Abstract
Induction of terminal differentiation represents a promising therapeutic approach to certain human malignancies. The peroxisome proliferator-activated receptor γ (PPARγ) and the retinoid X receptor α (RXRα) form a heterodimeric complex that functions as a central regulator of adipocyte differentiation. Natural and synthetic ligands for both receptors have been identified. We demonstrate here that PPARγ is expressed at high levels in each of the major histologic types of human liposarcoma. Moreover, primary human liposarcoma cells can be induced to undergo terminal differentiation by treatment with the PPARγ ligand pioglitazone, suggesting that the differentiation block in these cells can be overcome by maximal activation of the PPAR pathway. We further demonstrate that RXR-specific ligands are also potent adipogenic agents in cells expressing the PPARγ/RXRα heterodimer, and that simultaneous treatment of liposarcoma cells with both PPARγ- and RXR-specific ligands results in an additive stimulation of differentiation. Liposarcoma cell differentiation is characterized by accumulation of intracellular lipid, induction of adipocyte-specific genes, and withdrawal from the cell cycle. These results suggest that PPARγ ligands such as thiazolidinediones and RXR-specific retinoids may be useful therapeutic agents for the treatment of liposarcoma.
Liposarcoma is the most common soft tissue malignancy in adults, accounting for at least 20% of all sarcomas in this age group (1). Multiple histologic subtypes of liposarcoma are recognized, including well differentiated, dedifferentiated, myxoid, round cell, and pleomorphic. The histologic subtype is predictive of both the clinical course of the disease and the ultimate prognosis (2). Localized disease is treated primarily with surgery, often in combination with radiotherapy. Metastatic liposarcoma is associated with an extremely poor prognosis, with average 5-year survivals ranging from 70% to 25% depending on the type of tumor. Conventional chemotherapy for metastatic liposarcoma leads to complete response in only about 10% of cases, and thus for most patients is largely palliative (3, 4).
Induction of terminal differentiation represents a promising alternative to conventional chemotherapy for certain malignancies. For example, the retinoic acid receptor α, which plays an important role in the differentiation and malignant transformation of cells of the myelocytic lineage, has been used as a target for intervention in acute promyelocytic leukemia (5, 6). Differentiation therapy with all-trans retinoic acid has become the standard of care for this disease. Nuclear receptors that regulate growth and differentiation of other cell types may also represent potential targets for differentiation therapy.
The nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) plays a central role in the process of adipocyte differentiation. This receptor and its heterodimeric partner retinoid X receptor α (RXRα) form a DNA-binding complex that regulates transcription of adipocyte-specific genes (7–11). Expression and activation of PPARγ in fibroblastic cells triggers the adipocyte gene expression cascade and leads to the development of the adipose phenotype (12). The thiazolidinedione class of antidiabetic drugs and the nuclear prostanoid 15-deoxy-Δ12,14-prostaglandin J2 have recently been identified as ligands for PPARγ (13–15). Members of the C/EBP family of transcription factors have also been shown to promote adipocyte differentiation (16, 17), and recent evidence suggests that the adipogenic activity of some C/EBP family members is at least in part related to their ability to induce PPARγ expression (18).
All-trans retinoic acid is known to be an effective inhibitor of adipocyte differentiation (19, 20). Experiments using receptor-specific agonists have indicated that this effect is mediated primarily by the retinoic acid receptor α. The PPARγ partner RXRα, which binds 9-cis retinoic acid but not all-trans retinoic acid, responds to a distinct retinoid signaling pathway (21, 22). While studies have suggested that the transcriptional activity of the PPAR/RXR heterodimer is maximal in the presence of both PPAR and RXR activators (7, 8), it is not known how the binding of an RXR ligand modulates the adipogenic activity of the PPARγ/RXRα complex.
We report here that PPARγ is expressed consistently in each of the major histologic types of human liposarcoma, and that primary human liposarcoma cells can be induced to undergo terminal differentiation in vitro by treatment with thiazolidinediones and RXR-specific retinoids. Our results suggest that these compounds may be useful as differentiation therapy for liposarcoma.
MATERIALS AND METHODS
Tissue Samples and Cytogenetics.
Normal human tissues, liposarcomas, and other soft tissue sarcomas were obtained from surgical cases at the Brigham and Women’s Hospital (Boston). All tissue samples were taken from homogeneous and viable portions of the resected sample by the pathologist and frozen within 10 min of excision. Hematoxylin- and eosin-stained sections of each soft tissue sarcoma were reviewed by a single pathologist (C.D.M.F.) and classified according to histologic type and grade. Histologic classification was based solely on morphologic pattern recognition using conventional diagnostic criteria. For cytogenetic analysis tumors were disaggregated with collagenase and harvested after 3–7 days of culture in T25 flasks (23). Metaphase cells were analyzed by trypsin–Giemsa (24) and quinacrine mustard banding (25).
Cell Culture and RNA Analysis.
Primary liposarcoma cells were isolated from selected freshly harvested tumors as described (refs. 3 and 25, and references therein). Primary cells were cultured in 60-mm dishes in RPMI 1640 medium containing 15% Cosmic Calf Serum (HyClone), 20 μg/ml bovine pituitary extract (Collaborative Research), and 5 μg/ml insulin. Pioglitazone (Upjohn), troglitazone (Parke–Davis/Warner–Lambert), BRL49653 (Biomol, Plymouth Meeting, PA), and LG268 (Ligand Pharmaceuticals, La Jolla, CA) were dissolved in dimethyl sulfoxide and applied to cells in less than 0.1% of the media volume. The NIH PPARγ and NIH vector cells were derived by retroviral infection and cultured as described (12). Differentiated cells were fixed and stained for neutral lipid with oil red O (26). BrdUrd labeling was performed using the 5-bromo-2′-deoxyuridine Labeling and Detection Kit II (Boehringer Mannheim), according to the manufacturers’ instructions. RNA isolation and Northern blot analysis was carried out as described (8).
RESULTS
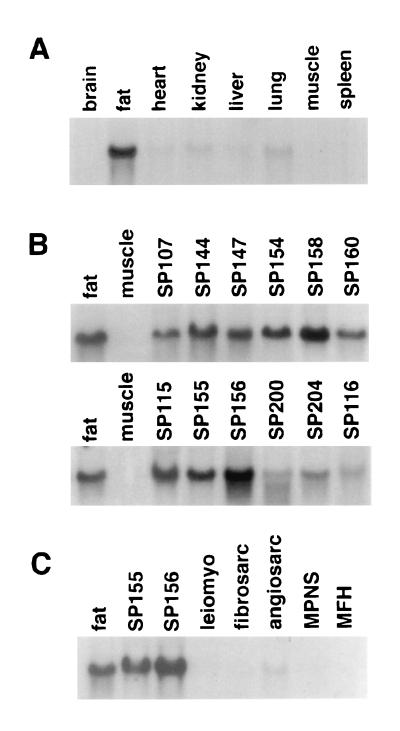
PPARγ is expressed at high levels in the adipose tissues of mouse and rat (8, 27). To determine the tissue distribution of this receptor in humans, we performed Northern blot analysis of RNA prepared from a variety of human tissues. As shown in Fig. 1A, human PPARγ is expressed at predominantly in adipose tissue and at lower levels in several other tissues.
Figure 1.
Expression of PPARγ mRNA in human tissues (A), human liposarcomas (B), and other soft tissue sarcomas (C). Total RNA (15 μg per lane) was isolated from human tumors, electrophoresed through formaldehyde-containing agarose gels, blotted to nylon, and hybridized with 32P-labeled hPPARγ cDNA. Equivalent amounts of intact RNA was run in each lane as indicated by hybridization to a 36B4 cDNA probe (not shown). MPNS, malignant peripheral nerve sheath tumor; MFH, malignant fibrous histiocytoma.
Tumorigenesis frequently involves the inactivation or down-regulation of genes responsible for initiating and maintaining a differentiated phenotype. As PPARγ appears to play a central role in the adipocyte differentiation process, we examined the expression of this receptor in a series of human liposarcomas. This series included RNA prepared from each of the three major histologic subtypes of liposarcoma: well differentiated/dedifferentiated, myxoid/round cell, and pleomorphic. The histologic and cytogenetic characteristics of each tumor are given in Table 1. For the most part, the well differentiated/dedifferentiated tumors exhibited ring chromosomes and giant marker chromosomes, the myxoid/round cell liposarcomas exhibited the characteristic t(12;16)(q13p11) translocation, and the pleomorphic forms exhibited complex rearrangements (28–30). Surprisingly, despite their block in differentiation, each liposarcoma examined was found to express significant levels of PPARγ RNA, comparable to that of normal fat (Fig. 1B). These results suggest that most if not all liposarcomas have been transformed at a point in the differentiation process after induction of PPARγ expression. In contrast, PPARγ RNA was not expressed at significant levels in any other type of soft tissue sarcoma examined including leiomyosarcoma (n = 4), fibrosarcoma (n = 1), angiosarcoma (n = 1), malignant peripheral nerve sheath tumor (n = 1), or malignant fibrous histiocytoma (n = 1) (Fig. 1C). Thus, PPARγ may be a sensitive marker for distinguishing liposarcoma from other histologic types of soft tissue sarcoma.
Table 1.
Histologic and cytogenetic characteristics of human liposarcomas
| Tumor | Histology | Cytogenetics | Cell culture |
|---|---|---|---|
| 107SP | Well differentiated | 47-48,XX,+1-2mars | NA |
| 115SP | High grade myxoid/ | 80-91,XXXX,t(12; | NA |
| round cell | 16)(q13;p11)x2 | ||
| 116SP | High grade, pleomorphic, | ND | NA |
| and myxoid areas | |||
| 200SP | High grade, mixed | ND | NA |
| pleomorphic/round cell | |||
| 203SP | Well differentiated | 48-50,XY,del(16) (q36),+2-4 r | LS175 |
| 204SP | Well differentiated | 48,XX,add(7)(q36), | LS857 |
| del(11)(p13),+2 mars | |||
| P144 | Well differentiated | 48,XX,+2r | NA |
| P147 | Well differentiated, lipoma-like, sclerosing | 46-49,XX, add(9)(q34),+1-2r, +1-2 mars | NA |
| P154 | Atypical lipoma/well | ND | NA |
| differentiated liposarc | |||
| P155 | Intermediate grade, myxoid > round cell | 46,XY,t(12;16)(q13; p11) | LS707 |
| P156 | Intermediate grade, | 49XY,+del(1)(p32), | NA |
| round cell > myxoid | +2,+8,t(12;16) (q13;p11) | ||
| P158 | Well differentiated | ND | NA |
| P160 | Well differentiated with dedifferentiated areas | 43-49,XX,add (1)(q43),−11, −13,−13,+1-3 r | NA |
ND, not determined; NA, not available
The thiazolidinedione antidiabetic drugs have recently been identified as ligand activators of murine PPARγ (13, 15). In data not shown, we have found that the thiazolidinediones are also effective activators of human PPARγ, and their relative potency parallels their potency as insulin sensitizing agents in vivo: BRL49653 > troglitazone > pioglitazone. These results are consistent with the hypothesis that PPARγ mediates the antidiabetic action of these compounds in humans.
Liposarcomas have presumably acquired one or more genetic defects that interfere with the course of normal adipocyte development. The observation that PPARγ is expressed consistently in these tumors raised the possibility that the malignant cells might be forced to complete the differentiation program by maximally activating the PPARγ pathway with thiazolidinedione ligand. To address this possibility, primary cells isolated from three human liposarcomas were cultured in vitro. Primary cell strains LS857 and LS175 were derived from well differentiated liposarcomas and LS707 was derived from an intermediate grade myxoid/round cell liposarcoma (Table 1). High grade pleomorphic liposarcoma cells could not be expanded to sufficient numbers to permit studies of differentiation. Cytogenetic analysis confirmed that the karyotype of the cells in each culture was characteristic of the parent liposarcoma.
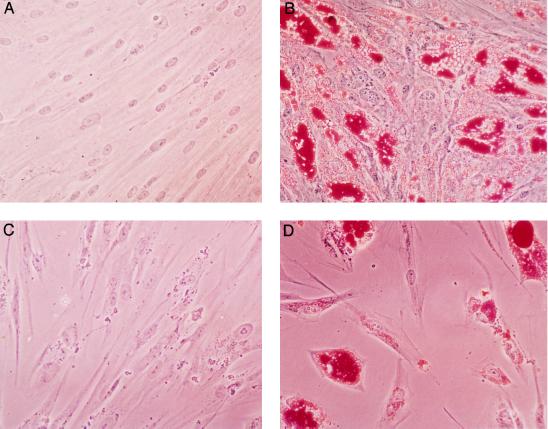
When cultured in the presence of fetal bovine serum and insulin, conditions permissive for adipocyte differentiation, all three cell strains maintain a fibroblastic morphology. When cultures were treated for 7 days with 10 μM of the PPARγ ligand pioglitazone, the cells readily accumulated lipid and adopted a morphology characteristic of mature cultured adipocytes (Fig. 2 and data not shown). The degree of morphologically recognizable differentiation varied from 40% in the LS857 cells to 75% in the LS175 cells. After induction for 7 days with thiazolidinedione, cells maintained their differentiated morphology even when pioglitazone was withdrawn. This experiment was performed at least twice with each cell strain with similar results. No differentiation was observed with cultured primary leiomyosarcoma cells, which do not express PPARγ (not shown).
Figure 2.
Pioglitazone induces differentiation of cultured human liposarcoma cells. Primary LS857 (A and B) and LS707 (C and D) cells were isolated and cultured in 60-mm dishes. At confluence, cells were cultured for 7 days in the presence (B and D) or absence (A and C) of 10 μM pioglitazone. After an additional 4 days of culture, cells were fixed and stained with oil red O. (×40.)
Previous work has suggested that maximal transcriptional activity of the PPAR/RXR heterodimer is achieved when both receptors are bound by their respective ligands (7, 8, 27). We hypothesized that simultaneous exposure of competent cells to both PPARγ and RXR-specific ligands might provide a stronger adipogenic signal than a PPARγ ligand alone. The ability of the RXR-specific ligand LG268 to promote adipocyte differentiation was investigated using NIH 3T3 fibroblasts that express PPARγ from a retroviral vector (12). We have previously shown that wild-type NIH 3T3 cells express RXRα but not PPARγ. As shown in Table 2, treatment of confluent NIH PPARγ cells for 7 days with 50 nM LG268 resulted in significant stimulation of adipocyte differentiation, comparable to that seen with 7 days of treatment with 1 μM pioglitazone alone. Simultaneous exposure to both activators resulted in an additive effect. LG268 had no effect on NIH vector cells, indicating that the adipogenic activity of this compound, like that of pioglitazone, is dependent on the presence of PPARγ. Similar results were obtained with the preadipocyte cell lines 3T3-L1 and 3T3-F442A, which express both PPARγ and RXRα (data not shown). Northern blot analysis confirmed that pioglitazone and LG268 had an additive effect on the induction of the adipocyte-specific genes aP2 and adipsin in NIH PPARγ cells (Fig. 3).
Table 2.
Thiazolidinedione and RXR-specific retinoids stimulate differentiation of PPARγ-expressing fibroblasts
| Cell line | % lipid containing cells
|
|||
|---|---|---|---|---|
| No activator | +PIO | +LG268 | +PIO+LG268 | |
| NIH vector | 0 | 0 | 0 | <1 |
| NIH PPARγ | 2-5 | 55-75 | 50-65 | >90 |
NIH vector and NIH PPARγ cells were treated at confluence for 7 days with no activator, 1 μM pioglitazone alone, 50 nM LG268 alone, or 5 μM thiazolidinedione and 50 nM LG268 as indicated. After an additional 4 days of culture cells were fixed and stained with oil red O. Data are presented as the range of morphologically recognizable differentiation observed for each cell line over three separate experiments.
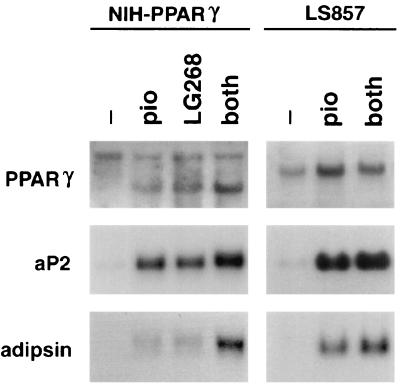
Figure 3.
PPARγ- and RXR-specific ligands induce expression of markers of terminal adipocyte differentiation in PPARγ-expressing fibroblasts and human liposarcoma cells. NIH PPARγ and LS857 cells were treated for 7 days with no activator, pioglitazone alone, LG268 alone, or pioglitazone and LG268 as indicated (both). Total RNA (10 μg per lane) was blotted to nylon and hybridized with 32P-labeled human or murine PPARγ, aP2, and adipsin cDNA. Equivalent amounts of intact RNA were run in each lane as indicated by hybridization to a 36B4 cDNA probe (not shown). Two PPARγ transcripts are present in NIH PPARγ cells, the viral transcript (Upper) and the endogenous transcript (Lower).
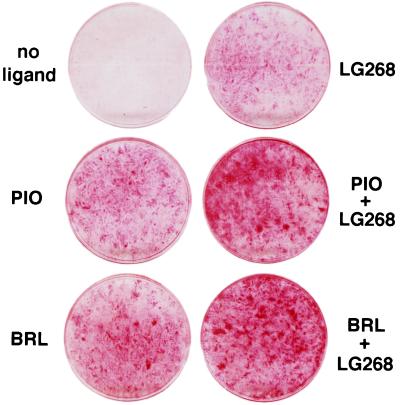
We next examined the ability of LG268 to promote differentiation of human liposarcoma cells. As shown in Fig. 4, treatment of LS857 cells for 7 days with 100 nM LG268 led to a significant degree of adipocyte differentiation, similar to that seen with 10 μM pioglitazone alone. When LS857 cells were treated simultaneously with LG268 and either pioglitazone or BRL49653, an additive effect on differentiation was observed. Expression of adipocyte-specific markers in these cells was confirmed by Northern blotting (Fig. 3). LS857 cells, like the tumor from which they were derived, express PPARγ mRNA (c.f., Fig. 1B, tumor 204SP). Treatment of LS857 cells with pioglitazone leads to the induction of the mRNAs encoding aP2 and adipsin (Fig. 3). Simultaneous treatment with pioglitazone and LG268 results in an additive effect. In summary, treatment of LS857 cells with thiazolidinediones and RXR-specific retinoids leads to changes in morphology and gene expression consistent with terminal adipocyte differentiation.
Figure 4.
Induction of differentiation in human liposarcoma cells by thiazolidinediones and RXR-specific retinoids. LS857 cells were treated at confluence for 7 days with no activator, 5 μM thiazolidinedione (BRL 49653 or pioglitazone), 50 nM LG268, or both as indicated. After an additional 4 days of culture cells were fixed and stained with oil red O. Macroscopic view of the 60-mM culture dishes is shown.
Terminal differentiation of white adipocytes in vitro and in vivo is characterized by permanent withdrawal from the cell cycle. A critical question is whether thiazolidinedione-induced differentiation of liposarcoma cells is accompanied by growth arrest. To address this issue, LS857 cells were cultured in the presence or absence of pioglitazone. Following induction of morphologic differentiation, pioglitazone was withdrawn. After 48 hr of continued culture in the absence of pioglitazone, cells were labeled for 48 hr with BrdUrd. In the two experiments presented in Table 3, 26–34% of the cells contained visible cytoplasmic lipid, and 40–51% of the cells in this culture stained positive for BrdUrd incorporation by light microscopy. However, of those cells containing lipid, only 3–4% stain positive for BrdUrd. These results demonstrate that thiazolidinedione-induced differentiation of LS857 cells leads to cell cycle withdrawal.
Table 3.
Differentiation of LS857 cells by thiazolidinediones and RXR-specific retinoids leads to cell cycle withdrawal
| Control | PIO/268 1 | PIO/268 2 | |
|---|---|---|---|
| Cells counted | 500 | 510 | 595 |
| BrdUrd + (%) | 232 (46) | 173 (34) | 156 (26) |
| Lipid + (%) | 0 | 204 (40) | 233 (51) |
| BrdUrd + lipid + (%) | NA | 22 (4) | 17 (3) |
LS857 cells were cultured in the presence or absence of pioglitazone. Following induction of morphologic differentiation, pioglitazone was withdrawn. After 48 hr of continued culture in the absence of pioglitazone, cells were labeled for 48 hr with BrdUrd, stained, and visualized microscopically. NA, not available.
DISCUSSION
Terminal differentiation of most specialized cell types, including white adipocytes, is linked to cell cycle withdrawal. Tumorigenesis is characterized by a loss of cell cycle control and a concordant block in the differentiation program. We have demonstrated here that most human liposarcomas express high levels of the adipocyte regulatory complex PPARγ/RXRα and that PPARγ- and RXRα-specific ligands are able to trigger terminal differentiation of primary human liposarcoma cells in vitro. These results suggest that the developmental defect in most liposarcomas is downstream of PPARγ expression, and that in at least some tumor cells this developmental block can be overcome by maximal activation of the PPARγ pathway.
While the precise nature of the developmental defects in liposarcoma is not yet clear, it is likely these defects ultimately lead to the inactivation or antagonism of one or more adipocyte transcriptional regulatory proteins. Members of both the C/EBP and PPAR transcription factor families have been shown to play central and complementary roles in adipogenesis in murine models (16, 18, 31–33). Interestingly, the C/EBP family has previously been implicated in the pathogenesis of human myxoid liposarcoma through the characterization of the t(12;16) translocation associated with this tumor. This rearrangement fuses the gene for the C/EBP family member CHOP on chromosome 12 to that of the RNA-binding protein TLS on chromosome 16 (34). The precise mechanism whereby TLS/CHOP contributes to differentiation arrest and tumorigenesis, however, remains to be elucidated.
The impact of RXR-specific activators on adipocyte differentiation has not been addressed previously. We have demonstrated that RXR-specific retinoids can function as adipogenic regulators through activation of the PPARγ/RXRα heterodimer, and that the adipogenic activity of the heterodimer is maximal when both receptors are bound by their respective ligands. Given that PPARγ is likely to be the biologic receptor mediating the insulin-sensitizing effects of the thiazolidinediones, this observation suggests that RXR-specific ligands may also have insulin-sensitizing activity in vivo. Moreover, the insulin-sensitizing effects of thiazolidinedione ligands for PPARγ might be enhanced by simultaneous administration of an RXR-specific ligand.
This study has important implications for the pharmacologic management of liposarcoma in humans. Despite conventional multimodality therapy, 25% to 75% of patients with advanced liposarcoma will die from their disease within 5 years. Our results suggest that the thiazolidinedione class of antidiabetic drugs and RXR-specific retinoids may be useful as a nontoxic alternative to conventional chemotherapy for the treatment of disseminated or locally advanced liposarcoma. Members of the thiazolidinedione class of drugs have undergone extensive preclinical testing as antidiabetic agents. Troglitazone is currently in phase three clinical trials in the United States, and studies have supported its usefulness in noninsulin-dependent diabetes mellitus (31). Although certain thiazolidinediones have been associated with some degree of toxicity in long-term use as insulin sensitizing agents, this should not preclude their use as antineoplastic agents because conventional chemotherapy is associated with far greater toxicity. The ability of thiazolidinediones and RXR-specific retinoids to induce differentiation of liposarcoma cells in vitro strongly suggests that these compounds may also be able to stimulate differentiation and growth arrest of human tumors in vivo. Finally, although expression of PPARγ is most prominent in fat, it is known to be expressed at some level in select other cell types such as liver and hematopoietic cells (8, 36–38). If PPARγ also plays a role in the regulation of growth and differentiation of these tissues, then this receptor may represent a target for therapeutic intervention in other human malignancies as well.
Acknowledgments
We thank Myles Brown for discussions and advice throughout the course of this work. We are grateful to Rolf Kletzien (Upjohn) for pioglitazone, Alan Saltiel (Parke–Davis/Warner–Lambert) for troglitazone, Richard Heyman (Ligand Pharmaceuticals) for LG268, and V. K. K. Chatterjee for the hPPARγ clone. This work was supported by grants from the National Institutes of Health (DK-31405, to B.M.S.), the American Cancer Society (EDT-87, to S.S.), National Research Service Awards T32 GM07753 (to P.T.) and DK-09090 (to R.P.B.), and the Tobacco-Related Disease Research Program (to B.M.F.) and Associazione Italiana per la Ricerca sul Cancro (Italy) (to E.M.). R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute for Biological Studies.
Footnotes
Abbreviations: PPARγ, peroxisome proliferator-activated receptor γ; RXRα, retinoid X receptor α.
References
- 1.Mack T M. Cancer. 1995;75:211–244. doi: 10.1002/1097-0142(19950101)75:1+<211::aid-cncr2820751309>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Chang H R, Hajdu S I, Collin C, Brennan M P. Cancer. 1989;64:1514–1520. doi: 10.1002/1097-0142(19891001)64:7<1514::aid-cncr2820640726>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Sreekantaiah C, Ladanyi M, Rodriguez E, Chaganti R S K. Am J Pathol. 1994;144:1121–1134. [PMC free article] [PubMed] [Google Scholar]
- 4.Patel S R, Burgess M A, Plager C, Papadopoulos N E, Linke K A, Benjamin R S. Cancer. 1994;74:1265–1269. doi: 10.1002/1097-0142(19940815)74:4<1265::aid-cncr2820740414>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Warrell R P, Jr, Frankel S R, Miller W H, Jr, Scheinberg D A, Itri L M, Hittleman W N, Vyas R, Andreef M, Tafuri A, Jakubowski A, Gabrilove J, Gordon M S, Dmitrovsky E. N Engl J Med. 1991;324:1385–1393. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- 6.Warrell R P, Jr, de The H, Wang Z-Y, Degos L. N Engl J Med. 1993;329:177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 7.Kliewer S A, Umesono K, Noonan D, Heyman R, Evans R M. Nature (London) 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tontonoz P, Hu E, Graves R A, Budavari A I, Spiegelman B M. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 9.Tontonoz P, Graves R A, Budavari A I, Erdjument-Bromage H, Lui M, Hu E, Tempst P, Spiegelman B M. Nucleic Acids Res. 1994;22:5628–5634. doi: 10.1093/nar/22.25.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tontonoz P, Hu E, Devine J, Beale E G, Spiegelman B M. Mol Cell Biol. 1995;15:351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sears I B, MacGinnitie M A, Kovacs L G, Graves R A. Mol Cell Biol. 1996;16:3410–3418. doi: 10.1128/mcb.16.7.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tontonoz P, Hu E, Spiegelman B M. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 13.Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 14.Kliewer S A, Lenhard J M, Wilson T M, Patel I, Morris D C, Lehmann J M. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann J M, Moore L B, Smith-Oliver T A, Wilkison W O, Wilson T M, Kliewer S A. J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 16.Freytag S O, Paielli D L, Gilbert J D. Genes Dev. 1994;8:1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- 17.Lin F, Lane D M. Proc Natl Acad Sci USA. 1994;91:8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, Xie Y, Bucher N L R, Farmer S R. Genes Dev. 1995;9:2350–2363. doi: 10.1101/gad.9.19.2350. [DOI] [PubMed] [Google Scholar]
- 19.Kamei Y, Kawada T, Mizukami J, Sugimoto E. Life Sci. 1994;55:307–312. doi: 10.1016/0024-3205(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 20.Xue J C, Schwarz E J, Chawla A, Lazar M A. Mol Cell Biol. 1996;16:1567–1575. doi: 10.1128/mcb.16.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin A A, Sturzenbecker L J, Kazmer S, Bosakowski T, Huselton C, Allenby G, Speck J, Kratzeisen C, Rosenberger M, Lovey A. Ann NY Acad Sci. 1992;669:70–86. doi: 10.1111/j.1749-6632.1992.tb17090.x. [DOI] [PubMed] [Google Scholar]
- 22.Heyman R A, Mangelsdorf D J, Dyck J A, Stein R B, Eichele G, Evans R M, Thaller C. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher J A, Kozakewich H P, Hoffer F A, Lage J M, Weidner N, Tepper R, Pinkus G S, Morton C C, Corson J M. N Engl J Med. 1991;324:436–443. doi: 10.1056/NEJM199102143240702. [DOI] [PubMed] [Google Scholar]
- 24.Seabright M A. Lancet. 1971;ii:971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher J A, Gibas Z, Donovan K, Perez-Atayde A, Genest D, Morton C C. Am J Pathol. 1991;138:515–520. [PMC free article] [PubMed] [Google Scholar]
- 26.Green H, Kehinde O. Cell. 1974;1:113–116. [Google Scholar]
- 27.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher C D M, Akerman M, Cin P D, de Wever I, Mandahl N, Mertens F, Mitelman F, Rosai J, Rydholm A, Sclot R, Tallini G, van den Berghe H, van de Ven W, Vanni R, Willen H. Am J Pathol. 1996;148:623–630. [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher J A, Kozakewich H P, Hoffer F A, Lage J M, Weidner N, Tepper R, Pinkus G S, Morton C C, Corson J M. N Engl J Med. 1991;324:436–442. doi: 10.1056/NEJM199102143240702. [DOI] [PubMed] [Google Scholar]
- 30.Knight J C, Renwick P J, Cin P D, Van den Berghe H, Fletcher C D. Cancer Res. 1995;55:24–27. [PubMed] [Google Scholar]
- 31.Cornelius P, MacDougald O A, Lane M D. Annu Rev Nutr. 1994;14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 32.Tontonoz P, Hu E, Spiegelman B M. Curr Opin Genet Dev. 1995;5:571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 33.Yeh W-C, Cao Z, Classon M, McKnight S L. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 34.Crozat A, Aman P, Mandahl N, Ron D. Nature (London) 1993;363:640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- 35.Nolan J J, Ludvik B, Beardson P, Joyce M, Olefsky J. N Engl J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 36.Kliewer S A, Forman B M, Blumberg B, Ong E S, Borgmeyer U, Mangelsdorf D J, Umesono K, Evans R M. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene M E, Blumberg B, McBride O W, Yi H F, Kronquist K, Kwan K, Hsieh L, Greene G, Nimer S D. Gene Exp. 1995;4:281–299. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Alvares K, Huang Q, Rao M S, Reddy J K. J Biol Chem. 1993;268:26817–26820. [PubMed] [Google Scholar]