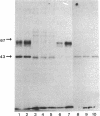
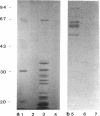
Abstract
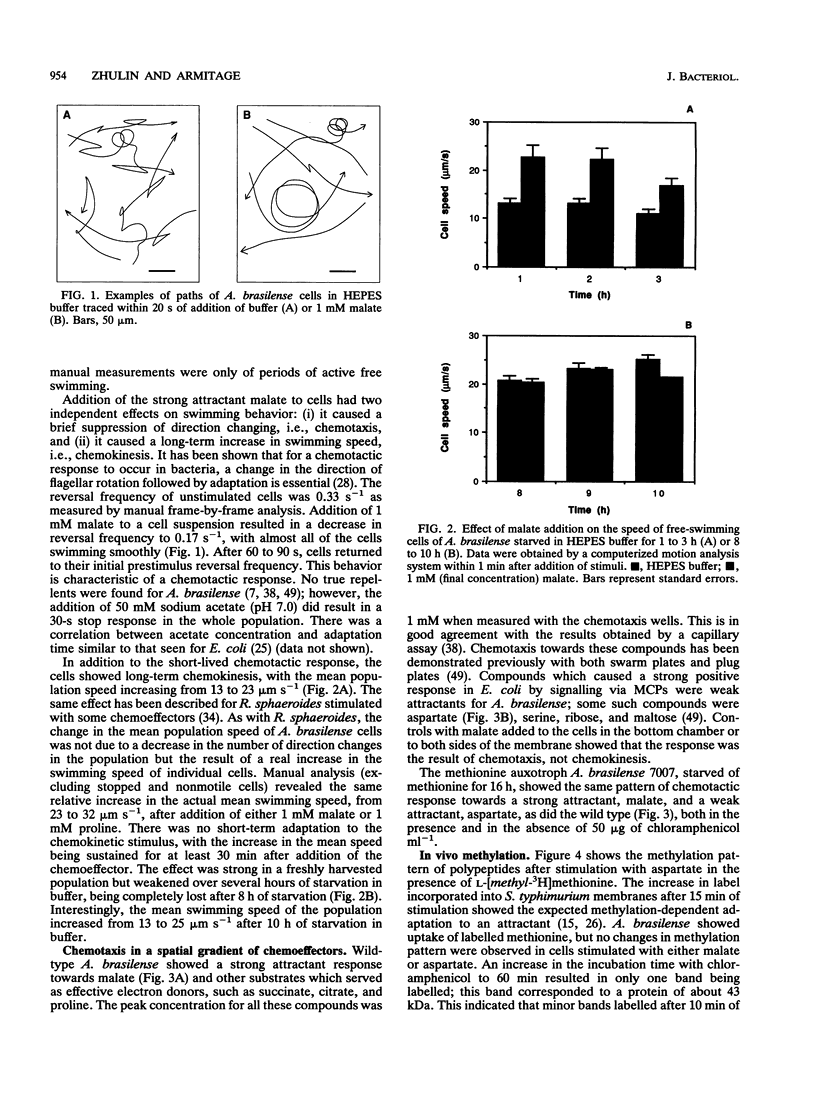
Observations of free-swimming and antibody-tethered Azospirillum brasilense cells showed that their polar flagella could rotate in both clockwise and counterclockwise directions. Rotation in a counterclockwise direction caused forward movement of free-swimming cells, whereas the occasional change in the direction of rotation to clockwise caused a brief reversal in swimming direction. The addition of a metabolizable chemoattractant, e.g., malate or proline, had two distinct effects on the swimming behavior of the bacteria: (i) a short-term decrease in reversal frequency from 0.33 to 0.17 s-1 and (ii) a long-term increase in the mean population swimming speed from 13 to 23 microns s-1. A. brasilense therefore shows both chemotaxis and chemokinesis in response to temporal gradients of some chemoeffectors. Chemokinesis was dependent on the growth state of the cells and may depend on an increase in the electrochemical proton gradient above a saturation threshold. Analysis of behavior of a methionine auxotroph, assays of in vivo methylation, and the use of specific antibodies raised against the sensory transducer protein Tar of Escherichia coli all failed to demonstrate the methylation-dependent pathway for chemotaxis in A. brasilense. The range of chemicals to which A. brasilense shows chemotaxis and the lack of true repellents indicate an alternative chemosensory pathway probably based on metabolism of chemoeffectors.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam M., Hazelbauer G. L. Structural features of methyl-accepting taxis proteins conserved between archaebacteria and eubacteria revealed by antigenic cross-reaction. J Bacteriol. 1991 Sep;173(18):5837–5842. doi: 10.1128/jb.173.18.5837-5842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M., Lebert M., Oesterhelt D., Hazelbauer G. L. Methyl-accepting taxis proteins in Halobacterium halobium. EMBO J. 1989 Feb;8(2):631–639. doi: 10.1002/j.1460-2075.1989.tb03418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage J. P. Behavioral responses in bacteria. Annu Rev Physiol. 1992;54:683–714. doi: 10.1146/annurev.ph.54.030192.003343. [DOI] [PubMed] [Google Scholar]
- Armitage J. P., Macnab R. M. Unidirectional, intermittent rotation of the flagellum of Rhodobacter sphaeroides. J Bacteriol. 1987 Feb;169(2):514–518. doi: 10.1128/jb.169.2.514-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak R., Nur I., Okon Y., Henis Y. Aerotactic response of Azospirillum brasilense. J Bacteriol. 1982 Nov;152(2):643–649. doi: 10.1128/jb.152.2.643-649.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Bernlohr R. W., Saha A. L., Young C. C., Toth B. R., Golden K. J. Nutrient-stimulated methylation of a membrane protein in Bacillus licheniformis. J Bacteriol. 1988 Sep;170(9):4113–4118. doi: 10.1128/jb.170.9.4113-4118.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block S. M., Segall J. E., Berg H. C. Impulse responses in bacterial chemotaxis. Cell. 1982 Nov;31(1):215–226. doi: 10.1016/0092-8674(82)90421-4. [DOI] [PubMed] [Google Scholar]
- Burgess-Cassler A., Ordal G. W. Functional homology of Bacillus subtilis methyltransferase II and Escherichia coli cheR protein. J Biol Chem. 1982 Nov 10;257(21):12835–12838. [PubMed] [Google Scholar]
- Craven R. C., Montie T. C. Chemotaxis of Pseudomonas aeruginosa: involvement of methylation. J Bacteriol. 1983 May;154(2):780–786. doi: 10.1128/jb.154.2.780-786.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström P., Hazelbauer G. L. Multiple methylation of methyl-accepting chemotaxis proteins during adaptation of E. coli to chemical stimuli. Cell. 1980 May;20(1):165–171. doi: 10.1016/0092-8674(80)90244-5. [DOI] [PubMed] [Google Scholar]
- Goldman D. J., Ordal G. W. Sensory adaptation and deadaptation by Bacillus subtilis. J Bacteriol. 1981 Jul;147(1):267–270. doi: 10.1128/jb.147.1.267-270.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. S. A methyl-accepting protein is involved in benzoate taxis in Pseudomonas putida. J Bacteriol. 1989 Sep;171(9):4603–4608. doi: 10.1128/jb.171.9.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. S., Fosnaugh K., Dispensa M. Flagellation of Pseudomonas putida and analysis of its motile behavior. J Bacteriol. 1989 Jul;171(7):4063–4066. doi: 10.1128/jb.171.7.4063-4066.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham C. J., Armitage J. P. Involvement of transport in Rhodobacter sphaeroides chemotaxis. J Bacteriol. 1987 Dec;169(12):5801–5807. doi: 10.1128/jb.169.12.5801-5807.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Greenberg E. P. Chemoattractants elicit methylation of specific polypeptides in Spirochaeta aurantia. J Bacteriol. 1983 Oct;156(1):95–100. doi: 10.1128/jb.156.1.95-100.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981 Mar;145(3):1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort E. N., Goy M. F., Larsen S. H., Adler J. Methylation of a membrane protein involved in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3939–3943. doi: 10.1073/pnas.72.10.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlin D. M., Nettleton D. O., Ordal G. W., Hazelbauer G. L. Chemotactic transducer proteins of Escherichia coli exhibit homology with methyl-accepting proteins from distantly related bacteria. J Bacteriol. 1985 Jul;163(1):262–266. doi: 10.1128/jb.163.1.262-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole P. S., Armitage J. P. Motility response of Rhodobacter sphaeroides to chemotactic stimulation. J Bacteriol. 1988 Dec;170(12):5673–5679. doi: 10.1128/jb.170.12.5673-5679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole P. S., Sinclair D. R., Armitage J. P. Real time computer tracking of free-swimming and tethered rotating cells. Anal Biochem. 1988 Nov 15;175(1):52–58. doi: 10.1016/0003-2697(88)90359-4. [DOI] [PubMed] [Google Scholar]
- Poole P. S., Smith M. J., Armitage J. P. Chemotactic signalling in Rhodobacter sphaeroides requires metabolism of attractants. J Bacteriol. 1993 Jan;175(1):291–294. doi: 10.1128/jb.175.1.291-294.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold B., Hurek T., Fendrik I. Strain-specific chemotaxis of Azospirillum spp. J Bacteriol. 1985 Apr;162(1):190–195. doi: 10.1128/jb.162.1.190-195.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimz A. Methylation of membrane proteins is involved in chemosensory and photosensory behavior of Halobacterium halobium. FEBS Lett. 1981 Mar 23;125(2):205–207. doi: 10.1016/0014-5793(81)80719-3. [DOI] [PubMed] [Google Scholar]
- Shaw P., Gomes S. L., Sweeney K., Ely B., Shapiro L. Methylation involved in chemotaxis is regulated during Caulobacter differentiation. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5261–5265. [PMC free article] [PubMed] [Google Scholar]
- Shioi J., Tribhuwan R. C., Berg S. T., Taylor B. L. Signal transduction in chemotaxis to oxygen in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988 Dec;170(12):5507–5511. doi: 10.1128/jb.170.12.5507-5511.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockett R. E., Armitage J. P., Evans M. C. Methylation-independent and methylation-dependent chemotaxis in Rhodobacter sphaeroides and Rhodospirillum rubrum. J Bacteriol. 1987 Dec;169(12):5808–5814. doi: 10.1128/jb.169.12.5808-5814.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. L., Koshland D. E., Jr Reversal of flagellar rotation in monotrichous and peritrichous bacteria: generation of changes in direction. J Bacteriol. 1974 Aug;119(2):640–642. doi: 10.1128/jb.119.2.640-642.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A. H., Ordal G. W. In vivo and in vitro chemotactic methylation in Bacillus subtilis. J Bacteriol. 1981 Feb;145(2):958–965. doi: 10.1128/jb.145.2.958-965.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. C., Alvarez J. D., Bernlohr R. W. Nutrient-dependent methylation of a membrane-associated protein of Escherichia coli. J Bacteriol. 1990 Sep;172(9):5147–5153. doi: 10.1128/jb.172.9.5147-5153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]