Abstract
Receptor recycling plays a critical role in the regulation of cellular responsiveness to environmental stimuli. Agonist-promoted phosphorylation of G protein-coupled receptors has been related to their desensitization, internalization, and sequestration. Dephosphorylation of internalized G protein-coupled receptors by cytoplasmic phosphatases has been shown to be pH-dependent, and it has been postulated to be necessary for receptors to recycle to the cell surface. The internalized V2 vasopressin receptor (V2R) expressed in HEK 293 cells is an exception to this hypothesis because it does not recycle to the plasma membrane for hours after removal of the ligand. Because this receptor is phosphorylated only by G protein-coupled receptor kinases (GRKs), the relationship between recycling and GRK-mediated phosphorylation was examined. A nonphosphorylated V2R, truncated upstream of the GRK phosphorylation sites, rapidly returned to the cell surface after removal of vasopressin. Less-drastic truncations of V2R revealed the presence of multiple phosphorylation sites and suggested a key role for a serine cluster present at the C terminus. Replacement of any one of Ser-362, Ser-363, or Ser-364 with Ala allowed quantitative recycling of full-length V2R without affecting the extent of internalization. Examination of the stability of phosphate groups incorporated into the recycling S363A mutant V2Rs revealed that the recycling receptor was dephosphorylated after hormone withdrawal, whereas the wild-type V2R was not, providing molecular evidence for the hypothesis that GRK sites must be dephosphorylated prior to receptor recycling. These experiments uncovered a role for GRK phosphorylation in intracellular sorting and revealed a GRK-dependent anchoring domain that blocks V2R recycling.
The V1a and V2 vasopressin receptors (V1aR and V2R) are members of the G protein-coupled receptor family and both become phosphorylated upon activation by agonist (1, 2). For several receptors of this family phosphorylation and internalization have been shown to be a consequence of activation by ligand and seem to play a role in reducing the cellular responses to repeated exposure to hormones. Phosphorylation of G protein-coupled receptors enhances their ability to bind arrestin, which uncouples the receptors from G proteins, and helps recruit them to the clathrin-coated pits, which mediate the internalization process (3–7). Although phosphorylation and internalization of G protein-coupled receptors have been known to play a role in receptor desensitization for several years, the biochemical steps involved are not fully known and are still the subject of intense investigation.
It has previously been observed that after removal of arginine vasopressin (AVP) from the medium and from the surface receptors with an acid wash, almost all of the internalized V1aR expressed in HEK 293 cells returns to the plasma membrane very rapidly (2), similar to what had been observed with isolated hepatocytes and smooth muscle vascular cells (8, 9). The human V2R expressed in transfected cells undergoes homologous but not heterologous desensitization (10), and recently, ligand promoted desensitization was shown to coincide with its phosphorylation as seen in COS and HEK 293 cells. Phosphorylation of cellular V2R was catalyzed only by G protein-coupled receptor kinases (GRKs) and found to be sustained for at least 30 min in the continuous presence of the ligand (1). In this report we present results of experiments designed to characterize ligand promoted internalization of the V2R. We found that the receptor lost from the cell surface fails to recycle after removal of the hormone and sought a possible correlation between the recently found sustained phosphorylation and absence of receptor recycling. Measurements of ligand-promoted phosphorylation in progressively truncated V2Rs showed the presence of distinct sites at the C terminus of the V2R that are phosphorylated by GRKs. Among these, a cluster of three serines is a key element in the intracellular retention of the V2R.
MATERIALS AND METHODS
Materials.
DMEM, MEM without sodium phosphate, Dulbecco’s PBS (D-PBS), penicillin/streptomycin, 0.5% trypsin in 5 mM EDTA, and fetal bovine serum were from Life Technologies; and cell culture plastic-ware was from Costar. [3H]AVP (specific activity, 60–80 Ci/mmol; 1 Ci = 37 GBq) and [32P]H3PO4 in water (pH 5–7) were purchased from DuPont/New England Nuclear. Okadaic acid was from Research Biochemicals; AVP and all other reagents were from Sigma.
Construction of Mutant V2 Receptors.
Mutant and chimeric receptors were prepared by using a PCR-based approach (1). The resulting constructs were sequenced and cloned into the expression vector pcDNA3 for eukaryotic cell expression (Invitrogen). Stop codons introduced in the cDNA are designated as t. The boundary of the V2–V1a chimera was located at the palmitoylated cysteines, the human-V2R-contributed amino acids 1–341, and the V1aR contributed amino acids 372–424 of the rat V1aR.
Transient Expression.
HEK 293 cells were plated at a density of 8 × 106 cells per 150-mm dish and transfected the following day with 14 ml of a mixture of 100 μM chloroquine and DEAE-dextran (0.25 mg/ml) in DMEM/10% fetal bovine serum containing 9 μg of plasmid DNA. After 2 h at 37°C, the solution was removed and the cells were treated for 1 min at room temperature with 10% dimethyl sulfoxide in PBS. The cells were rinsed twice with PBS and incubated further in DMEM/10% fetal bovine serum.
Phosphorylation of V2R in Intact Cells.
Transfected cells were plated in six-well plates (1 × 106 cells per well) 24 h after transfection. After 18 h, growth medium was removed, and the cells were washed and starved for 30 min in phosphate-free MEM, followed by 2 h labeling with 0.1 mCi of [32P]orthophosphate per well. Vehicle or 100 nM AVP was added during the last 20 min of the pulse. After treatment the cells were solubilized for 1 h at 4°C in 300 μl of RIPA buffer (150 mM NaCl/50 mM Tris⋅HCl, pH 8/5 mM EDTA/1% Nonidet P-40/0.1% sodium deoxycholate/0.1% SDS) with protease inhibitors [0.1 mM phenylmethylsulfonyl fluoride/soybean trypsin inhibitor (1 μg/ml)/leupeptin (1 μg/ml)] and phosphatase inhibitors (10 mM sodium pyrophosphate/10 mM NaF/300 nM okadaic acid) with the help of sonication as described (1, 2). The extracts were incubated overnight at 4°C with affinity-purified rabbit polyclonal antibody 3 (9 μg/ml) (1), and the antigen–antibody complexes were isolated on protein A-Sepharose (1). The Sepharose beads were washed for five 4-min periods with 750 μl of RIPA buffer and retrieved by centrifugation. The proteins were eluted with 80 μl of 2× Laemmli buffer containing 10% 2-mercaptoethanol for 20 min at room temperature. After electrophoresis in SDS/10% polyacrylamide gels, radioactivity was detected by exposing the dried gels to Kodak X-Omat film at −70°C. Quantification of the 32P incorporated into proteins was performed with a Molecular Dynamics PhosphoImager.
Hormone Treatments.
Cells were treated with 100 nM AVP (or vehicle) for 20 min at 37°C to promote V2R sequestration. The hormone remaining on the cell surface was removed by two washes with PBS, two washes with 150 mM NaCl/5 mM acetic acid, and three washes with PBS, all at 4°C. Fresh DMEM/10% fetal bovine serum was added, and the cells were returned to the 37°C incubator. The receptor present on the plasma membrane was measured with binding assay at 4°C in 20 nM [3H]AVP at the indicated times. Two hours after sequestration and incubation in hormone-free medium, there was no significant increase in surface binding sites for wild-type and 369t mutant V2R, indicating that there was minimal contribution of de novo synthesis to the abundance of cell surface receptors during this period. As a consequence the effect of protein synthesis inhibitors was not tested.
Hormone Binding to Intact Cells.
Transfected HEK 293 cells were plated 24 h after transfection in polylysine-coated 24-well plates (1.5–2.5 × 105 cells per well). The next day, hormone treatments were performed followed by acid washes and neutralizing washes as described above. The cells were washed twice with ice-cold D-PBS before adding 0.5 ml of ice-cold binding mixture (D-PBS/2% BSA/25 nM [3H]AVP). After a 2-h incubation on top of crushed ice in the cold room, the binding mixture was removed by aspiration, the wells were rinsed twice with ice-cold D-PBS, and 0.5 ml of 0.1 M NaOH was added to extract bound radioactivity. After 30 min at 37°C, the fluid from each well was transferred to a scintillation vial containing 3.5 ml of Ultima-Flo M scintillation fluid (Packard) for radioassay. Nonspecific binding was determined under the same conditions in the presence of 10 μM unlabeled AVP.
RESULTS AND DISCUSSION
Ligand-promoted internalization of the V1aR expressed transiently in HEK 293 cells was followed by fast recycling of this receptor to the cell surface after removal of the hormone from the medium and from cell surface receptors with an acid wash (2). These results were similar to the recycling of the V1aR that had been observed in isolated hepatocytes and vascular smooth muscle cells (7, 8). In contrast to this rapid recycling, the human V2R expressed transiently in HEK 293 cells and internalized by a 20-min treatment with 100 nM AVP remained inside the cell after removal of the hormone. As shown in Fig. 1A, the wild-type receptor failed to return to the cell surface 2 h after removal of the hormone. Identical results were obtained after recovery periods of 6 h (data not shown). Wild-type V2R expressed transiently in COS cells and stably in HEK or Madin–Darby canine kidney cells also failed to recycle to the cell surface after ligand-induced sequestration, indicating that the phenomenon was not peculiar to the transiently transfected HEK cells (data not shown).
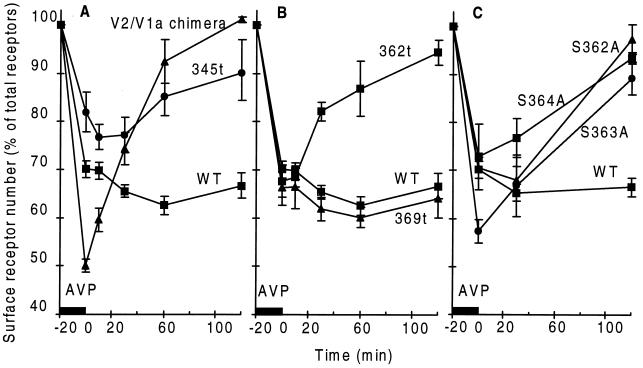
Figure 1.
Time course of receptor recycling. After AVP-promoted sequestration, the hormone was removed by acid wash and the sites on the cell surface were measured before and after incubation at 37°C. In all experiments the expression of the mutant receptors was determined by performing binding assays with a known number of intact cells. The number of receptors expressed by the different constructs as percent of wild type (WT) were 102 ± 10% for the V2–V1a chimera, 33 ± 5% for 345t, 86 ± 11% for 369t, 88 ± 5% for 362t, 102 ± 7% for S362A, 99 ± 12% for S363A, and 94 ± 8% for S362A V2R. (A) Recycling of WT (n = 16), V2–V1a chimera (n = 3), and 345t mutant (n = 6) receptors. (B) Recycling of WT (n = 16), 369t (n = 4), and 362t (n = 3) mutant receptors. (C) Recycling of WT (n = 16) and three mutant V2Rs carrying a single Ala substitution in the Ser cluster at positions 362 (n = 3), 363 (n = 3), or 364 (n = 3). Numbers in parentheses specify number of experiments performed.
For many G protein-coupled receptors, including the V2R, the C terminus has been shown to play an important role in receptor internalization rather than participating in the activation of G proteins (1, 11–13). This role has been related to the phosphorylation of this segment of the receptors by G protein-coupled receptor kinases. The segment of the V2R containing the acceptor sites for GRK phosphorylation can be removed by introducing a stop codon at position 345 (Fig. 2) to generate the truncated receptor 345t-V2R (1). The truncated V2R that was no longer phosphorylated was sequestered when exposed to high concentrations of AVP, although to a lesser extent than the full-length protein (Fig. 1A). In contrast to the full-length receptor, the 345t mutant V2R returned to the cell surface after the removal of AVP as shown in Fig. 1A.
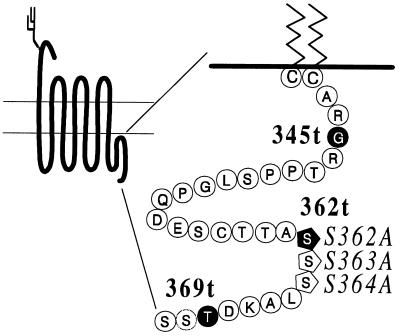
Figure 2.
Schematic representation of the V2R and amino acid composition of the C terminus. The amino acids downstream of the palmitoylation sites of the V2R, Cys-341 and Cys-342 are identified (10). The residues on a black background indicate the sites substituted by a stop codon to produce the truncated receptors in boldface type. The residues represented by pentagons indicate single amino acid substitutions that were introduced to obtain the constructs in italic type.
To examine the possibility that recycling of the 345t-V2R could have been the consequence of less efficient sequestration, a chimera was constructed in which the V2R C terminus was replaced by the corresponding portion of the V1aR starting at the palmitoylation sites identified in Fig. 2 (14). The V2–V1a chimeric receptor resembled the wild-type V2R in terms of level of expression, AVP binding affinity and coupling to the G protein Gs (data not shown and ref. 15). Like the V1aR, the chimeric receptor was internalized to a greater extent than the V2R (Fig. 1A and ref. 2), but contrary to the latter, it efficiently recycled to the plasma membrane. One hour after removal of ligand, almost 100% of the chimeric receptor had returned to the cell surface. The behavior of the chimera implied that neither the extent of internalization nor the length of the C terminus were responsible for blocking the return of the wild-type V2R to the cell surface and suggested the presence of a retention signal in the V2R terminal segment.
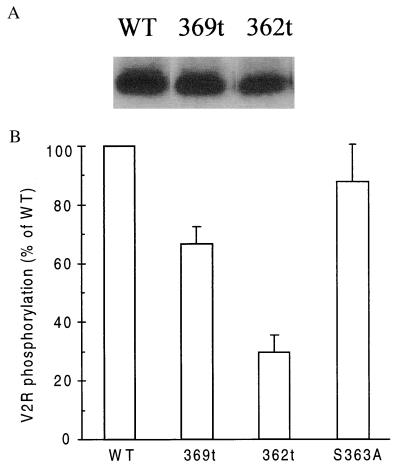
All the phosphorylation acceptor sites seem to be located within the last 15 amino acids of the V2R, and the receptor appeared to be phosphorylated only by GRKs (1). To determine whether these sites are responsible for the observed retention, the nine putative phosphate acceptor sites present in the full-length receptor were reduced to six or three sites by the insertion of stop codons at positions 369 and 362 (Fig. 2). The radioactive phosphate incorporated into these mutant receptors upon AVP stimulation was compared with the amount of phosphate incorporated into the full-length protein and quantified by a PhosphoImager. As illustrated in Fig. 3 A and B, 369t-V2R incorporated 30% less radioactive phosphate than the full-length receptor, suggesting that the two Ser residues and the Thr residue missing are acceptor sites for phosphorylation. The 362t-V2R incorporated 60% less radioactive phosphate than the full-length receptor, indicating that the additional three Ser residues missing were also phosphorylated. The reduced phosphorylation of the truncated receptors was proportional to the number of missing Ser and Thr residues, indicating that the tail of the receptor protein contains multiple phosphorylation sites, probably nine sites. The exact location of the estimated three phosphates incorporated into the 362t-V2R remains conjectural, although the two Thr residues and single Ser residue that remain at the end of the protein are the likely acceptor sites.
Figure 3.
Phosphorylation of wild-type and mutant V2Rs. The phosphate incorporated into the proteins in response to 100 nM AVP was quantified in the immunoprecipitated receptors for the WT, 369t, 362t, and S363A mutant receptors. (A) Autoradiographic image of WT, 369t, and 362t phosphorylated V2Rs in a representative experiment. (B) Summary of the quantification of receptor phosphorylation. S363A V2R incorporated 88 ± 12%, 369t incorporated 60 ± 8%, and 362t incorporated 33 ± 7% of the phosphate incorporated by WT V2R. The values for each construct are expressed as percentage (mean ± SEM, n = 4) of the phosphate incorporated into the wild-type V2R after normalization for the number of binding sites detected with each construct.
The extent of internalization of the 369t- and 362t-V2R mutant receptors was similar to the wild-type receptor as shown in Fig. 1B, indicating that the last 10 amino acids of the V2R were not involved in internalization. When the truncated proteins were analyzed in recycling experiments, they yielded different results. The 362t-V2R recycled to the cell surface after removal of the ligand, but the 369t-V2R remained inside the cell, as does the full-length receptor (Fig. 1B). These experiments revealed that the last 3 amino acids of the V2R were not implicated in the intracellular trapping of the receptor, whereas the serine cluster at positions 362, 363, and 364 seemed to be required to observe retention of the receptor inside the cells.
As mentioned above, the extent of phosphorylation of the truncated receptors indicated that the three serines present as a cluster at positions 362, 363, and 364 were phosphorylated. To examine the role of the phosphorylation sites between codons 362 and 369 in the context of the full-length protein, Ser at position 362, 363, or 364 was replaced with Ala, one at a time, in the full-length V2R (Fig. 2). Substitution of a single amino acid in any of the three positions had different impacts on the magnitude of ligand-promoted phosphorylation of the mutant receptors. Compared with the wild-type receptor, the S363A mutant V2R incorporated 88 ± 12% as much phosphate, a reduction equivalent to the loss of one phosphate acceptor site over the putative nine present in the last 14 amino acids of the receptor. The S362A and S364A mutant V2Rs suffered a more significant reduction in phosphate incorporation to 61 ± 10% and 74 ± 12% as much as the wild type, respectively. The decrease in phosphate incorporation for these mutants was greater than the loss of phosphate expected from the disappearance of a single site and suggested a hierarchy of phosphorylation acceptor sites in the V2R. Sites with a greater impact in phosphate uptake than the loss of one amino acid susceptible to phosphorylation are probably the locations at which the GRKs begin to incorporate phosphates into the protein. Despite these differences in phosphate uptake, the full-length V2Rs missing one Ser residue at position 362, 363, or 364 recycled to the cell surface as well as the 362t-V2R (Fig. 1C). This indicates that the full cluster of three Ser residues is a key element of a previously unrecognized retention domain. Phosphorylation of this domain is necessary to promote the interaction of the V2R with an unidentified intracellular component that prevents the receptor from recycling.
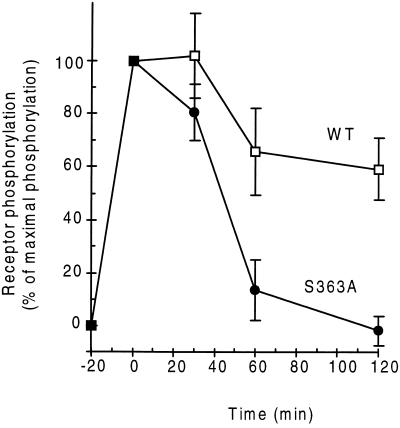
In experiments presented elsewhere, the rapidly recycling V1aR was found to lose rapidly the radioactive phosphate incorporated by GRKs (2). Because the V2R had been found to remain maximally phosphorylated for as long as 30 min in the continuous presence of AVP, the possibility that the presence of phosphate in the Ser cluster interfered with dephosphorylation was investigated. To test the hypothesis of a possible role of the Ser cluster in protein dephosphorylation the S363A mutant V2R was chosen because its high level of phosphorylation suggested that the two remaining Ser residues at the adjacent positions are phosphorylated and only the phosphate at position 363 is missing. The cellular nucleotide pools were labeled with radioactive phosphate and the receptor was activated with 100 nM AVP for 20 min to obtain a fully phosphorylated receptor. Exposure to agonist was followed by removal of the hormone with a mild acid wash and further incubation of the cells at 37°C, a protocol identical to that of the receptor recycling experiments. The intensity of the 32P signal associated with the immunoprecipitated receptors was measured at the indicated times; 100% was the phosphorylation value found after the 20-min AVP treatment. As illustrated in Fig. 4, the recycling mutant receptor S363A was almost completely dephosphorylated after 1 h of recovery, but after 2 h the amount of phosphate carried by the trapped wild-type V2R was only partially reduced. The nonrecycling 369t-V2R also retained most of its phosphate 2 h after removal of the hormone (data not shown). The slight decrease in phosphate observed in the wild type was probably due to loss of some phosphates because no significant degradation of 35S-labeled wild-type receptor was detected after 2 h in the presence or absence of AVP (data not shown).
Figure 4.
Time course of receptor dephosphorylation. After 100 nM AVP promoted phosphorylation, excess hormone was removed by acid wash, and the cells were allowed to recover. The amount of radioactive phosphate present in each receptor protein was quantified at the indicated times by immunoprecipitating receptors from detergent cell extracts. Results obtained for wild-type and S363A mutant receptors are shown. The values for each construct are expressed as the mean ± SEM of the percent phosphate incorporated in the immunoprecipitated receptor before the recovery. Number of experiments were n = 7 for −20 min (untreated) and time 0, n = 3 for 30 min, n = 4 for 60 min, and n = 5 for 120 min of recycling.
These results indicate that after activation by AVP, the resulting phosphorylation of the serine cluster in the C terminus of the V2R blocks dephosphorylation of the internalized receptor and, thus, recycling to the cell surface. The mechanism that protects this segment of the internalized receptor from the action of cytoplasmic phosphatases is under investigation. A favored hypothesis at the moment is that tight binding of a cytoplasmic component to the phosphorylated Ser cluster blocks access of protein phosphatases and prevents hydrolysis of the ester bond. These data provide molecular evidence for the hypothesis that recycling needs to be preceded by receptor dephosphorylation (16), revealed by mild mutagenesis of the V2R. Preventing complete phosphorylation of the Ser cluster made the phosphorylated V2R sensitive to phosphatases and allowed the dephosphorylated protein to recycle to the cell surface.
The data suggest the existence of two intracellular pathways for internalized G protein-coupled receptors as revealed by the V2R: internalization followed by recycling or prolonged retention (sequestration) of the receptor inside the cell. The stability of the phosphate attached to the internalized receptor and the stability of the 35S-labeled protein suggests that the receptor had not been targeted to a compartment rich in proteases, i.e., that the retention signal did not result in quick degradation. Examination of the C terminus of receptors from the rhodopsin family identified a few containing a Ser cluster that was always in a context of amino acids very different from the V2R terminal segment. Whether arrestin, clathrin, and dynamin are involved in the internalization of nonrecycling receptors remains to be defined. The existence of mechanisms of receptor internalization not requiring arrestin–clathrin complexes has been suggested recently for the m2 muscarinic receptor (17). Whether the same or an alternative pathway is used by the V2R should be examined in the future. Another conclusion from these results is that GRK phosphorylation plays a role in intracellular targeting in addition to its known effects on receptor uncoupling and internalization.
Acknowledgments
This work was supported in part by National Institutes of Health Grant DK 41-244 to M.B.
ABBREVIATIONS
- V1aR and V2R
V1a and V2 vasopressin receptors, respectively
- GRK
G protein-coupled receptor kinase
- AVP
arginine vasopressin
References
- 1.Innamorati G, Sadeghi H, Eberle A, Birnbaumer M. J Biol Chem. 1997;272:2486–2492. doi: 10.1074/jbc.272.4.2486. [DOI] [PubMed] [Google Scholar]
- 2.Innamorati, G., Sadeghi, H. & Birnbaumer, M. (1998) J. Biol. Chem. 273, in press. [DOI] [PubMed]
- 3.Goodman O B, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. Nature (London) 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 4.Goodman O B, Jr, Krupnick J G, Gurevich V V, Benovic J L, Keen J H. J Biol Chem. 1997;272:15017–15022. doi: 10.1074/jbc.272.23.15017. [DOI] [PubMed] [Google Scholar]
- 5.von Zastrow M, Kobilka B K. J Biol Chem. 1992;267:3530–3538. [PubMed] [Google Scholar]
- 6.Chuang T T, Iacovielli L, Sallese M, De Blasi A. Trends Pharmacol Sci. 1996;17:416–421. doi: 10.1016/s0165-6147(96)10048-1. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson S S G, Zhang J, Barak L S, Caron M G. News Physiol Sci. 1997;12:145–151. [Google Scholar]
- 8.Fishman J B, Dickey B F, Bucher N L, Fine R E. J Biol Chem. 1985;260:12641–12646. [PubMed] [Google Scholar]
- 9.Briner V A, Williams B, Tsai P, Schrier R W. Proc Natl Acad Sci USA. 1992;89:2854–2858. doi: 10.1073/pnas.89.7.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaumer M, Antaramian A, Themmen A P N, Gilbert S. J Biol Chem. 1992;267:11783–11788. [PubMed] [Google Scholar]
- 11.Lattion A, Diviani D, Cotecchia S. J Biol Chem. 1994;269:22887–22893. [PubMed] [Google Scholar]
- 12.Ashworth R, Yu R, Nelson E J, Dermer S, Gershengorn M C, Hinkle P M. Proc Natl Acad Sci USA. 1995;92:512–516. doi: 10.1073/pnas.92.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouvier M, Hausdorff W P, De Blasi A, O’Dowd B F, Kobilka B K, Caron M G, Lefkowitz R J. Nature (London) 1988;333:370–373. doi: 10.1038/333370a0. [DOI] [PubMed] [Google Scholar]
- 14.Sadeghi H, Innamorati G, Dagarag M, Birnbaumer M. Mol Pharmacol. 1997;52:21–29. doi: 10.1124/mol.52.1.21. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Wess J. J Biol Chem. 1996;271:8772–8778. doi: 10.1074/jbc.271.15.8772. [DOI] [PubMed] [Google Scholar]
- 16.Krueger K M, Daaka Y, Pitcher J A, Lefkowitz R J. J Biol Chem. 1997;272:5–8. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Pals-Rylaarsdam R, Gurevich V V, Lee K B, Ptasienski J A, Benovic J L, Hosey M M. J Biol Chem. 1997;272:23682–23689. doi: 10.1074/jbc.272.38.23682. [DOI] [PubMed] [Google Scholar]