Abstract
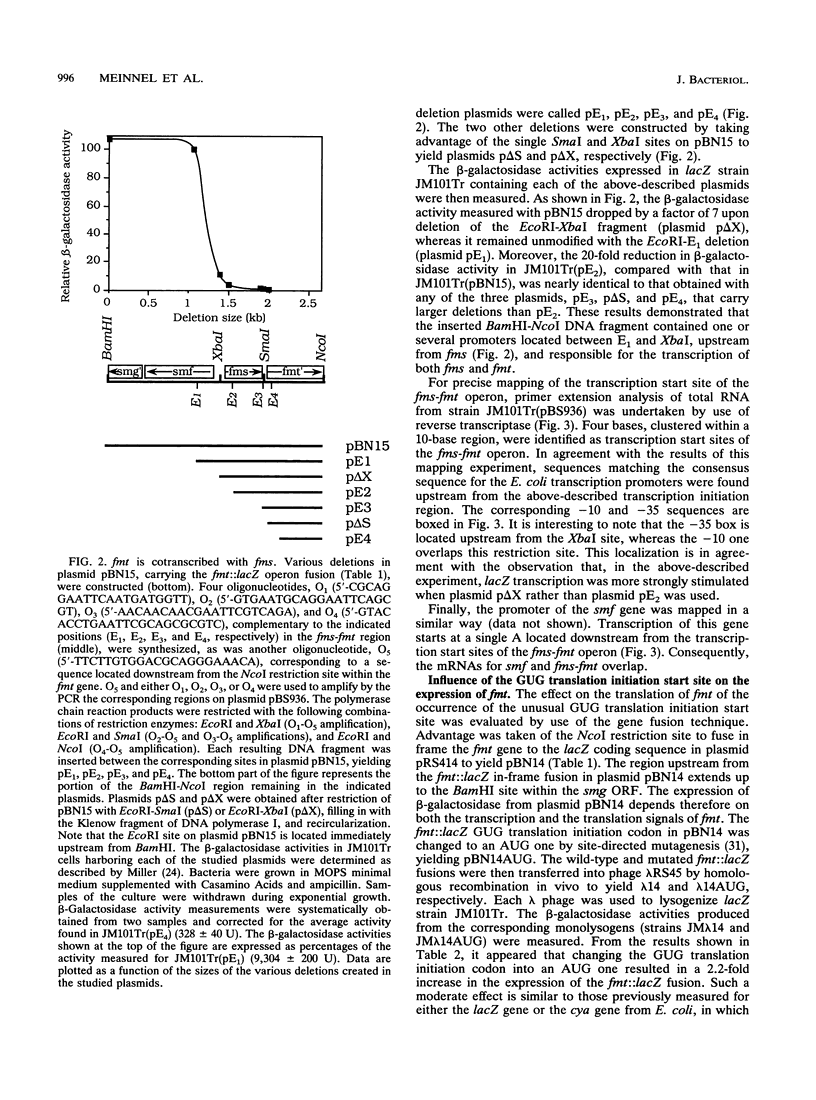
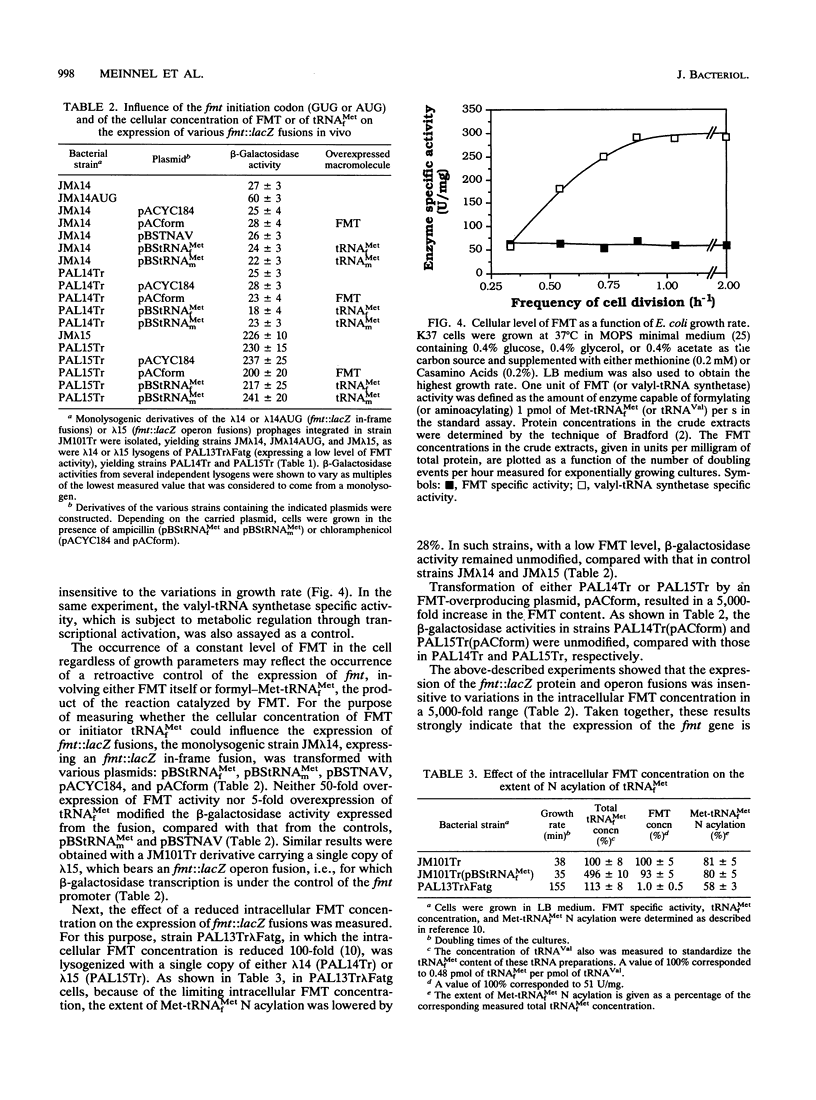
The genetic organization near the recently cloned fmt gene, encoding Escherichia coli methionyl-tRNA(fMet) formyltransferase (J. M. Guillon, Y. Mechulam, J. M. Schmitter, S. Blanquet, and G. Fayat, J. Bacteriol. 174:4294-4301, 1992), has been studied. The fmt gene, which starts at a GUG codon, is cotranscribed with another gene, fms, and the transcription start site of this operon has been precisely mapped. Moreover, the nucleotide sequence of a 1,379-bp fragment upstream from fmt reveals two additional open reading frames, in the opposite polarity. In the range of 0.3 to 2 doublings per h, the intracellular methionyl-tRNA(fMet) formyltransferase concentration remains constant, providing, to our knowledge, the first example of a gene component of the protein synthesis apparatus escaping metabolic control. When the gene fusion technique was used for probing, no effect on fmt expression of the concentrations of methionyl-tRNA(fMet) formyltransferase or tRNA(fMet) could be found. The possibility that the fmt gene, the product of which is present in excess to ensure full N acylation of methionyl-tRNA(fMet), could be expressed in a constitutive manner is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanquet S., Dessen P., Kahn D. Properties and specificity of methionyl-tRNAfMet formyltransferase from Escherichia coli. Methods Enzymol. 1984;106:141–152. doi: 10.1016/0076-6879(84)06013-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brombach M., Pon C. L. The unusual translational initiation codon AUU limits the expression of the infC (initiation factor IF3) gene of Escherichia coli. Mol Gen Genet. 1987 Jun;208(1-2):94–100. doi: 10.1007/BF00330428. [DOI] [PubMed] [Google Scholar]
- Butler J. S., Springer M., Grunberg-Manago M. AUU-to-AUG mutation in the initiator codon of the translation initiation factor IF3 abolishes translational autocontrol of its own gene (infC) in vivo. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4022–4025. doi: 10.1073/pnas.84.12.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings H. S., Sands J. F., Foreman P. C., Fraser J., Hershey J. W. Structure and expression of the infA operon encoding translational initiation factor IF1. Transcriptional control by growth rate. J Biol Chem. 1991 Sep 5;266(25):16491–16498. [PubMed] [Google Scholar]
- Gualerzi C. O., Pon C. L. Initiation of mRNA translation in prokaryotes. Biochemistry. 1990 Jun 26;29(25):5881–5889. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- Guillon J. M., Mechulam Y., Schmitter J. M., Blanquet S., Fayat G. Disruption of the gene for Met-tRNA(fMet) formyltransferase severely impairs growth of Escherichia coli. J Bacteriol. 1992 Jul;174(13):4294–4301. doi: 10.1128/jb.174.13.4294-4301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon J. M., Meinnel T., Mechulam Y., Lazennec C., Blanquet S., Fayat G. Nucleotides of tRNA governing the specificity of Escherichia coli methionyl-tRNA(fMet) formyltransferase. J Mol Biol. 1992 Mar 20;224(2):359–367. doi: 10.1016/0022-2836(92)91000-f. [DOI] [PubMed] [Google Scholar]
- Hirel P. H., Lévêque F., Mellot P., Dardel F., Panvert M., Mechulam Y., Fayat G. Genetic engineering of methionyl-tRNA synthetase: in vitro regeneration of an active synthetase by proteolytic cleavage of a methionyl-tRNA synthetase--beta-galactosidase chimeric protein. Biochimie. 1988 Jun;70(6):773–782. doi: 10.1016/0300-9084(88)90107-1. [DOI] [PubMed] [Google Scholar]
- Jakubowski H., Goldman E. Quantities of individual aminoacyl-tRNA families and their turnover in Escherichia coli. J Bacteriol. 1984 Jun;158(3):769–776. doi: 10.1128/jb.158.3.769-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn D., Fromant M., Fayat G., Dessen P., Blanquet S. Methionyl-transfer-RNA transformylase from Escherichia coli. Purification and characterisation. Eur J Biochem. 1980 Apr;105(3):489–497. doi: 10.1111/j.1432-1033.1980.tb04524.x. [DOI] [PubMed] [Google Scholar]
- Khudyakov YuE, Neplyueva V. S., Kalinina T. I., Smirnov V. D. Effect of structure of the initiator codon on translation in E. coli. FEBS Lett. 1988 May 23;232(2):369–371. doi: 10.1016/0014-5793(88)80771-3. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Eskin B., Redfield B., Weissbach H. DNA-directed in vitro synthesis of beta-galactosidase: requirement for formylation of methionyl-tRNAf. Arch Biochem Biophys. 1979 Jul;195(2):396–400. doi: 10.1016/0003-9861(79)90366-7. [DOI] [PubMed] [Google Scholar]
- Lee C. P., Seong B. L., RajBhandary U. L. Structural and sequence elements important for recognition of Escherichia coli formylmethionine tRNA by methionyl-tRNA transformylase are clustered in the acceptor stem. J Biol Chem. 1991 Sep 25;266(27):18012–18017. [PubMed] [Google Scholar]
- Lévêque F., Gazeau M., Fromant M., Blanquet S., Plateau P. Control of Escherichia coli lysyl-tRNA synthetase expression by anaerobiosis. J Bacteriol. 1991 Dec;173(24):7903–7910. doi: 10.1128/jb.173.24.7903-7910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinnel T., Mechulam Y., Fayat G. Fast purification of a functional elongator tRNAmet expressed from a synthetic gene in vivo. Nucleic Acids Res. 1988 Aug 25;16(16):8095–8096. doi: 10.1093/nar/16.16.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinnel T., Mechulam Y., Le Corre D., Panvert M., Blanquet S., Fayat G. Selection of suppressor methionyl-tRNA synthetases: mapping the tRNA anticodon binding site. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):291–295. doi: 10.1073/pnas.88.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinnel T., Schmitt E., Mechulam Y., Blanquet S. Structural and biochemical characterization of the Escherichia coli argE gene product. J Bacteriol. 1992 Apr;174(7):2323–2331. doi: 10.1128/jb.174.7.2323-2331.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. I., Friedman D. I. An E. coli gene product required for lambda site-specific recombination. Cell. 1980 Jul;20(3):711–719. doi: 10.1016/0092-8674(80)90317-7. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Reddy P., Peterkofsky A., McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5656–5660. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter J. M., Mechulam Y., Fayat G., Anselme M. Rapid purification of DNA fragments by high-performance size-exclusion chromatography. J Chromatogr. 1986 Jun 13;378(2):462–466. doi: 10.1016/s0378-4347(00)80743-4. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]