Abstract
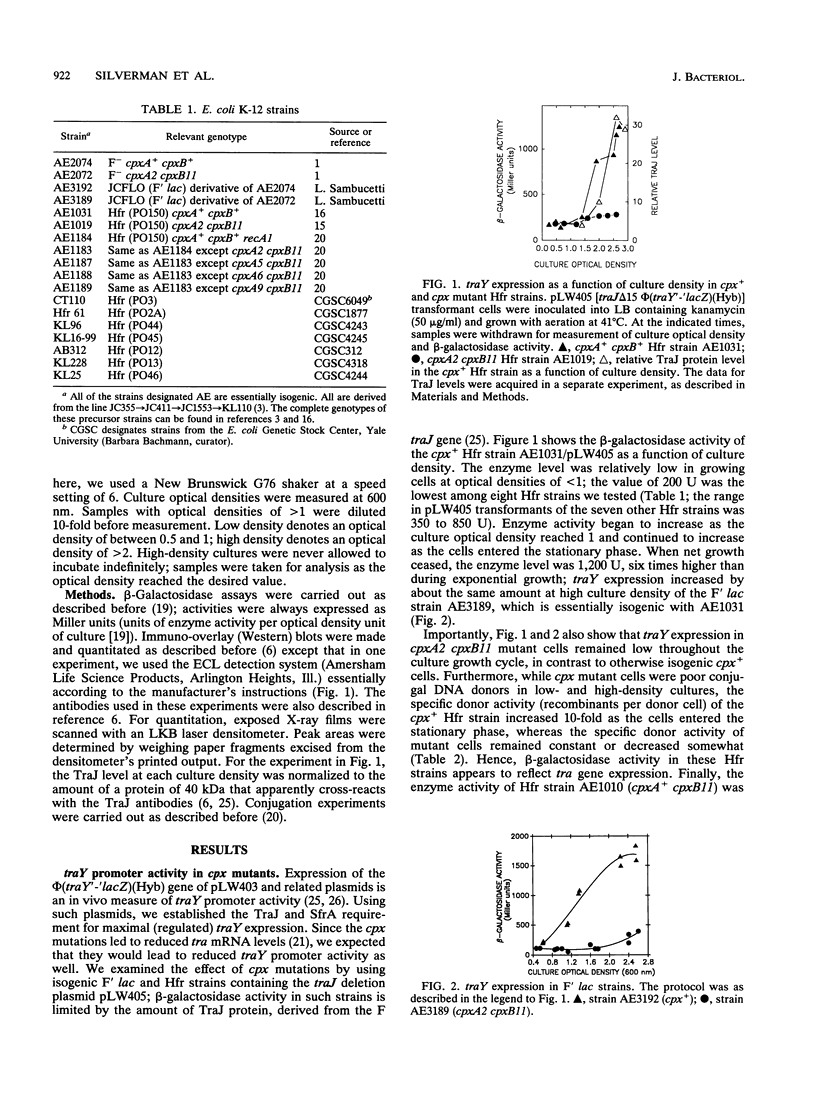
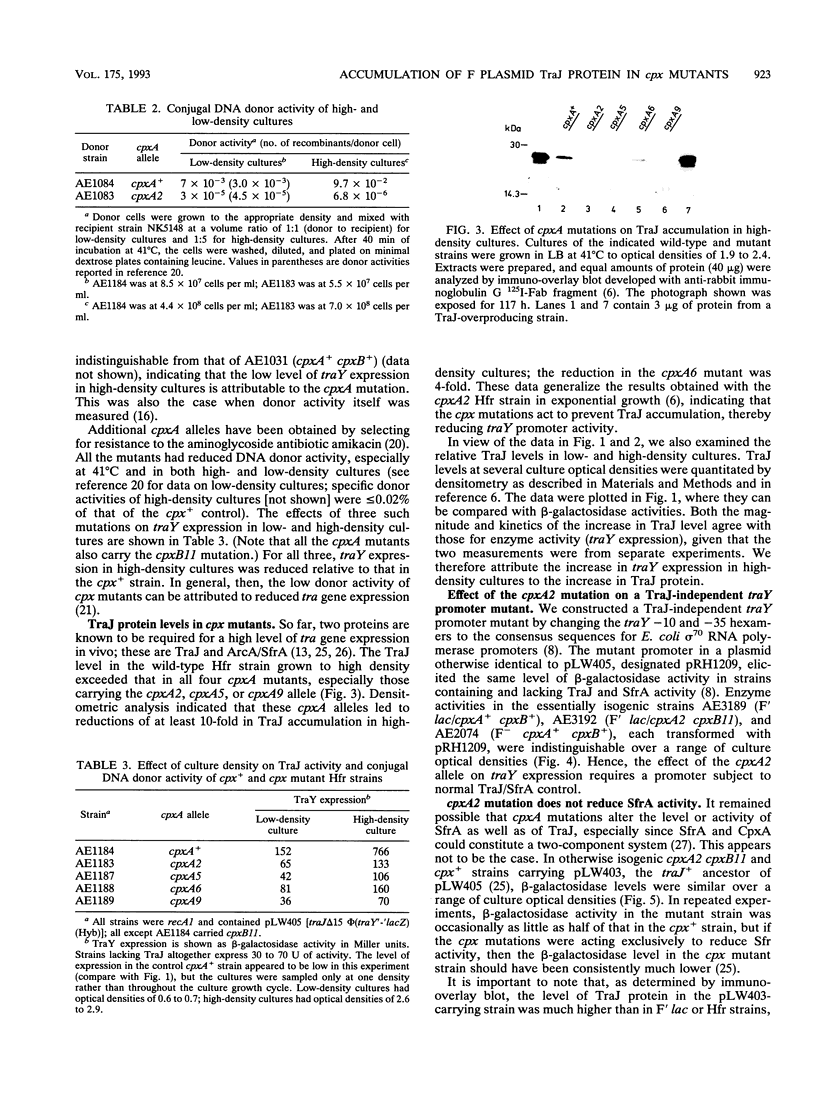
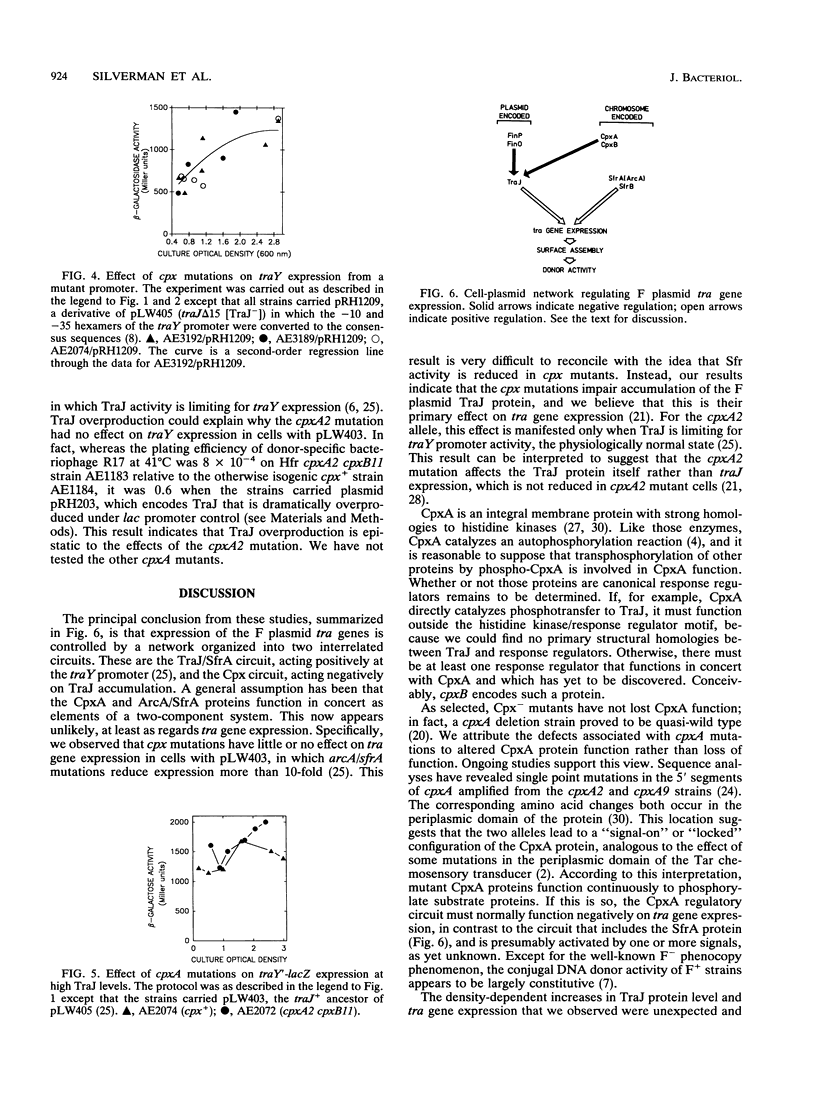
We report here studies of the cellular control of F plasmid TraJ protein levels, focusing on the effects of chromosomal cpx mutations. The principal conclusion from our results is that the cpx mutations impair accumulation of the TraJ protein, thereby reducing tra gene expression. We measured TraJ activity in vivo by expression of a traY'-'lacZ fusion gene and TraJ protein by immuno-overlay blot. In strains with normal TraJ levels, traY expression and donor-related functions were reduced in cells carrying any of four cpxA mutations. In the strain background used to isolate cpx mutants, these reductions were especially evident in cells grown to high density, when traY expression and donor activity both increased in cpx+ cells. In each of the four cpxA mutants tested, TraJ levels were lower than in the otherwise isogenic cpxA+ strain. In cells grown to high density, the differences ranged from 4-fold in the cpxA6 strain to > 10-fold in the cpxA2, cpxA5, and cpxA9 strains. The cpxA2 mutation had little or no effect on traY expression or on donor-related functions when TraJ was present in excess of its limiting level in F' or Hfr cells or on a mutant traY promoter whose expression in vivo was independent of TraJ.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin R., Silverman P. M. Physical and genetic structure of the glpK-cpxA interval of the Escherichia coli K-12 chromosome. Mol Gen Genet. 1984;197(2):261–271. doi: 10.1007/BF00330972. [DOI] [PubMed] [Google Scholar]
- Ames P., Parkinson J. S. Transmembrane signaling by bacterial chemoreceptors: E. coli transducers with locked signal output. Cell. 1988 Dec 2;55(5):817–826. doi: 10.1016/0092-8674(88)90137-7. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broda P. Transience of the donor state in an Escherichia coli K12 strain carrying a repressed R factor. Mol Gen Genet. 1975;138(1):65–69. doi: 10.1007/BF00268828. [DOI] [PubMed] [Google Scholar]
- Cuozzo M., Silverman P. M. Characterization of the F plasmid TraJ protein synthesized in F' and Hfr strains of Escherichia coli K-12. J Biol Chem. 1986 Apr 15;261(11):5175–5179. [PubMed] [Google Scholar]
- Curtiss R., 3rd, Caro L. G., Allison D. P., Stallions D. R. Early stages of conjugation in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1091–1104. doi: 10.1128/jb.100.2.1091-1104.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Furlong D., Lin E. C. Differentiation of arcA, arcB, and cpxA mutant phenotypes of Escherichia coli by sex pilus formation and enzyme regulation. J Bacteriol. 1989 May;171(5):2889–2893. doi: 10.1128/jb.171.5.2889-2893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. Purification and phosphorylation of the Arc regulatory components of Escherichia coli. J Bacteriol. 1992 Sep;174(17):5617–5623. doi: 10.1128/jb.174.17.5617-5623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Matsuda Z., Fujiwara T., Lin E. C. The arcB gene of Escherichia coli encodes a sensor-regulator protein for anaerobic repression of the arc modulon. Mol Microbiol. 1990 May;4(5):715–727. doi: 10.1111/j.1365-2958.1990.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Maneewannakul K., Maneewannakul S., Ippen-Ihler K. Sequence alterations affecting F plasmid transfer gene expression: a conjugation system dependent on transcription by the RNA polymerase of phage T7. Mol Microbiol. 1992 Oct;6(20):2961–2973. doi: 10.1111/j.1365-2958.1992.tb01755.x. [DOI] [PubMed] [Google Scholar]
- McEwen J., Sambucetti L., Silverman P. M. Synthesis of outer membrane proteins in cpxA cpxB mutants of Escherichia coli K-12. J Bacteriol. 1983 Apr;154(1):375–382. doi: 10.1128/jb.154.1.375-382.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J., Silverman P. M. Mutations in genes cpxA and cpxB alter the protein composition of Escherichia coli inner and outer membranes. J Bacteriol. 1982 Sep;151(3):1553–1559. doi: 10.1128/jb.151.3.1553-1559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J., Silverman P. Chromosomal mutations of Escherichia coli that alter expression of conjugative plasmid functions. Proc Natl Acad Sci U S A. 1980 Jan;77(1):513–517. doi: 10.1073/pnas.77.1.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J., Silverman P. Genetic analysis of Escherichia coli K-12 chromosomal mutants defective in expression of F-plasmid functions: identification of genes cpxA and cpxB. J Bacteriol. 1980 Oct;144(1):60–67. doi: 10.1128/jb.144.1.60-67.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J., Silverman P. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in isoleucine and valine syntheses. J Bacteriol. 1980 Oct;144(1):68–73. doi: 10.1128/jb.144.1.68-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainwater S., Silverman P. M. The Cpx proteins of Escherichia coli K-12: evidence that cpxA, ecfB, ssd, and eup mutations all identify the same gene. J Bacteriol. 1990 May;172(5):2456–2461. doi: 10.1128/jb.172.5.2456-2461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambucetti L., Eoyang L., Silverman P. M. Cellular control of conjugation in Escherichia coli K12. Effect of chromosomal cpx mutations on F-plasmid gene expression. J Mol Biol. 1982 Oct 15;161(1):13–31. doi: 10.1016/0022-2836(82)90275-3. [DOI] [PubMed] [Google Scholar]
- Silverman P. M., Rother S., Gaudin H. Arc and Sfr functions of the Escherichia coli K-12 arcA gene product are genetically and physiologically separable. J Bacteriol. 1991 Sep;173(18):5648–5652. doi: 10.1128/jb.173.18.5648-5652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman P. M., Wickersham E., Harris R. Regulation of the F plasmid traY promoter in Escherichia coli by host and plasmid factors. J Mol Biol. 1991 Mar 5;218(1):119–128. doi: 10.1016/0022-2836(91)90878-a. [DOI] [PubMed] [Google Scholar]
- Silverman P. M., Wickersham E., Rainwater S., Harris R. Regulation of the F plasmid traY promoter in Escherichia coli K12 as a function of sequence context. J Mol Biol. 1991 Jul 20;220(2):271–279. doi: 10.1016/0022-2836(91)90012-u. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R. F., Silverman P. M. The cpx proteins of Escherichia coli K12. Structure of the cpxA polypeptide as an inner membrane component. J Mol Biol. 1988 Sep 20;203(2):467–478. doi: 10.1016/0022-2836(88)90013-7. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]




