Abstract
Increased pulmonary artery pressure (PAP) can complicate the postoperative care of children undergoing surgical repair of congenital heart defects. Endogenous NO regulates PAP and is derived from arginine supplied by the urea cycle. The rate-limiting step in the urea cycle is catalyzed by a mitochondrial enzyme, carbamoyl-phosphate synthetase I (CPSI). A well-characterized polymorphism in the gene encoding CPSI (T1405N) has previously been implicated in neonatal pulmonary hypertension. A consecutive modeling cohort of children (N=131) with congenital heart defects requiring surgery was prospectively evaluated to determine key factors associated with increased postoperative PAP, defined as a mean PAP > 20mmHg for at least one hour during the 48 hours following surgery measured by an indwelling pulmonary artery catheter. Multiple Dimensionality Reduction (MDR) was used to both internally validate observations and develop optimal two-variable through five-variable models that were tested prospectively in a validation cohort (N= 41). Unconditional logistic regression analysis of the modeling cohort revealed that age (OR= 0.92, p=0.01), CPSI T1405N genotype (AC vs. AA: OR=4.08, p=0.04, CC vs. AA: OR=5.96, p=0.01), and Down syndrome (OR=5.25, p=0.04) were independent predictors of this complex phenotype. MDR predicted that the best two-variable model consisted of age and CPSI T1405N genotype (p<0.001). This two-variable model correctly predicted 73% of the outcomes from the validation cohort. A five-variable model that added race, gender and Down’s syndrome was not significantly better than the two-variable model. In conclusion, the CPSI T1405N genotype appears to be an important new factor in predicting susceptibility to increased PAP following surgical repair of congenital cardiac defects in children.
Keywords: Mitochondrial enzyme, CPS I, pulmonary artery pressure
1. Introduction
Congenital heart defects affect nearly 1% of all newborns (American Heart Association, 2005; Hoffman, 1995). Corrective or palliative surgery utilizing cardiopulmonary bypass is often performed during the first year of life (Aylin et al, 2004). Increased pulmonary artery pressure (PAP) can complicate the surgical repair of many of these cardiac defects by further compromising cardiac output in the immediate postoperative period (Russell, et al, 1998; Bandla et al, 1999; Steinhorn and Fineman, 1999; Gentles et al, 1997; Freeman et al, 1995; Nakajima et al, 1996; Adatia et al, 1996; Schulze-Neick, 2001). Invasive and expensive interventions, including mechanical ventilation with inhaled nitric oxide (NO), are often required to treat this complication (Russell, et al, 1998; Adatia et al, 1996; Journois et al, 1994; Miller et al, 2000; Yagahi et al, 1998; Zobel et al, 1998; Metzler and Beitzke, 1997).
Endogenous NO is critical for the maintenance of normal PAP (Steinhorn and Fineman, 1999; Schulze-Neick et al, 1999; Kirshbom et al, 1996). Endothelial cells generate NO from arginine, a precursor amino acid supplied by the urea cycle (Moncada and Higgs, 1993). We have previously demonstrated that infants undergoing surgical repair of congenital heart defects have significantly decreased serum arginine levels in the immediate postoperative period (Barr et al, 2003). This decrease in availability of a key substrate for NO following cardio-pulmonary bypass may increase the risk of postoperative pulmonary hypertension (Barr et al, 2003).
The rate limiting step in arginine production occurs inside the mitochondrion and is catalyzed by the enzyme, carbamyl-phosphate synthetase I (CPSI). This process is outlined in Figure 1 (Summar et al, 2003). The CPSI gene, located on human chromosome 2q35, consists of 38 exons and 37 introns that span more than 120 kilobases of genomic DNA (Summar et al, 2003). A specific single nucleotide polymorphism (SNP) designated as T1405N in this gene results in a threonine to asparagine amino acid substitution at an important cofactor binding site (Summar et al, 2003). We have previously shown that CPSI T1405N genotype affects plasma arginine levels (Pearson et al, 2001). In addition, the CPSI T1405N genotype distribution was significantly different in neonates who developed persistent pulmonary hypertension of the newborn (PPHN) compared to unaffected neonates (Pearson et al, 2001). Specifically, neonates with at least one copy of the A allele (Asparagine 1405 variant) appeared to be less likely to develop PPHN.
Figure 1.
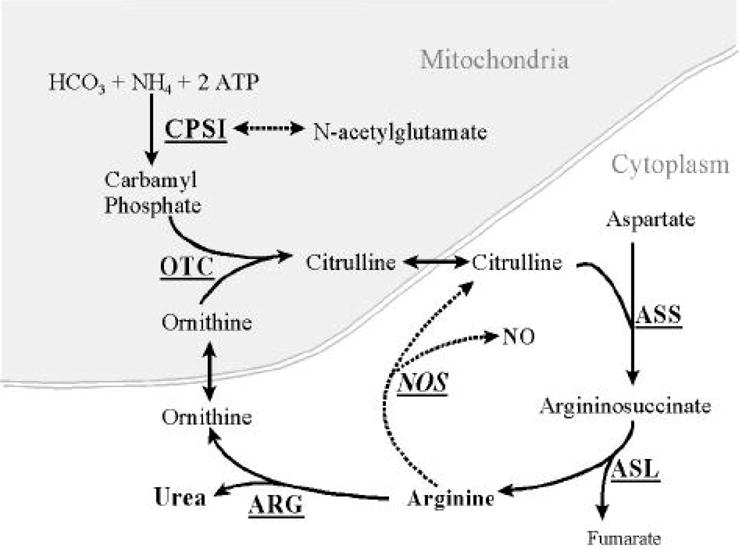
The urea cycle. Carbamyl phosphate synthetase I is the rate limiting step in the urea cycle. The CPSI T1405N polymorphism is located in the N-acetylglutamate cofactor binding site. Note that arginine formed by the urea cycle serves as the substrate for NO.
We hypothesized that the CPSI T1405N genotype would affect susceptibility to increased PAP in children undergoing surgical repair of congenital heart defects. This cohort study was initially constructed to determine if the T1405N polymorphism was an independent predictor of increased PAP measured by an indwelling pulmonary artery catheter during the postoperative period. A model incorporating key variables predicting this phenotype was then prospectively tested in a second validation patient cohort. Our objective was to determine potential clinical and genetic factors that predict the occurrence of increased PAP in this especially vulnerable population.
2. Methods
2.1 Ascertainment of Patient Populations
Two separate cohorts of children undergoing surgical correction of their congenital heart defects at Vanderbilt Children’s Hospital were prospectively enrolled in this study. The first group was a continuous modeling cohort of 131 children enrolled between August 2000 and September 2002. The second group was a continuous validation cohort of 41 children enrolled between April 2003 and August 2004. Inclusion criteria were identical for both groups. Infants undergoing any of the following 5 surgical procedures for repair of congenital heart defects were eligible: atrioventricular septal defect (AVSD) repair, ventricular septal defect (VSD) repair, bidirectional Glenn procedure, modified Fontan procedure, and arterial switch procedure for transposition of the great vessels. Written consent was obtained from the parents of patients at the time of preoperative evaluation in accordance with a protocol and consent document approved by the Vanderbilt University Institutional Review Board. All surgical procedures were performed by one of two pediatric cardiac surgeons using similar cardiopulmonary bypass and cardioplegia preparations.
2.2 Definition of Increased Pulmonary Artery Pressure
All patients in this cohort had pulmonary artery pressure measured continuously during the postoperative period via a 3 French single lumen non-thermodilution pulmonary artery catheter (or 4 French double lumen central line in the superior vena cava in the case of patients undergoing a modified Glenn or Fontan procedure) inserted at the time of surgery. All patients had PA pressure monitoring performed for clinically indicated reasons at the discretion of the attending surgeon, no patient had a PA catheter placed solely for research purposes. Thermodilution catheters were not used due to risk of complications from large thermodilution catheters in infants. Using previously published perioperative criteria, increased PAP was defined as a sustained increase in mean pulmonary artery pressure (MPAP) of > 20mmHg for at least an hour in duration during the 48 hours following surgery (Kulick, 1998).
2.3 Genotyping of the CPSI T1405N Polymorphism
Three milliliters of arterial blood was obtained from patients preoperatively and stored in citrated tubes. The buffy coat was isolated and genomic DNA extracted from white blood cells utilizing a genomic DNA isolation kit (Promega Corp, Madison WI). Isolated DNA from patients and known controls was subjected to PCR utilizing previously described T1405N primers and optimal PCR conditions as previously described (Summar et al, 2003). PCR products were isolated by mutation detection enhancement (MDE) electrophoresis utilizing a MDE heteroduplex kit to clearly visualize single base substitutions (AT Biochem, Malvern, PA).
2.4 Statistical Analysis
Means for continuous variables were compared by means of the Student’s t test for normally distributed variables. In this case, data are presented as means with standard deviations. If the assumption of a normal distribution could not be made for a given continuous variable, the Mann-Whitney U-test was used to compare results between PAP outcome categories. Categorical variables were compared using either the Chi-square test or Fisher’s exact test. Multivariate logistic regression was used to estimate the adjusted odds ratio of the risk of individual CPSI genotypes after taking other factors into consideration. The level of significance in this study was set prior to analysis at 0.05. STATA 9.0 (STATA Corp., College Station, TX) statistical software was used to accomplish all of the analyses in this study. To determine whether the CPSI T1405N polymorphism was in Hardy-Weinberg equilibrium an analysis of the genotypes was performed by goodness-of-fit chi-square analysis with 2 degrees of freedom. Expected values for the distribution of the T1405N polymorphism were based on allele frequencies from a sample of 400 unrelated adults from the same geographic area and with the same racial distribution as the children in this study (Summar et al, 2003). The mathematical model derived from unconditional logistic regression was then tested prospectively in the validation cohort. If the model’s predicted probability was greater than 0.50 for the primary outcome in a given individual, then this was considered as a prediction of that outcome. The results of this model are presented as % accuracy of predicted values.
2.5 Multifactor Dimensionality Reduction (MDR) Analysis
Multifactor Dimensionality Reduction (MDR) was used in this analysis to both internally validate findings in the modeling cohort and to develop optimal two-variable through five-variable models for testing in the validation cohort (Hahn et al, 2003; Ritchie et al, 2001; Coffey et al, 2004; Cho et al 2004; Bastone et al, 2004; Moore, 2004; Moore and Williams, 2002; Ritchie et al, 2003). In the first step of Multifactor Dimensionality Reduction (MDR), the data is divided into a training set and an independent testing set for cross validation. Second, a set of n genetic and/or environmental factors is selected. These factors and their multi-factor classes are divided in n-dimensional space. Then the ratio of affecteds to unaffecteds is calculated within each multifactor class. Each multifactor cell class is then labeled “high risk” or “low risk” based on the ratio calculated, therefore reducing the n-dimensional space to one dimension with two levels. The collection of these multifactor classes composes the MDR model for a particular combination of factors. For each possible model size (one-variable, two-variable, etc.) a single MDR model is chosen that has the lowest number of misclassified individuals. In order to evaluate the predictive ability of the model, prediction error is calculated using 10-fold cross-validation. The result is a set of models, one for each model size considered. From these models, a final model is chosen based on minimization of prediction error and maximization of cross validation consistency (number of times a particular set of factors is identified across the cross validation subsets). The statistical significance of the final best model is determined through permutation testing. Permutation testing involves creating 1000 permuted datasets by randomizing the disease status labels. The entire MDR procedure is repeated for each, generating a distribution of prediction errors and cross validation consistencies that could be expected by chance alone. The significance of the final model is determined by comparing the prediction error and cross validation consistency of the final model to the distribution. A p-value is extracted for the model by its theoretical location in the distribution (Ritchie et al, 2001).
3. Results
3.1 Model Study Population
During the modeling study period, 131 consecutive children met inclusion criteria and were enrolled. Of these patients, 78 (60%) developed increased postoperative PAP. There were no significant differences between children with and without increased PAP with respect to gender, race or length of cardiopulmonary bypass. On univariate analysis, there were statistically significant differences between children with and without increased PAP with respect to age, weight, and Down’s syndrome (Table 1). As expected, age at surgery and weight were highly correlated (r=0.87) and therefore weight was not included in subsequent analyses.
Table 1. Demographic and Clinical Characteristics of Patients in the Modeling Cohort (N=131).
The primary outcome variable in the modeling cohort was the postoperative development of increased pulmonary artery pressure (PAP). Of the 131 patients in the modeling cohort, 78 (60%) developed increased PAP. Age, weight, and Down’s syndrome were independent risk factors for the development of increased postoperative PAP on univariate analysis. Weight was determined to highly correlated with age (r=0.87) and was not included in subsequent multivariate analyses. Race, gender, and length of cardiopulmonary bypass (CPB time) did not affect this outcome. Patients with increased postoperative PAP had longer duration of postoperative mechanical ventilation, and higher use of postoperative inhaled nitric oxide. Age, length of mechanical ventilation, and length of hospitalization are expressed as median and interquartile range, weight and cardiopulmonary bypass time are expressed as means ± standard deviation.
| Pulmonary Artery Pressure Following Surgery | |||
|---|---|---|---|
| Variable | Increased (N=78) | Not Increased (N= 53) | P-value |
| Age, months | 4.5 (2.3–6.3) | 6.5 (5–11) | <0.01 |
| Race, | |||
| %Caucasian | 77% | 85% | 0.96 |
| % African-American | 12% | 11% | |
| Gender, | |||
| % Female | 38% | 38% | 0.96 |
| Weight, kg | 5.4 (±2.7) | 7.4 (±3.7) | <0.01 |
| Down’s Syndrome, % | 22.5% | 3.6% | <0.01 |
| CPB Time, minutes | 110 (±42) | 100 (±32) | 0.12 |
| Duration of Mechanical Ventilation (hours) | 62 (22–142) | 13 (7–43.5) | <0.01 |
| Use of Inhaled Nitric Oxide | 31/78 (39.7%) | 6/53 (11.3%) | <0.01 |
| Length of Hospitalization (days) | 8 (6–15) | 7 (5–10) | 0.06 |
| Mortality | 8/78 (10.3%) | 2/53 (3.8%) | 0.20 |
Patients with increased postoperative PAP tended to require increased duration of mechanical ventilation and increased utilization of inhaled nitric oxide (Table 1). There were also non-significant trends towards increased length of hospitalization and increased mortality in patients with increased postoperative PAP.
In the modeling cohort, the distribution of CPSI T1405N genotypes was different in patients who developed increased PAP compared to those who did not, p=0.031 (Table 2). The cohort N=131 was in Hardy-Weinberg equilibrium (p=0.70). Multivariate logistic regression analysis of the list of potential variables contributing to this phenotype revealed that T1405N genotype was an independent predictor of increased postoperative PAP (Table 3). Other variables that remained independent predictors included age at the time of surgery (OR= 0.92, 95% CI 0.87–0.98) and Down’s syndrome (OR= 5.25, 95% CI 1.11–24.76) following adjustment for both CPSI T1405N status and cardiopulmonary bypass time.
Table 2. CPSI T1405N Genotype Distribution for Children Developing Increased Pulmonary Artery Pressure (PAP) Following Surgical Repair of Congenital Heart Defects.
The CPSI T1405N genotype distribution for children developing increased postoperative pulmonary artery pressure (PAP) following surgical repair of congenital heart anomalies is different between outcome groups (p=0.031) and supports our hypothesis that variation in this genotype may have a role in the development of postoperative elevation in PAP. Possible CPSI T1405N genotypes include homozygote (CC), heterozygote (AC), and homozygote (AA).
| CPSI Genotype | ||||
|---|---|---|---|---|
| Outcome Variable | CC | AC | AA | |
| Increased PAP, (%) | 44 (56%) | 30 (39%) | 4 (5%) | (N=78) |
| No increase in PAP, (%) | 20 (38%) | 24 (45%) | 9 (17%) | (N=53) |
| P-value=0.031 | ||||
Table 3. Multivariate Logistic Regression Analysis Demonstrating That CPSI Genotype Independently Predicts Increased Postoperative Pulmonary Artery Pressure After Adjustment For Age, Cardiopulmonary Bypass Time and Down’s Syndrome.
This table demonstrates the results of the multivariate analysis of the listed variables and their role in predicting the outcome. Note that CPSI genotype independently predicts the development on increased postoperative PAP after adjustment for age, cardiopulmonary bypass time (CPB time) and Down’s syndrome. OR= odds ratio, CI= confidence interval. CPSI T1405N genotypes are compared as heterozygote (AC) vs homozygote (AA) and homozygote (CC) vs homozygote (AA).
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Age | 0.92 | 0.87–0.98 | 0.01 |
| CPB Time | 1.00 | 0.99–1.01 | 0.53 |
| Down’s Syndrome | 5.25 | 1.11–24.76 | 0.04 |
| CPSI T1405N Genotype | |||
| AC vs. AA | 4.08 | 1.04–16.04 | 0.04 |
| CC vs. AA | 5.96 | 1.53–23.15 | 0.01 |
MDR analysis of the modeling cohort concluded that a two-variable model incorporating age and CPSI T1405N genotype status had the highest cross-validation consistency and lowest prediction error (p<0.001). This is summarized in Figure 2. The equation for this model is presented in Figure 3. The next best model generated from the MDR analysis was a five-variable model consisting of age, CPSI T1405N genotype status, Down’s syndrome, race and gender (p=0.049).
Figure 2.
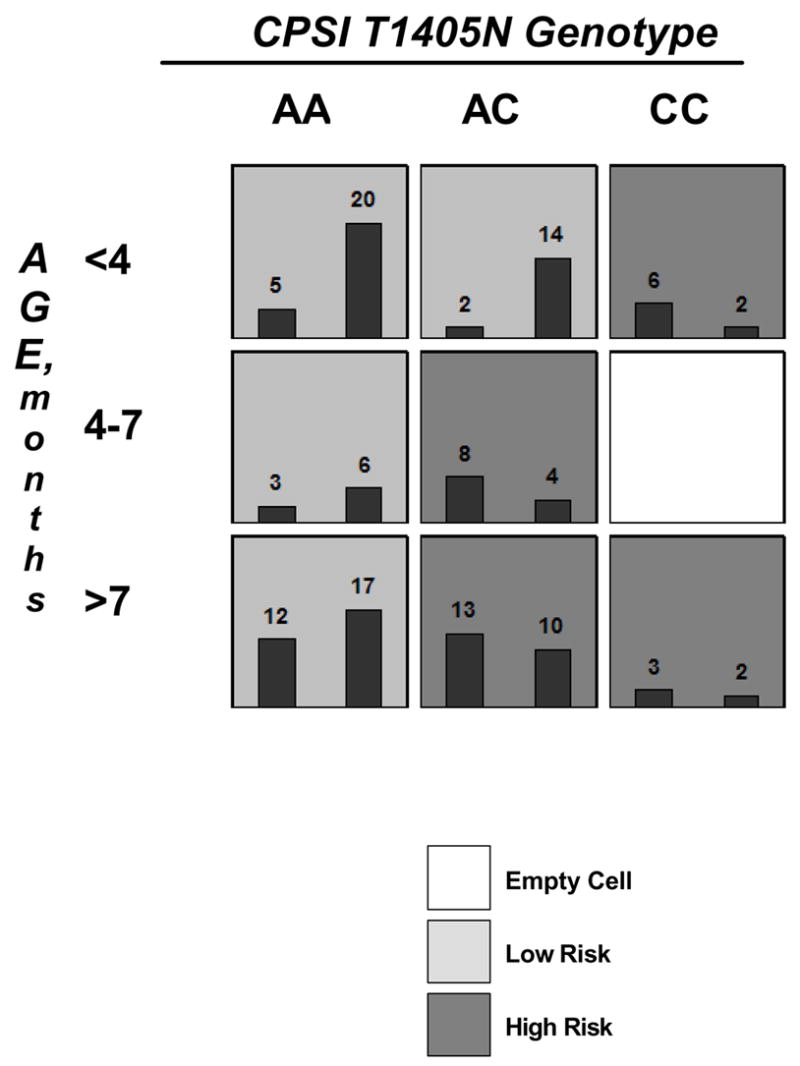
MDR model demonstrating an interaction between CPSI 1405N genotype and patient age that predicted disease status. The average prediction error of the MDR model was only 32.5 % and the average prediction accuracy was 67.5 % (p<0.001). Light grey cells are low-risk, dark grey cells are high risk, and white cells contain no data points. Within each cell, the number of patients with increased PAP are shown in the left histogram while patients without increased PAP are shown by the right histogram.
Figure 3.
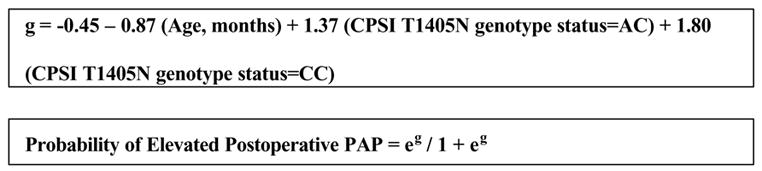
The equation in the upper box represents the best two-variable model derived from the modeling cohort. The lower box represents the equation that transforms the modeling equation into the predicted probability of the outcome. This model was the best predictor of outcomes in the validation cohort with an accuracy of 73%.
3.2 Validation Cohort
The validation cohort for this study consisted of 41 children at the same institution followed prospectively. These children had the same inclusion and exclusion criteria. They were collected and enrolled prior to the analysis outlined above. Using the two-variable model derived from the MDR analysis, age and CPSI T1405N genotype status, the model predicted with 73% accuracy the development of increased PAP. The model with age alone predicted the primary outcome with 66% accuracy. The five-variable model consisting of age, CPSI T1405N genotype status, Down’s syndrome, race and gender predicted outcome in the validation cohort with 71% accuracy. The validation cohort had significantly fewer children with Down’s syndrome develop increased PAP than the modeling cohort.
4. Discussion
This study of two consecutive cohorts of children undergoing surgical repair of congenital heart defects provided a special opportunity to further understand the clinical and genetic factors involved in the development of increased postoperative pulmonary artery pressure. Analysis of the initial modeling cohort again demonstrated the clinical relevance of the CPSI T1405N polymorphism in a phenotype where endogenous NO is critically important. Previously, this same polymorphism was implicated in neonatal pulmonary hypertension and hepatovenocclusive disease (HVOD) following bone marrow transplantation (Pearson et al, 2001, Kallianpur et al, 2005). The CPSI T1405N polymorphism is the result of C to A transversion at base 4332 in the 3′ region of the CPSI mRNA, changing the triplet code from the evolutionarily conserved ACC to AAC. This results in an amino acid change from threonine to asparagine at residue 1405 (referred to as T1405N) in the CPSI enzyme. Residue 1405 is located within the binding site of an essential CPSI cofactor, N-acetylglutamate (NAG) (Summar et al, 2001). CPSI is the key, rate-limiting enzyme that catalyzes the first step in the hepatic urea cycle. In addition to removal of waste nitrogen, the urea cycle also synthesizes arginine which is the main substrate for NO production by nitric oxide synthase. Preliminary enzyme-kinetic studies of recombinant carbamoyl-phosphate synthase in vitro showed that the Asn1405 variant was more efficient than the Thr1405 variant (Summar et al, 2004). This corresponds with the observation that neonates with the AA T1405N genotype (homozygous for the Asn1405 variant) had significantly higher levels of arginine and nitric oxide metabolites (Pearson et al, 2001). It is therefore plausible that the CPSI T1405N polymorphism may result in a significant change in CPSI enzymatic function.
In a previous study of infants undergoing congenital heart surgery, we found significantly decreased postoperative levels of arginine, citrulline (a urea cycle intermediate and precursor of arginine) and nitric oxide metabolites (Barr, 2003). In that study, arginine levels fell by more than 50% and did not recover until 48 hours following surgery (Barr, 2003). In a related study, we demonstrated the effectiveness of oral citrulline in preserving preoperative levels of arginine and decreasing the risk of postoperative pulmonary hypertension in a similar population of children undergoing repair of congenital heart defects (Smith et al, 2006).
Previous reports have highlighted the complexity of this phenotype (Russell, et al, 1998; Bandla et al, 1999; Steinhorn and Fineman, 1999; Gentles et al, 1997; Freeman et al, 1995; Nakajima et al, 1996; Adatia et al, 1996; Schulze-Neick, 2001). In these previous reports, age at the time of surgery has been identified as an important factor in the development of elevated PAP, with younger children being at increased risk for this complication (Steinhorn and Fineman, 1999; Schulze-Neick et al, 2001). In this study, we identified a genetic factor, CPSI T1405N, that is also an independent risk factor for the development of increased PAP. Since many genetic association studies have been difficult to reproduce, we tested the model derived from our initial cohort on a subsequent validation cohort. Both cohorts were population-based in that all children were consecutively enrolled prior to their surgery to minimize selection bias. Additionally, we chose to target a single nucleotide polymorphism that had already been implicated in a similar phenotype thus limiting the potential effect of multiple comparisons. To further limit the possibility of a false-positive association, we utilized Multiple Dimensionality Reduction (MDR) in our model development (Hahn et al, 2003; Ritchie et al, 2001). Using this combination of cohorts and methods, we developed and validated a novel model, combining both clinical and genetic data, which contributes to the prediction of postoperative PAP.
The primary outcome variable in this study was a postoperative increase in mean PAP. We evaluated patients who had a 3 French single lumen non-thermodilution pulmonary artery catheter (or a central venous line in the superior vena cava for single ventricle patients) inserted at the end of surgery for clinically indicated reasons by the attending surgeon. We defined increased pulmonary artery pressure as a sustained increase in the mean PAP > 20 mmHg for at least 1 hour within 48 hours of surgery (Kulik, 1998). The threshold of a mean PAP>20 is an important outcome for several reasons (Steinhorn and Fineman, 1999; Kulik, 1998). In patients undergoing repair of single ventricle lesions, pulmonary blood flow is passive relying on low downstream pressures. Therefore, mean pulmonary pressures exceeding 20 to 25 mmHg would not be tolerated physiologically, leading to therapeutic intervention and reevaluation (Gentles et al, 1997; Metzler and Beitzke, 1997; Petrosian et al, 1999; Amodeo et al, 1997; Mosca et al, 2000). For patients undergoing repair of two ventricle lesions, in the immediate postoperative period mildly elevated pulmonary artery pressures may worsen right ventricular function. Finally, severe elevations in pulmonary artery pressure to systemic or near systemic levels elicit prompt therapeutic intervention with inhaled NO. For these reasons we utilized the above definition to capture patients with a mild to moderate increase in PAP. Unfortunately, without a thermodilution catheter, we were not able to measure pulmonary vascular resistance in these patients. Commercially available thermodilution catheters are too large to routinely place in small infants and children and because this was an observational study, we did not attempt to change the practice for postoperative pulmonary artery pressure monitoring from what is normally done by the cardiac surgeons.
While the model consisting of CPSI T1405N genotype and age at surgery was able to predict outcome in 73% of patients in the validation cohort, there are clearly many other factors involved in the development of postoperative PAP. Certainly other genetic, biochemical, and clinical factors will be identified that will increase predictive ability. Of particular interest will be the transport functions associated with the critically important portion of the urea cycle occurring inside the mitochondrion. Large, prospective, multi-centered studies with appropriate blinding will be necessary in order to begin to stratify for broad classifications of congenital heart disease. Only when we have knowledge of these individual factors and how they interact will we be able to accurately predict the development of this complication. In summary, future predictive models of increased pulmonary artery pressure following surgery to correct congenital cardiac defects should incorporate CPSI T1405N genotype.
Footnotes
Funding Support: NIH/NHLBI R01 HL073317-01 (FEB)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adatia I, Atz A, Jonas RA, Wessel DL. Diagnostic use of inhaled nitric oxide after neonatal cardiac operations. J Cardiovasc Surg. 1996;112:1403–1405. doi: 10.1016/S0022-5223(96)70166-6. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association. Heart disease and stroke statistics – 2005 update. American Heart Association; Dallas, TX: 2005. [Google Scholar]
- 3.Amodeo A, Galetti L, Marianeschi S, Picardo S, Giannico S, Di Renzi P, Marcelletti C. Extracardiac Fontan operation for complex cardiac anomalies: seven years experience. J Cardiovasc Surg. 1997;114:1020–1031. doi: 10.1016/S0022-5223(97)70016-3. [DOI] [PubMed] [Google Scholar]
- 4.Aylin P, Bottle A, Jarman B, Elliot P. Paediatric cardiac surgical mortality in England after Bristol: descriptive analysis of hospital episode statistics 1991–2002. BMJ. 2004;329:825. doi: 10.1136/bmj.329.7470.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandla HPR, Hopkins RL, Beckerman RC, Gozal D. Pulmonary risk factors compromising postoperative recovery after surgical repair for congenital heart disease. Chest. 1999;116:740–747. doi: 10.1378/chest.116.3.740. [DOI] [PubMed] [Google Scholar]
- 6.Barr FE, Beverley H, VanHook K, Cermak E, Christian K, Drinkwater D, Dyer K, Raggio NT, Moore JH, Christman B, Summar M. Effect of Cardiopulmonary Bypass on Urea Cycle Intermediates and Nitric Oxide Levels after Congenital Heart Surgery. J Pediatrics. 2003;142:26–30. doi: 10.1067/mpd.2003.mpd0311. [DOI] [PubMed] [Google Scholar]
- 7.Bastone L, Reilly M, Rader DJ, Foulkes AS. MDR and PRP: a comparison of methods for high-order genotype-phenotype associations. Hum Hered. 2004;58:82–92. doi: 10.1159/000083029. [DOI] [PubMed] [Google Scholar]
- 8.Cho YM, Ritchie MD, Moore JH, Park JY, Lee KU, Shin HD, Lee HK, Park KS. Multifactor dimensionality reduction reveals interactions among the UCP2 and PPARg genes in type 2 diabetes. Diabetologia. 2004;47:549–554. doi: 10.1007/s00125-003-1321-3. [DOI] [PubMed] [Google Scholar]
- 9.Coffey CS, Hebert PR, Ritchie MD, Krumholz HM, Gaziano JM, Ridker PM, Brown NJ, Vaughan DE, Moore JH. An application of conditional logistic regression and multifactor dimensionality reduction for detecting gene-gene interactions on risk of myocardial infarction: the importance of model validation. BMC Bioinformatics. 2004;5:49. doi: 10.1186/1471-2105-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman J, DeLeon SY, Miles RH, Downey FX, Hofstra J, Quinones JA, Fisher EA, Pifarre R. Acute pulmonary hypertension complicating the arterial switch procedure. Pediatr Cardiol. 1995;16:297–300. doi: 10.1007/BF00798066. [DOI] [PubMed] [Google Scholar]
- 11.Gentles TL, Mayer JE, Jr, Gauvreau K, Gentles TL, Mayer JE, Jr, Gauvreau K, Newburger JW, Lock JE, Kupferschmid JP, Burnett J, Jonas RA, Castaneda AR, Wernovsky G. Fontan operations in five hundred consecutive patients: factors affecting early and late outcome. J Cardiovasc Surg. 1997;114:376–391. doi: 10.1016/s0022-5223(97)70183-1. [DOI] [PubMed] [Google Scholar]
- 12.Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics. 2003;19:376–382. doi: 10.1093/bioinformatics/btf869. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman JI. Incidence of congenital heart disease: I. Postnatal incidence. Pediatr Cardiol. 1995;16:103–113. doi: 10.1007/BF00801907. [DOI] [PubMed] [Google Scholar]
- 14.Journois D, Pouard P, Mauriat P, Malhere T, Vouhe P, Safran D. Inhaled nitric oxide as a therapy for pulmonary hypertension after operations for congenital heart defects. J Thorac Cardiovasc Surg. 1994;107:1129–1135. [PubMed] [Google Scholar]
- 15.Kallianpur AR, Hall LD, Yadav M, Byrne DW, Speroff T, Dittus RS, Haines JL, Christman BW, Summar ML. The hemochromatosis C282Y allele: a risk factor for hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:1155–1164. doi: 10.1038/sj.bmt.1704943. [DOI] [PubMed] [Google Scholar]
- 16.Kirshbom P, Jacobs M, Tsui S, DiBernardo LR, Schwinn DA, Ungerleider RM, Gaynor JW. Effects of cardiopulmonary bypass and circulatory arrest on endothelium-dependent vasodilatation in the lung. J Thorac Cardiovasc Surg. 1996;111:1248–1256. doi: 10.1016/s0022-5223(96)70228-3. [DOI] [PubMed] [Google Scholar]
- 17.Kulik TJ. Pulmonary Hypertension. In: Chang AC, Hanley FL, Wernovsky G, Wessel DL, editors. Pediatric Cardiac Intensive Care. 1. Lippincott Williams & Wilkins; Baltimore, MD, USA: 1998. p. 497. [Google Scholar]
- 18.Metzler H, Beitzke A. Inhaled nitric oxide in patients with critical pulmonary perfusion after Fontan-type procedures and bidirectional Glenn anastomosis. J Cardiovasc Surg. 1997;113:435–442. doi: 10.1016/S0022-5223(97)70355-6. [DOI] [PubMed] [Google Scholar]
- 19.Miller OI, Tang SF, Keech A, Pigott NB, Beller E, Celermajer DS. Inhaled nitric oxide as a therapy for pulmonary hypertension after congenital heart surgery: a randomized double-blind study. Lancet. 2000;356:1464–9. doi: 10.1016/S0140-6736(00)02869-5. [DOI] [PubMed] [Google Scholar]
- 20.Moore JH. Computational analysis of gene-gene interactions in common human diseases using multifactor dimensionality reduction. Expert Rev Mol Diagn. 2004;4:795–803. doi: 10.1586/14737159.4.6.795. [DOI] [PubMed] [Google Scholar]
- 21.Moore JH, Williams SM. New strategies for identifying gene-gene interactions in hypertension. Ann Med. 2002;34:88–95. doi: 10.1080/07853890252953473. [DOI] [PubMed] [Google Scholar]
- 22.Moncada S, Higgs A. The L-Arginine-Nitric Oxide Pathway. N Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 23.Mosca RS, Kulik TJ, Goldberg CS, Vermilion RP, Charpie JR, Crowley DC, Bove EL. Early results of the Fontan procedure in one hundred consecutive patients with hypoplastic left heart syndrome. J Cardiovasc Surg. 2000;119:1110–1118. doi: 10.1067/mtc.2000.106656. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima Y, Momma K, Seguchi M, Nakazawa M, Imai Y. Pulmonary hypertension in patients with complete transposition of the great arteries: midterm results after surgery. Pediatr Cardiol. 1996;17:104–107. doi: 10.1007/BF02505092. [DOI] [PubMed] [Google Scholar]
- 25.Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, Bazyk A, Scott N, Summar ML. The role of urea cycle intermediates, carbamyl phosphate synthetase I function, and nitric oxide production in neonatal pulmonary hypertension. N Engl J Med. 2001;344:1832–1838. doi: 10.1056/NEJM200106143442404. [DOI] [PubMed] [Google Scholar]
- 26.Petrossian E, Reddy M, McElhinney DB, Akkersdijk GP, Moore P, Parry AJ, Thompson LD, Hanley FL. Early results of the extracardiac conduit Fontan operation. J Cardiovasc Surg. 1999;117:688–695. doi: 10.1016/S0022-5223(99)70288-6. [DOI] [PubMed] [Google Scholar]
- 27.Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, Moore JH. Multifactor dimensionality reduction reveals high-order interactions among estrogen metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69:138–147. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritchie MD, Hahn LW, Moore JH. Power of multifactor dimensionality reduction for detecting gene-gene interactions in the presence of genotyping error, missing data, phenocopy, and genetic heterogeneity. Genet Epidemiol. 2003;24:150–157. doi: 10.1002/gepi.10218. [DOI] [PubMed] [Google Scholar]
- 29.Russell IAM, Zwass M, Fineman JR, Balea M, Rouine-Rapp K, Brook M, Hanley FL, Silverman NH, Cahalan MK. The effects of inhaled nitric oxide on postoperative pulmonary hypertension in infants and children undergoing surgical repair of congenital heart disease. Anesthesia Analgesia. 1998;87:46–51. doi: 10.1097/00000539-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Schulze-Neick I, Li J, Penny D, Redington A. Pulmonary vascular resistance after cardiopulmonary bypass in infants: effect on postoperative recovery. J Thorac Cardiovasc Surg. 2001;121:1033–1039. doi: 10.1067/mtc.2001.113747. [DOI] [PubMed] [Google Scholar]
- 31.Schulze-Neick I, Penny D, Rigby MML, Morgan C, Kelleher A, Collins P, Li J, Bush A, Shinebourne EA, Redington AN. L-arginine and substance P reverse the pulmonary endothelial dysfunction caused by congenital heart surgery. Circulation. 1999;100:749–755. doi: 10.1161/01.cir.100.7.749. [DOI] [PubMed] [Google Scholar]
- 32.Smith HA, Canter JA, Christian KG, Drinkwater DC, Scholl FG, Christman BW, Rice GD, Barr FE, Summar ML. Nitric oxide precursors and congenital heart surgery: a randomized controlled study of oral citrulline. J Thorac Cardiovasc Surg. 2006;132(1):58–65. doi: 10.1016/j.jtcvs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Summar ML, Hall LD, Eeds AM, Hutcheson HB, Kuo AN, Willis AS, Rubio V, Arvin MK, Schofield JP, Dawson EP. Characterization of genomic structure and polymorphisms in the human CPSI gene. Gene. 2003;311:51–57. doi: 10.1016/s0378-1119(03)00528-6. [DOI] [PubMed] [Google Scholar]
- 34.Summar ML, Hall L, Christman B, Barr F, Smith H, Kallianpur A, Brown N, Yadav M, Willis A, Eeds A, Cermak E, Summar S, Wilson A, Arvin M, Putnam A, Wills M, Cunningham G. Environmentally determined genetic expression: clinical correlates with molecular variants of carbamyl phosphate synthetase I. Mol Genet Metab. 2004;81(S1):S12–S19. doi: 10.1016/j.ymgme.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Steinhorn RH, Fineman JR. The pathophysiology of pulmonary hypertension in congenital heart disease. Artificial Organs. 1999;23:970–974. doi: 10.1046/j.1525-1594.1999.06447.x. [DOI] [PubMed] [Google Scholar]
- 36.Yahagi N, Kumon K, Tanigami H, Watanabe Y, Haruna M, Hayashi H, Imanaka H, Takeuchi M, Takamoto S. Cardiac surgery and inhaled nitric oxide: Indication and follow-up (2–4 years) Artificial Organs. 1998;22:886–891. doi: 10.1046/j.1525-1594.1998.06186.x. [DOI] [PubMed] [Google Scholar]
- 37.Zobel G, Gamillscheg A, Schwinger W, Berger J, Urlesberger B, Dacar D, Rigler B, Metzler H, Beitzke A. Inhaled nitric oxide in infants and children after open heart surgery. J of Cardiovasc Surg (Torino) 1998;39:79–86. [PubMed] [Google Scholar]


