the predominant clinical symptoms, cortical location, architectural pattern and outcome were considered insufficient to designate this entity as an ependymoma variant. Given the uncertainties regarding histogenesis, angiocentric glioma was grouped with astroblastoma and chordoid glioma of the third ventricle in the category of ‘Other neuroepithelial tumours’, previously designated ‘Tumours of uncertain origin’. Due to its benign clinical behaviour and the possibility of curative surgery, the neoplasm was assigned to WHO grade I.
Fig. 1.
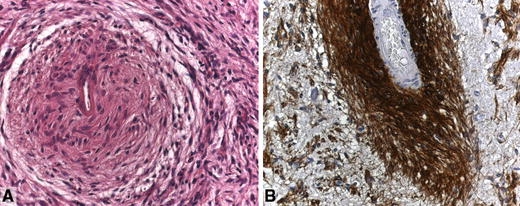
Angiocentric glioma. a Elongated tumour cells with concentric perivascular arrangement. b Perivascular tumour cells strongly express GFAP. Courtesy of Dr. V. H. Hans
Atypical choroid plexus papilloma
ICD-O 9390/1, WHO grade II
Most intraventricular papillary neoplasms derived from choroid plexus epithelium are benign in nature and can be cured by surgery (choroid plexus papilloma, WHO Grade I, ICD-O 9390/0). At the other side of the spectrum is the choroid plexus carcinoma (WHO Grade III, ICD-O 9390/3) with frank signs of malignancy, including brisk mitotic activity, increased cellularity, blurring of the papillary pattern, necrosis and frequent invasion of brain parenchyma. The WHO Working group proposes to introduce an additional entity with intermediate features, designated ‘atypical choroid plexus papilloma’ which is primarily distinguished from the choroid plexus papilloma by increased mitotic activity. Curative surgery is still possible but the probability of recurrence appears to be significantly higher. To reflect this concern, the proposed ICD-O code is the same as for other choroid plexus tumours but carries the /1 extension (ICD-O 9390/1).
Extraventricular neurocytoma
ICD-O 9506/1, WHO grade II
The term central neurocytoma describes a neuronal tumour with pathological features distinct from cerebral neuroblastoma, occurring in young adults, with preferential location in the lateral ventricles in the region of the foramen of Monro and a generally favourable prognosis. Central neurocytomas are composed of uniform round cells with immunohistochemical and ultrastructural evidence of neuronal differentiation. Additional features include fibrillary areas mimicking neuropil, and a low proliferation rate. During the past decade several reports have shown that neoplasms occur in brain parenchyma outside the ventricular system with similar biological behaviour and histopathological characteristics although the latter appear to exhibit a somewhat larger morphological spectrum. For these neoplasms, the 2007 WHO classification recommends the term ‘extraventricular neurocytoma’ and proposes an ICD-O code identical to that of central neurocytoma (9506/1).
Papillary glioneuronal tumour (PGNT)
ICD-O 9509/1, WHO grade I
The papillary glioneuronal tumour (Fig. 2) was established as a distinct clinico-pathologic entity by Komori et al. in 1998 [28]. Histopathologically similar tumours had previously been described as pseudopapillary ganglioglioneurocytoma [29] and pseudopapillary neurocytoma with glial differentiation [23]. PGNT manifests over a wide age range (mean 27 years) and is a clinically benign neoplasm that typically corresponds to WHO grade I. Its preferential location is the temporal lobe. On CT and MR images, it appears as a contrast-enhancing, well delineated mass, occasionally showing a cyst-mural nodule pattern. Histologically, it is characterized by a single or pseudostratified layer of flat to cuboidal, GFAP-positive astrocytes surrounding hyalinized vascular pseudopapillae and by synaptophysin-positive interpapillary sheets of neurocytes, large neurons and intermediate size “ganglioid” cells. This tumour is part of the growing list of relatively benign glioneuronal tumours.
Fig. 2.
Novel glioneuronal tumour entities. a Papillary glioneuronal tumour (PGNT). Layers of tumour cells surround vessels, forming pseudopapillary structures with b pseudopapillae covered by inner cells with hyperchromatic and outer cells with vesicular nuclei. Courtesy of Dr. Y. Nakazato. c Rosette-forming glioneuronal tumour of the fourth ventricle (RGNT). Pseudorosette with ring-like arrangement of neurocytic tumour cell nuclei around an eosinophilic neuropil core which d shows strong immunoreactivity to synaptophysin. Courtesy of Dr. J. A. Hainfellner
Rosette-forming glioneuronal tumour of the fourth ventricle
ICD-O 9509/1, WHO grade I
This newly included entity, initially described as dysembryoplastic neuroepithelial tumour (DNT) of the cerebellum [32], was established as distinct disease entity in a report of 11 cases by Komori et al. in 2002 [30]. Since then, a total of 17 cases have been reported. Rosette-forming glioneuronal tumour of the fourth ventricle (RGNT) is defined as a rare, slowly growing tumour of the fourth ventriclular region that predominantly affects young adults (mean age 33 years) and causes obstructive hydrocephalus, ataxia being the most common clinical manifestation. RGNT typically arises in the midline and primarily involves the cerebellum and wall or floor of the fourth ventricle. It often occupies the fourth ventricle and/or aqueduct, and may show parenchymal extension. T2-weighted MR imaging reveal a well delineated, hyperintense tumour. Histopathologically, RGNTs are characterized by a biphasic neurocytic and glial architecture [18, 30, 40]. The neuronal component consists of neurocytes that form neurocytic rosettes with eosinophilic, synaptophysin-positive cores and/or perivascular pseudorosettes. The glial component dominates and typically exhibits features of pilocytic astrocytoma. Given its benign clinical behaviour with the possibility of surgical cure, the rosette-forming glioneuronal tumour (RGNT) corresponds to WHO grade I. It shares the new ICD-O code 9509/1 with the papillary glioneuronal tumour (PGNT).
Papillary tumour of the pineal region
ICD-O 9395/3, WHO grade II/III
This recently described rare neuroepithelial tumour (Fig. 3) of the pineal region manifests in children and adults (mean age 32 years), is relatively large (2.5–4 cm), and well-circumscribed, with MR imaging showing a low T1 and increased T2 signal as well as contrast enhancement. In addition to the initial 2003 report of six cases by Jouvet et al. [20], a total of 38 cases have been published to date. Histologically, papillary tumours of the pineal region are characterized by a papillary architecture and epithelial cytology, with immunoreactivity for cytokeratin and, focally, GFAP. Although macroscopically indistinguishable from pineocytoma, the histology is incompatible with a pineal parenchymal tumour. Ultrastructural features suggest ependymal differentiation and a possible origin from specialized ependymal cells of the subcommissural organ (SCO) has been suggested [20]. The biological behaviour of papillary tumour of the pineal region (PTPR) is variable and may correspond to WHO grades II or III, but precise histological grading criteria remain to be defined. The code 9395/3 has been proposed for the fourth edition of ICD-O.
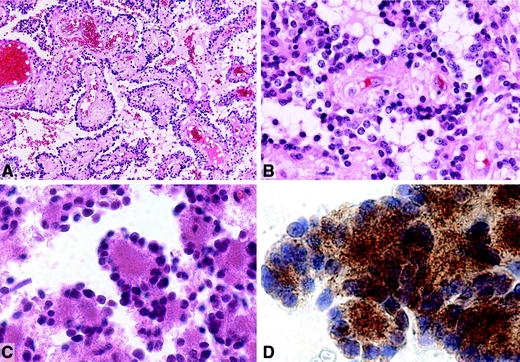
Fig. 3.
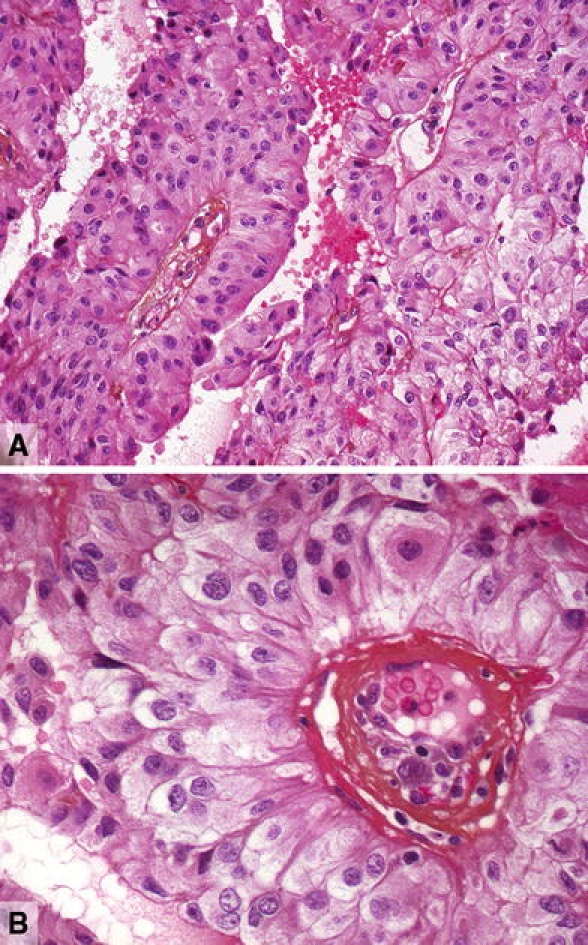
Papillary tumour of the pineal region. a Histology shows the typical papillary architecture and epithelial cytology. b In papillary areas, the tumour cells are large with columnar or cuboidal shape. Courtesy of Dr. M. Fevre-Montange
Pituicytoma
ICD-O 9432/1, WHO grade I
Pituicytoma is a rare, solid, low grade, spindle cell, glial neoplasm of adults that originates in the neurohypophysis or infundibulum (Fig. 4). In the past, the term pituicytoma was also applied to other tumours in the sellar and suprasellar region, particularly granular cell tumours and pilocytic astrocytomas. Presently, it is reserved for low-grade glial neoplasms that originate in the neurohypophysis or infundibulum and are distinct from pilocytic astrocytoma. Less preferred terms for pituicytoma include ‘posterior pituitary astrocytoma’ and, for lesions arising in the pituitary stalk, ‘infundibuloma’. The WHO Working Group felt that inclusion of the diagnostic term pituicytoma would help to more clearly delineate neoplasms manifesting in the neurohypophysis and pituitary stalk.
Fig. 4.
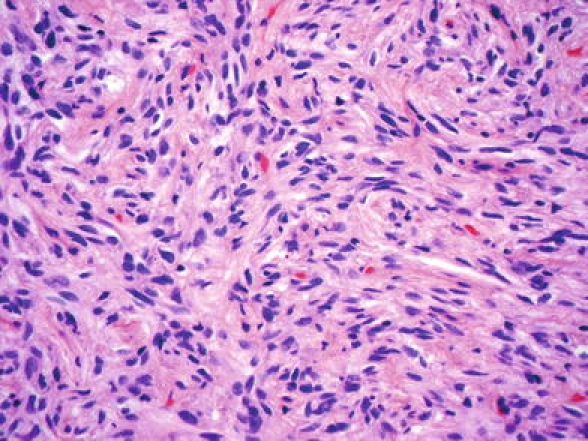
Pituicytoma with elongate, bipolar spindle cells arranged in interlacing fascicles. Courtesy of Dr. D. Brat
To date, less than 30 bona fide examples have been described, often as case reports. Clinical signs and symptoms include visual disturbance, headache and features of hypopituitarism. Pituicytomas are well-circumscribed, solid masses that can measure up to several centimetres. Histologically, they show a compact architecture consisting of elongate, bipolar spindle cells arranged in interlacing fascicles or assuming a storiform pattern [2, 48]. Mitotic figures are absent or rare. Pituicytomas are generally positive for vimentin, S-100 protein and, to a variable degree, GFAP. In contrast to spindle cell oncocytoma (see below), oncocytic change is lacking. Due to their slow growth and the possibility of curative surgery, pituicytomas correspond to WHO grade I. The code 9432/1 has been proposed for the fourth edition of ICD-O.
Spindle cell oncocytoma of the adenohypophysis
ICD-O 8291/0, WHO grade II
Spindle cell oncocytoma (Fig. 5) is defined as an oncocytic, non-endocrine neoplasm of the anterior pituitary that manifests in adults (mean age 56 years). These tumours may macroscopically be indistinguishable from a non-functioning pituitary adenoma and follow a benign clinical course, corresponding to WHO grade I. The eosinophilic, variably oncocytic cytoplasm contains numerous mitochondria, is immunoreactive for the anti-mitochondrial antibody 113-I as well as to S-100 protein and EMA, but is negative for pituitary hormones. Since the initial 2002 report of five cases by Roncaroli et al. [43], five additional cases have been published from three institutions [4, 27, 49]. The code 8291/0 has been proposed for the fourth edition of ICD-O.
Fig. 5.
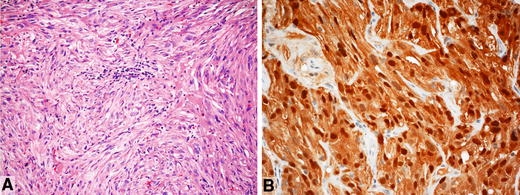
Spindle cell oncocytoma. a Note spindle and somewhat epithelioid cells with abundance of variably granular cytoplasm, different degrees of nuclear atypia and focal inflammatory reaction. b Generalized staining for S-100 protein is a regular feature of this rare tumour
New variants
Throughout the history of the WHO Blue Book series, histological variants have been introduced if there was evidence that a particular morphological pattern was associated with a different biological or clinical behaviour. Admittedly, in retrospect, this criterion was not always fulfilled. In the fourth edition of the WHO Classification, three new variants were introduced that can be reliably identified histologically and which have some relevance in terms of clinical outcome.
Pilomyxoid astrocytoma
ICD-O 9425/3, WHO grade II
Originally described by Jänisch et al. in 1985 as ‘diencephalic pilocytic astrocytoma with clinical onset in infancy’ [19], the term pilomyxoid astrocytoma was introduced in 1999 by Tihan et al. [47] who also defined its characteristic histopathological features. Pilomyxoid astrocytoma (PMA) (Fig. 6) occurs typically in the hypothalamic/chiasmatic region, sites that are also affected by classical pilocytic astrocytomas. PMA is histologically characterized by a prominent myxoid matrix and angiocentric arrangement of monomorphous, bipolar tumour cells. The close relationship to pilocytic astrocytoma is underscored by a report of two cases that occurred in the setting of neurofibromatosis type 1 (NF1). PMA affects predominantly infants and children (median age, 10 months) and appears to have a less favourable prognosis. Local recurrences and cerebrospinal spread are more likely to occur in pilomyxoid than in pilocytic astrocytomas. The Working Group therefore recommended an assignment to WHO grade II and the new code 9425/3 has been proposed for the fourth edition of ICD-O.
Fig. 6.
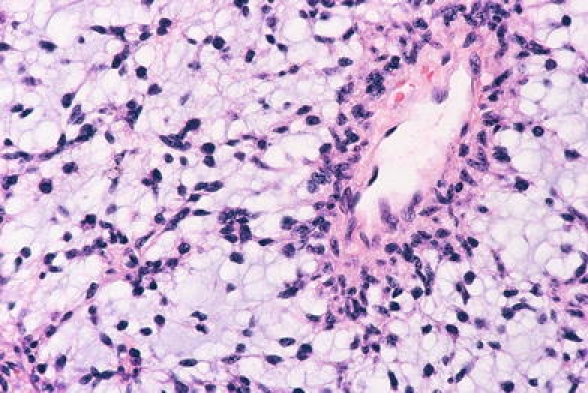
Pilomyxoid astrocytoma showing a monomorphous population of tumour cells in a homogenously myxoid background with angiocentric accumulation
Anaplastic medulloblastoma
ICD-O 9474/3, WHO grade IV
This variant of medulloblastoma is characterized histologically by marked nuclear pleomorphism, nuclear moulding, cell–cell wrapping, and high mitotic activity, often with atypical forms. Although all medulloblastomas show some degree of atypia, these changes are particularly pronounced and widespread in the anaplastic variant. Histological progression from classic to anaplastic medulloblastomas may be observed, even within the same biopsy. The highly malignant large cell medulloblastomas and anaplastic medulloblastomas have considerable cytological overlap. The large cell variant features often spherical cells with round nuclei, open chromatin and prominent central nucleoli. The patterns may coexist. Indeed, in several studies a combined large cell/anaplastic category has been used. It is proposed that in the fourth edition of ICD-O anaplastic medulloblastoma and large cell medulloblastoma share the same code 9474/3.
Medulloblastoma with extensive nodularity
ICD-O 9471/3, WHO grade IV
The medulloblastoma with extensive nodularity is closely related to the desmoplastic/nodular medulloblastoma (9471/3) and was previously designated ‘cerebellar neuroblastoma’. It occurs in infants and differs from the desmoplastic nodular variant by exhibiting a markedly expanded lobular architecture, due to the fact that the reticulin-free zones become unusually large and rich in neuropil-like tissue. Such zones contain a population of small cells resembling those of a central neurocytoma and exhibit a streaming pattern. The internodular reticulin-rich component, which dominates in the desmoplastic/nodular variant, is markedly reduced. Following radiotherapy and/or chemotherapy, medulloblastomas with extensive nodularity may occasionally undergo further maturation to tumours dominated by ganglion cells. A more favourable outcome than for patients with classic medulloblastomas is recognized for both, the desmoplastic/nodular medulloblastoma variant and medulloblastoma with extensive nodularity. It is proposed that medulloblastoma with extensive nodularity and desmoplastic/nodular medulloblastoma share the same ICD-O code 9471/3.
Variants versus patterns
Tumours of the CNS often display a significant histopathological heterogeneity. Surgical pathologists need to know such divergent patterns of differentiation but not every pattern warrants designation as a variant, since some patterns do not have distinct clinical or genetic features. The WHO Working Group for the fourth edition proposed that some recognized tumours should be considered divergent patterns of differentiation rather than distinct clinico-pathological variants.
Small cell glioblastoma
Although small cells are common in glioblastoma, they are predominant or exclusive in a subset known as small cell glioblastoma [38]. This glioblastoma subtype displays a monomorphic cell population characterized by small, round to slightly elongated, densely packed cells with mildly hyperchromatic nuclei, high nuclear:cytoplasmic ratios and only minor atypia. On occasions, these neoplasms superficially resemble anaplastic oligodendroglioma. Microvascular proliferation, necrosis and GFAP immunoreactivity may be minimal while marked proliferative activity is typical. The small cell glioblastoma phenotype frequently shows EGFR amplification [3, 16, 38], p16INK4a homozygous deletion [16], PTEN mutations [16] and LOH 10q [38]. In one study [38], these tumours more commonly expressed the EGFR (83 vs. 35%) and its variant EGFR-vIII (50 vs. 21%) as compared to non-small cell glioblastomas. This glioblastoma type appears to have a poor prognosis [38] although in one population-based study there was no significant overall survival difference when compared to non-small cell glioblastoma [16]. If future clinical trials show a difference in prognosis and/or response to therapy, the small cell glioblastoma may be reconsidered as a variant of glioblastoma with a distinct ICD-O code.
Glioblastoma with oligodendroglioma component
Occasional glioblastomas contain foci that resemble oligodendroglioma. These areas are variable in size and frequency. Two large studies of malignant gliomas suggest that necrosis is associated with significantly worse prognosis in anaplastic gliomas with both oligodendroglial and astrocytic components [36, 50]: patients whose tumours showed necrosis had a substantially shorter median overall survival compared to patients whose tumours did not. At the WHO consensus meeting, the designation of these tumours was controversial. Some participants proposed the term ‘oligoastrocytoma WHO grade IV’ or ‘oligodendroglial glioblastoma multiforme’. The majority of pathologists thought that more clinico-pathological data should be available before this tumour is considered a new disease entity. In particular, it should be established whether or not these tumours carry a better prognosis than standard glioblastomas [13, 16, 31]. For the time being, the new WHO Classification recommends classifying such tumours as ‘glioblastoma with oligodendroglioma component’.
Glioneuronal tumour with neuropil-like islands
Rare infiltrating gliomas contain focal, sharply delineated, round to oval islands composed of a delicate, neuropil-like matrix with granular immunolabeling for synaptophysin. Reported cases were located supratentorially [46], with the exception of one example situated in the cervicothoracic spinal cord [12]. As a rule, the underlying lesions were astrocytomas WHO grade II or III [39, 46]; one case was associated with ependymoma [10].
The neuropil-like islands typically contain neurocytic cells but occasionally also mature-appearing neurons. Usually [46], but not always [22], these cells show a lower proliferative activity than the predominant glial component which typically shows a high degree of atypia, and consists mainly of GFAP-positive, fibrillary and gemistocytic elements identical to those populating conventional astrocytomas. Glioneuronal tumours with neuropil-like islands seem to behave in a manner comparable to astrocytomas with similar WHO grade. The Working Group felt that they constitute a distinct pattern of differentiation but that more cases with genetic analysis and clinical follow-up would be required to consider these lesions as a distinct new variant or entity.
Medulloblastoma with myogenic differentiation versus medullomyoblastoma
Medulloblastoma with myogenic differentiation was previously termed medullomyoblastoma (ICD-O: 9472/3). The code remains but since its clinical features and genetic profile are similar to those of other medulloblastomas, this lesion is no longer considered a distinct entity [14, 34] and it is suggested that the code for medulloblastoma (9470/3) be applied. The descriptive term ‘medulloblastoma with myogenic differentiation’ may be used for any variant (desmoplastic/nodular, large cell medulloblastoma, etc.) containing focal rhabdomyoblastic elements with immunoreactivity to desmin [15, 44], myoglobin [6, 15, 45], and fast myosin [21], but not smooth muscle α-actin alone [15, 44].
Medulloblastoma with melanotic differentiation versus melanotic medulloblastoma
Medulloblastoma with melanotic differentiation was previously termed melanocytic medulloblastoma, with an ICD-O code identical to that of medulloblastoma (9470/3). The melanotic tumour cells may appear undifferentiated or epithelial, with formation of tubules or papillae [1, 7, 9]. They usually express S-100 protein [1, 9]. Since clusters of melanotic tumour cells can occur in any variant of medulloblastoma, such lesions are not regarded as a distinct variant or entity.
CNS primitive neuroectodermal tumours
This term refers to a heterogeneous group of embryonal tumours that occur predominantly in children and adolescents and show aggressive clinical behaviour. They may be phenotypically poorly differentiated, or show divergent differentiation along neuronal, astrocytic and ependymal lines. In the previous edition of the WHO classification [26], these tumours were termed ‘supratentorial primitive neuroectodermal tumours’. In order to include similar tumours located in the brain stem and spinal cord, the more general term (PNET) is recommended. Since this designation is also used for similar, but not identical tumours at extracerebral sites, the WHO Working Group proposes to add the prefix CNS to these entities, in order to avoid any confusion. The term CNS PNET, not otherwise specified (NOS) is synonymous with the current ICD-O term Supratentorial PNET (9473/3) as used for undifferentiated or poorly differentiated embryonal tumours that occur at any extracerebellar site in the CNS.
Tumours with only neuronal differentiation are termed CNS neuroblastomas (9500/3) or, if neoplastic ganglion cells are also present, CNS ganglioneuroblastomas (9490/3). Tumours that display features of the embryonal neural tube formation retain the term medulloepithelioma (9501/3), and those with ependymoblastic rosettes the designation ependymoblastoma (9392/3).
WHO grading
Histological grading is a means of predicting the biological behaviour of a neoplasm. In the clinical setting, tumour grade is a key factor influencing the choice of therapies, particularly determining the use of adjuvant radiation and specific chemotherapy protocols. The WHO classification of tumours of the nervous system includes a grading scheme that is a ‘malignancy scale’ ranging across a wide variety of neoplasms rather than a strict histological grading system [25, 52]. It is widely used, but not a requirement for the application of the WHO classification. The WHO Working Group responsible for the fourth edition included some novel entities (see above); however, since the number of cases of some newly defined entities is limited, the assignment of grades remains preliminary, pending publication of additional data and long-term follow-up.
Grading across tumour entities
Grade I applies to lesions with low proliferative potential and the possibility of cure following surgical resection alone. Neoplasms designated grade II are generally infiltrative in nature and, despite low-level proliferative activity, often recur. Some type II tumours tend to progress to higher grades of malignancy, for example, low-grade diffuse astrocytomas that transform to anaplastic astrocytoma and glioblastoma. Similar transformation occurs in oligodendroglioma and oligoastrocytomas. The designation WHO grade III is generally reserved for lesions with histological evidence of malignancy, including nuclear atypia and brisk mitotic activity. In most settings, patients with grade III tumours receive adjuvant radiation and/or chemotherapy. The designation WHO grade IV is assigned to cytologically malignant, mitotically active, necrosis-prone neoplasms typically associated with rapid pre- and postoperative disease evolution and a fatal outcome. Examples of grade IV neoplasms include glioblastoma, most embryonal neoplasms and many sarcomas as well. Widespread infiltration of surrounding tissue and a propensity for craniospinal dissemination characterize some grade IV neoplasms.
Grading of astrocytic tumours
Grading has been systematically evaluated and successfully applied to a spectrum of diffusely infiltrative astrocytic tumours. These neoplasms are graded in a three-tiered system similar to that of the Ringertz [42], St Anne-Mayo [5] and the previously published WHO schemes [52]. The WHO defines diffusely infiltrative astrocytic tumours with cytological atypia alone as grade II (diffuse astrocytoma), those also showing anaplasia and mitotic activity as grade III (anaplastic astrocytoma), and tumours additionally showing microvascular proliferation and/or necrosis as WHO grade IV. This system is similar to the St Anne/Mayo classification [5], with the only major difference being grade I; in the WHO system, grade I is assigned to the more circumscribed pilocytic astrocytoma, whereas the St Anne/Mayo classification assigns grade 1 to an exceedingly rare diffuse astrocytoma without atypia. Since the finding of a solitary mitosis in an ample specimen does not confer grade III behaviour, separation of grade II from grade III tumours may be more reliably achieved by determination of MIB-1 labelling indices [11, 17, 37]. For WHO grade IV, some authors accept only the criterion of endothelial proliferation, i.e. an apparent multi-layering of endothelium. The WHO classification also accepts glomeruloid microvascular proliferations. Necrosis may be of any type; perinecrotic palisading need not be present.
Tumour grade as a prognostic factor
WHO grade is one component of a combination of criteria used to predict a response to therapy and outcome. Other criteria include clinical findings, such as age of the patient, neurologic performance status and tumour location; radiological features such as contrast enhancement; extent of surgical resection; proliferation indices; and genetic alterations. For each tumour entity, combinations of these parameters contribute to an overall estimate of prognosis. Despite these variables, patients with WHO grade II tumours typically survive more than 5 years and those with grade III tumours survive 2–3 years. The prognosis of patients with WHO grade IV tumours depends largely upon whether effective treatment regimens are available. The majority of glioblastoma patients, particularly the elderly, succumb to the disease within a year. For those with other grade IV neoplasms, the outlook may be considerably better. For example, cerebellar medulloblastomas and germ cell tumours such as germinomas, both WHO grade IV lesions, are rapidly fatal if untreated, while state-of-the-art radiation and chemotherapy result in 5-year survival rates exceeding 60 and 80%, respectively.
Acknowledgments
We wish to thank our colleagues for providing photomicrographs: Dr. Daniel J. Brat, Department of Pathology and Laboratory Medicine, Emory University Hospital, Atlanta, GA 30322, USA; Dr. Volkmar H. Hans, Institute of Neuropathology Evangelisches Krankenhaus, 33617 Bielefeld, Germany; Dr. Johannes A. Hainfellner, Institute of Neurology, Medical University of Vienna, 1097 Vienna, Austria; Dr. M. Fevre-Montange, INSERM U433, Medical Faculty, RTH Laennec, 69003 Lyon, France; Dr. Yoichi Nakazato, Department of Human Pathology, Gunma University, Maebashi, Gunma 371–5811, Japan.
References
- 1.Baylac F, Martinoli A, Marie B, Bracard S, Marchal JC, Bey P, Sommelet D, Hassoun J, Plenat F (1997) [An exceptional variety of medulloblastoma: melanotic medulloblastoma]. Ann Pathol 17:403–405 [PubMed]
- 2.Brat DJ, Scheithauer BW, Staugaitis SM, Holtzman RN, Morgello S, Burger PC (2000) Pituicytoma: a distinctive low-grade glioma of the neurohypophysis. Am J Surg Pathol 24:362–368 [DOI] [PubMed]
- 3.Burger PC, Pearl DK, Aldape K, Yates AJ, Scheithauer BW, Passe SM, Jenkins RB, James CD (2001) Small cell architecture—a histological equivalent of EGFR amplification in glioblastoma multiforme? J Neuropathol Exp Neurol 60:1099–1104 [DOI] [PubMed]
- 4.Dahiya S, Sarkar C, Hedley-Whyte ET, Sharma MC, Zervas NT, Sridhar E, Louis DN (2005) Spindle cell oncocytoma of the adenohypophysis: report of two cases. Acta Neuropathol (Berl) 110:97–99 [DOI] [PubMed]
- 5.Daumas-Duport C, Varlet P (2003) [Dysembryoplastic neuroepithelial tumors]. Rev Neurol (Paris) 159:622–636 [PubMed]
- 6.Dickson DW, Hart MN, Menezes A, Cancilla PA (1983) Medulloblastoma with glial and rhabdomyoblastic differentiation. A myoglobin and glial fibrillary acidic protein immunohistochemical and ultrastructural study. J Neuropathol Exp Neurol 42:639–647 [DOI] [PubMed]
- 7.Dolman CL (1988) Melanotic medulloblastoma. A case report with immunohistochemical and ultrastructural examination. Acta Neuropathol (Berl) 76:528–531 [DOI] [PubMed]
- 8.Fritz A et al (eds) (2000) ICD-O International classification of diseases for oncology. World Health Organization, Geneva
- 9.Garcia Bragado F, Cabello A, Guarch R, Ruiz de Azua Y, Ezpeleta I (1990) Melanotic medulloblastoma. Ultrastructural and histochemical study of a case. Arch Neurobiol Madr 53:8–12 [PubMed]
- 10.Gessi M, Marani C, Geddes J, Arcella A, Cenacchi G, Giangaspero F (2005) Ependymoma with neuropil-like islands: a case report with diagnostic and histogenetic implications. Acta Neuropathol (Berl) 109:231–234 [DOI] [PubMed]
- 11.Giannini C, Scheithauer BW, Steinberg J, Cosgrove TJ (1998) Intraventricular perineurioma: case report. Neurosurgery 43:1478–1481 [DOI] [PubMed]
- 12.Harris BT, Horoupian DS (2000) Spinal cord glioneuronal tumor with “rosetted” neuropil islands and meningeal dissemination: a case report. Acta Neuropathol (Berl) 100:575–579 [DOI] [PubMed]
- 13.He J, Mokhtari K, Sanson M, Marie Y, Kujas M, Huguet S, Leuraud P, Capelle L, Delattre JY, Poirier J, Hoang-Xuan K (2001) Glioblastomas with an oligodendroglial component: a pathological and molecular study. J Neuropathol Exp Neurol 60:863–871 [DOI] [PubMed]
- 14.Helton KJ, Fouladi M, Boop FA, Perry A, Dalton J, Kun L, Fuller C (2004) Medullomyoblastoma: a radiographic and clinicopathologic analysis of six cases and review of the literature. Cancer 101:1445–1454 [DOI] [PubMed]
- 15.Holl T, Kleihues P, Yasargil MG, Wiestler OD (1991) Cerebellar medullomyoblastoma with advanced neuronal differentiation and hamartomatous component. Acta Neuropathol (Berl) 82:408–413 [DOI] [PubMed]
- 16.Homma T, Fukushima T, Vaccarella S, Yonekawa Y, Di Patre PL, Franceschi S, Ohgaki H (2006) Correlation among pathology, genotype, and patient outcomes in glioblastoma. J Neuropatho Exp Neurol 65:846–854 [DOI] [PubMed]
- 17.Huang CI, Chiou WH, Ho DM (1987) Oligodendroglioma occurring after radiation therapy for pituitary adenoma. J Neurol Neurosurg Psychiatry 50:1619–1624 [DOI] [PMC free article] [PubMed]
- 18.Jacques TS, Eldridge C, Patel A, Saleem NM, Powell M, Kitchen ND, Thom M, Revesz T (2006) Mixed glioneuronal tumour of the fourth ventricle with prominent rosette formation. Neuropathol Appl Neurobiol 32:217–220 [DOI] [PubMed]
- 19.Janisch W, Schreiber D, Martin H, Gerlach H (1985) Diencephalic pilocytic astrocytoma with clinical onset in infancy. Biological behavior and pathomorphological findings in 11 children. Zentralbl Allg Pathol 130:31–43 [PubMed]
- 20.Jouvet A, Fauchon F, Liberski P, Saint-Pierre G, Didier-Bazes M, Heitzmann A, Delisle MB, Biassette HA, Vincent S, Mikol J, Streichenberger N, Ahboucha S, Brisson C, Belin MF, Fevre-Montange M (2003) Papillary tumor of the pineal region. Am J Surg Pathol 27:505–512 [DOI] [PubMed]
- 21.Kalimo H, Paljarvi L, Ekfors T, Pelliniemi LJ (1987) Pigmented primitive neuroectodermal tumor with multipotential differentiation in cerebellum (pigmented medullomyoblastoma). A case with light- and electron-microscopic, and immunohistochemical analysis. Pediatr Neurosci 13:188–195 [DOI] [PubMed]
- 22.Keyvani K, Rickert CH, von Wild K, Paulus W (2001) Rosetted glioneuronal tumor: a case with proliferating neuronal nodules. Acta Neuropathol (Berl) 101:525–528 [DOI] [PubMed]
- 23.Kim DH, Suh YL (1997) Pseudopapillary neurocytoma of temporal lobe with glial differentiation. Acta Neuropathol (Berl) 94:187–191 [DOI] [PubMed]
- 24.Kleihues P, Burger PC, Scheithauer BW (eds) (1993) Histological typing of tumours of the central nervous system. World Health Organization international histological classification of tumours. Springer, Heidelberg
- 25.Kleihues P, Burger PC, Scheithauer BW (1993) The new WHO classification of brain tumours. Brain Pathol 3:255–268 [DOI] [PubMed]
- 26.Kleihues P, Cavenee WK (eds) (2000) World Health Organization Classification of Tumours. Pathology and genetics of tumours of the nervous system. IARC Press, Lyon
- 27.Kloub O, Perry A, Tu PH, Lipper M, Lopes MB (2005) Spindle cell oncocytoma of the adenohypophysis: report of two recurrent cases. Am J Surg Pathol 29:247–253 [DOI] [PubMed]
- 28.Komori T, Scheithauer BW, Anthony DC, Rosenblum MK, McLendon RE, Scott RM, Okazaki H, Kobayashi M (1998) Papillary glioneuronal tumor: a new variant of mixed neuronal-glial neoplasm. Am J Surg Pathol 22:1171–1183 [DOI] [PubMed]
- 29.Komori T, Scheithauer BW, Anthony DC, Scott RM, Okazaki H, Kobayashi M (1996) Pseudopapillary ganglioneurocytoma. J Neuropathol Exp Neurol 55:654
- 30.Komori T, Scheithauer BW, Hirose T (2002) A rosette-forming glioneuronal tumor of the fourth ventricle: infratentorial form of dysembryoplastic neuroepithelial tumor? Am J Surg Pathol 26:582–591 [DOI] [PubMed]
- 31.Kraus JA, Lamszus K, Glesmann N, Beck M, Wolter M, Sabel M, Krex D, Klockgether T, Reifenberger G, Schlegel U (2001) Molecular genetic alterations in glioblastomas with oligodendroglial component. Acta Neuropathol (Berl) 101:311–320 [DOI] [PubMed]
- 32.Kuchelmeister K, Demirel T, Schlorer E, Bergmann M, Gullotta F (1995) Dysembryoplastic neuroepithelial tumour of the cerebellum. Acta Neuropathol (Berl) 89:385–390 [DOI] [PubMed]
- 33.Lellouch-Tubiana A, Boddaert N, Bourgeois M, Fohlen M, Jouvet A, Delalande O, Seidenwurm D, Brunelle F, Sainte-Rose C (2005) Angiocentric neuroepithelial tumor (ANET): a new epilepsy-related clinicopathological entity with distinctive MRI. Brain Pathol 15:281–286 [DOI] [PMC free article] [PubMed]
- 34.Leonard JR, Cai DX, Rivet DJ, Kaufman BA, Park TS, Levy BK, Perry A (2001) Large cell/anaplastic medulloblastomas and medullomyoblastomas: clinicopathological and genetic features. J Neurosurg 95:82–88 [DOI] [PubMed]
- 35.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) (2007) WHO Classification of tumours of the central nervous system. IARC, Lyon [DOI] [PMC free article] [PubMed]
- 36.Miller CR, Dunham CP, Scheithauer BW, Perry A (2006) Significance of necrosis in grading of oligodendroglial neoplasms: a clinicopathologic and genetic study of newly diagnosed high-grade gliomas. J Clin Oncol 24:5419–5426 [DOI] [PubMed]
- 37.Neder L, Colli BO, Machado HR, Carlotti CG Jr, Santos AC, Chimelli L (2004) MIB-1 labeling index in astrocytic tumors–a clinicopathologic study. Clin Neuropathol 23:262–270 [PubMed]
- 38.Perry A, Aldape KD, George DH, Burger PC (2004) Small cell astrocytoma: an aggressive variant that is clinicopathologically and genetically distinct from anaplastic oligodendroglioma. Cancer 101:2318–2326 [DOI] [PubMed]
- 39.Prayson RA, Abramovich CM (2000) Glioneuronal tumor with neuropil-like islands. Hum Pathol 31:1435–1438 [DOI] [PubMed]
- 40.Preusser M, Dietrich W, Czech T, Prayer D, Budka H, Hainfellner JA (2003) Rosette-forming glioneuronal tumor of the fourth ventricle. Acta Neuropathol (Berl) 106:506–508 [DOI] [PubMed]
- 41.Preusser M, Holschen A, Novak K, Czech T, Prayer O, Hainfellner A, Baumgartner C, Woermann FG, Tuxhorn IE, Pannek HW, Bergmann M, Radlwimmer B, Villagran R, Weber RG, Hans VH (2007) Angiocentric glioma: report of clinico-pathologic and genetic findings in 8 cases. Am J Surg Pathol (in press) [DOI] [PubMed]
- 42.Ringertz J (1950) Grading of gliomas. Acta Pathol Microbiol Scand 27:51–64 [PubMed]
- 43.Roncaroli F, Scheithauer BW, Cenacchi G, Horvath E, Kovacs K, Lloyd RV, Abell-Aleff P, Santi M, Yates AJ (2002) ‘Spindle cell oncocytoma’ of the adenohypophysis: a tumor of folliculostellate cells? Am J Surg Pathol 26:1048–1055 [DOI] [PubMed]
- 44.Schiffer D, Giordana MT, Pezzotta S, Pezzulo T, Vigliani MC (1992) Medullomyoblastoma: report of two cases. Childs Nerv Syst 8:268–272 [DOI] [PubMed]
- 45.Smith T, Davidson R (1984) Medullomyoblastoma. A histologic, immunohistochemical, and ultrastructural study. Cancer 54:323–332 [DOI] [PubMed]
- 46.Teo JG, Gultekin SH, Bilsky M, Gutin P, Rosenblum MK (1999) A distinctive glioneuronal tumor of the adult cerebrum with neuropil-like (including “rosetted”) islands: report of 4 cases. Am J Surg Pathol 23:502–510 [DOI] [PubMed]
- 47.Tihan T, Fisher PG, Kepner JL, Godfraind C, McComb RD, Goldthwaite PT, Burger PC (1999) Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol 58:1061–1068 [DOI] [PubMed]
- 48.Ulm AJ, Yachnis AT, Brat DJ, Rhoton AL Jr (2004) Pituicytoma: report of two cases and clues regarding histogenesis. Neurosurgery 54:753–757 [DOI] [PubMed]
- 49.Vajtai I, Sahli R, Kappeler A (2006) Spindle cell oncocytoma of the adenohypophysis: report of a case with a 16-year follow-up. Pathol Res Pract 202:745–750 [DOI] [PubMed]
- 50.van den Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJ, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L, Haaxma-Reiche H, Kros JM, van Kouwenhoven MC, Vecht CJ, Allgeier A, Lacombe D, Gorlia T (2006) Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol 24:2715–2722 [DOI] [PubMed]
- 51.Wang M, Tihan T, Rojiani AM, Bodhireddy SR, Prayson RA, Iacuone JJ, Alles AJ, Donahue DJ, Hessler RB, Kim JH, Haas M, Rosenblum MK, Burger PC (2005) Monomorphous angiocentric glioma: a distinctive epileptogenic neoplasm with features of infiltrating astrocytoma and ependymoma. J Neuropathol Exp Neurol 64:875–881 [DOI] [PubMed]
- 52.Zülch KJ (ed) (1979) Histological typing of tumours of the central nervous system. World Health Organization, Geneva


