Summary
There is a significant genetic component in age-related macular degeneration (AMD). CX3CR1, which encodes the fractalkine (chemokine, CX3CL1) receptor, has two single nucleotide polymorphisms (SNPs): V249I and T280M. These SNPs are correlated with other aged-related diseases such as atherosclerosis. We have reported an association of CX3CR1 SNP and AMD. In this study we examined CX3CR1 SNP frequencies and protein expression on archived sections of AMD and normal eyes. We microdissected non-retinal, peripheral retinal and macular cells from archived slides of eyes of AMD patients and normal subjects. CX3CR1 SNP typing was conducted by PCR and restriction fragment length polymorphism analysis. CX3CR1 transcripts from retinal cells were also measured using RT-PCR. CX3CR1 protein expression was evaluated using avidin-biotin complex immunohistochemistry. We successfully extracted DNA from 32/40 AMD cases and 2/2 normal eyes. Among the 32 AMD cases, 18 had neovascular AMD and 14 had non-neovascular AMD. The M280 allele was detected in 19/64 (32 cases x2) with a frequency of 29.7%, which was significantly higher as compared to the frequency in the normal population (11.2%). We detected CX3CR1 expression in the various retinal cells. CX3CR1 transcript and protein levels were diminished in the macular lesions. This study successfully analyzed CX3CR1 SNP and transcript expression in microdissected cells from archived paraffin fixed slides. Our data suggest that the M280 allele, a SNP resulting in aberrant CX3CR1 and CX3CL1 interaction, as well as lowered expression of macular CX3CR1, may contribute to the development of AMD.
Keywords: Age-related macular degeneration, Single nuclear polymorphism, CX3CR1, Chemokine, Macrophage
Introduction
Age-related macular degeneration (AMD) is the leading cause of visual impairment and blindness in the United States and the developed countries among people 65 years and older (Klaver et al., 1998b; la Cour et al., 2002). However, the etiology of AMD is still unknown. The development and pathogenesis of AMD is determined by a complex interaction between environmental factors and genetic backgrounds. In addition to age, epidemiological studies have identified cigarette smoking and diet as risk factors for AMD development (AREDS, 2000; Seddon et al., 2001; Husain et al., 2002; Hyman and Neborsky, 2002). The tendency for familial aggregation in AMD cases, with approximately 20% of AMD patients having a positive family history, suggests a significant genetic component in disease development as well (De Jong et al., 2001).
The potential role of genetic variation in the development of AMD has received increased attention. Gene variation has been reported in association with various other human age-related diseases such as cancer and the cardiovascular diseases (Halushka et al., 1999; Gulcher et al., 2001; Brennan, 2002). A few studies have also demonstrated an association between AMD and various gene polymorphisms (Schmidt et al., 2000). Examples of genes with single nucleotide polymorphisms (SNPs) that have been found in association with AMD include SOD2, a manganese eroxide dismustase (MnSOD) isoform (Kimura et al., 2000), paraoxonase (Ikeda et al., 2001), and apolipoprotein E (ApoE) (Klaver et al., 1998a; Souied et al., 1998; Simonelli et al., 2001).
Macrophage activity has been shown to play a significant role in AMD pathogenesis (Dastgheib and Green, 1994; Espinosa-Heidmann et al., 2003; Forrester, 2003). For this reason, investigators have begun to further explore the potential role of immunological mechanisms in this disease (Ambati et al., 2003b). Chemokines are a group of small, pro-inflammatory molecules first described for their pivotal role in the mobilization and subsequent activation upon arrival of specific leukocyte subsets to sites of inflammation (Taub and Oppenheim, 1994). Due to their vast functional responsibilities, chemokines have been linked to the pathogeneses of many seemingly unrelated diseases such as HIV infection, cancer, atherosclerosis, and various autoimmune diseases (Howard et al., 1996; Berger et al., 1999; Balkwill, 2003). The CX3CR1 gene, which encodes a chemokine receptor for fractalkine (CX3CL1), is known to be polymorphic with two non-synonymous SNPs in the open reading frame. These two polymorphisms, involve a valine to isoleucine substitution at position 249 and a threonine to methionine at position 280 respectively. Several investigators have reported associations between these CX3CR1 polymorphisms and human HIV progression and arteriosclerosis susceptibility (Faure et al., 2000; McDermott et al., 2000, 2003).
Recently we reported an association between the CX3CR1 T280M and V249I polymorphisms and AMD risk (Tuo et al., 2004a,b). In a small multiple control study, we reported an increased prevalence of the I249 and M280 alleles among AMD cases when compared to the control populations (p<0.05). In order to further investigate the involvement of CX3CR1 in AMD, we evaluated CX3CR1 messenger and protein expression in addition to performing SNP typing assay on archived slides obtained from patients with histopathologically classified neovascular or non-neovascular AMD.
Materials and methods
Cases
Archived and paraffin-embedded slides of 42 autopsied eyes were collected. Forty eyes with a pathological diagnosis of AMD were obtained from the Wilmer Eye Institute (35 cases) and National Eye Institute (5 cases). All eyes were serially sectioned through the macula via the pupillary optic nerve head axis. Only 1-2 slides per case were available from the material collected from the Wilmer Eye Institute; therefore, only SNP analysis could be performed for these 35 cases. For the 5 cases obtained from the National Eye Institute, SNP analysis, immunohisto-chemistry, and RT-PCR could all be performed.
All slides were stained for hematoxylin and eosin. Two normal autopsied eyes collected from 70 and 78 year-old Caucasians from the National Eye Institute served as controls. The Institutional Review Board of the National Eye Institute approved this study for human subjects.
Microdissection
The cover slips of the hematoxylin-eosin stained slides were removed. The non-retinal (corneal and/or iris), peripheral retinal and macular retinal cells were then carefully microdissected either manually under a light microscope or using the PixCell IIe laser capture microscope (Arturus, Mountain View, CA). Approximately similar numbers of peripheral and macular retinal cells were obtained from each case. The PixCell IIe uses a low power infrared laser to collect selected cells on a membrane located on the cap of a 1.5 ml tube. In manual microdissection, the selected cells were gently scraped, detached and lifted from the slide using a 30-gauge needle (Shen et al., 1998).
Single nucleotide polymorphism (SNP) assay
The non-retinal cells were immediately placed in proteinase K enriched DNA extraction buffer. PCR amplification was performed using the primers containing the polymorphic CX3CR1 site (sense, 5’-CCG AGG TCC TTC AGG AAA TCT-3’ and anti-sense, 3’-GAG TTC CTG AAC CTG ATG CTG A-5’). SNP assay was performed through PCR amplification followed by restriction fragment length polymorphism (RFLP) analysis.
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA was extracted from both peripheral and macular retinal cells of the 5 cases obtained from the NEI in order to perform RT-PCR for CX3CR1 and β-actin mRNA. The primers used were 5’-CAG ATC CAG AGG TTC CCT TG-3’ and 5’-TAA CAG GCC TCA GCC AAA TC-3’ for CX3CR1 and 5’-TAA CAG GCC TCA GCC AAA TC-3’ and 5’-ACA TCT GCT GGA AGG TGG AC-3’ for β-actin. Microdissected peripheral and macular retinal cells from two normal autopsied eyes served as controls and were assayed in the same fashion.
Immunohistochemistry
The avidin-biotin-complex immunoperoxidase technique was utilized on the unstained, de-paraffinized slides of 5 NEI cases in which macular sections were available. A normal autopsied eye was also stained and used as a control. The primary antibody was rabbit anti-human CX3CR1 polyclonal antibody (Chemicon International, Inc., Temecular, CA) or control rabbit IgG. The secondary antibody was biotin-conjugated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA). The substrate was avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA), and the chromogen was diaminobenzidine and nickel sulfate. A deparaffinized section from a normal eye served as a positive control for immunostaining against the CX3CR1 antibody. The positive reaction will result in the production of a blue-blackish color.
Results
Histopathology
All 40 cases demonstrated classical AMD lesions in the macula as defined in the literature (Green and Enger, 1993; Green, 1999). In general, both eyes of the same case presented similar and rather symmetrical lesions. Subretinal choroidal neovascularization and photoreceptor loss with or without disciform scars were found in 23 of the 40 cases. These cases were diagnosed with neovascular AMD. The remaining 17 cases were diagnosed with AMD showing areolar (geographic) atrophy without neovascularization. These cases were characterized by a loss of photoreceptors, alteration or loss of RPE cells, drusen formation, and/or calcification in the macula. In this paper, we refer to these two groups as either with or without neovascular AMD respectively.
Detection of CX3CR1 T280M polymorphism
Genotyping was carried out in 32 of the 40 cases from which DNA could be successfully extracted from the microdissected ocular cells. A mean age of 82.8 years (ranging from 63-96 years old) was obtained for these patients. Further demographic information was unavailable for 5 of these 32 cases. General demographic information of the 27 known patients includes a gender distribution of 20 females and 7 males and a race distribution of 26 Caucasians and 1 African American. Histopathology diagnosed 18 of these cases with neovascular AMD and 14 without neovascular AMD. Sixteen of the 32 cases were found to be CX3CR1 M280 carriers: 13 cases were heterozygous (Thr/Met; 8 with neovascular AMD and 5 without neovascular AMD) and 3 were homozygous for the variant allele (Met/Met; two with neovascular and one without neovascular AMD). The M280 allele frequency was 29.7%. Out of the 16 M280 carriers, 10 had neovascular AMD (allele frequency of 33%) and 6 had non-neovascular AMD (allele frequency of 25%). Although there was a slightly higher prevalence of the M280 allele in the neovascular AMD cases, our findings suggest that this SNP may not correlate well with phenotype. In other words, CX3CR1 M280 carriers could belong to either the neovascular or non-neovascular AMD group.
Lowered expression of CX3CR1 in the AMD macula
Four (3 neovascular and 1 without neovascular AMD) of the 5 NEI cases carried the CX3CR1 M280 (Thr/Met) allele. The remaining case (with neovascular AMD) was Thr/Thr in genotype (Fig. 1). CX3CR1 messengers were successfully recovered from 3 (2 with neovascular and 1 without neovascular AMD) of these 5 cases. Of these three cases, each carried the M280 allele (Thr/Met) and demonstrated an absence of CX3CR1 mRNA expression in the macula as compared to the peripheral retina. In contrast, CX3CR1 mRNA expression in the pairs of normal eyes (with ages of 70 and 78 years) detected no difference of CX3CR1 transcripts between the macular and perimacular and peripheral retina regions (Fig. 2).
Fig. 1.
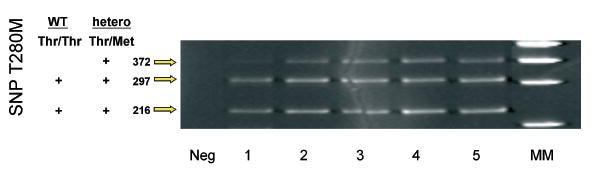
Autoradiography showing CX3CR1 SNP typing of 5 AMD cases from the National Eye Institute: one with Thr/Thr (case 1) and 4 with Thr/Met genotypes. The restriction enzyme used to digest the PCR T280M products was the BsmBI endonuclease.
Fig. 2.
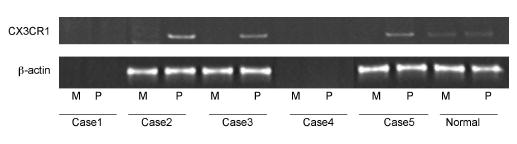
Autoradiography showing CX3CR1 mRNA expression in perimacular but not macular cells of three AMD cases with CX3CR1 M280. CX3CR1 transcripts were detected in both macular (M) and perimacular (P) cells of a normal eye. Two other cases (Thr/Thr and Thr/Met) did not yield RT-PCR products.
CX3CR1 stained positively at the RPE, Müller cell, microglia and outer segment of the photoreceptors in the retina of normal eye. However, positive reactivity of CX3CR1 was decreased in number and intensity in the macula of the eyes affected by both with and without neovascular AMD (Fig. 3). No changes in intensity, number, or staining pattern were found in the peripheral retina in the eyes with AMD as compared to the normal eyes.
Fig. 3.
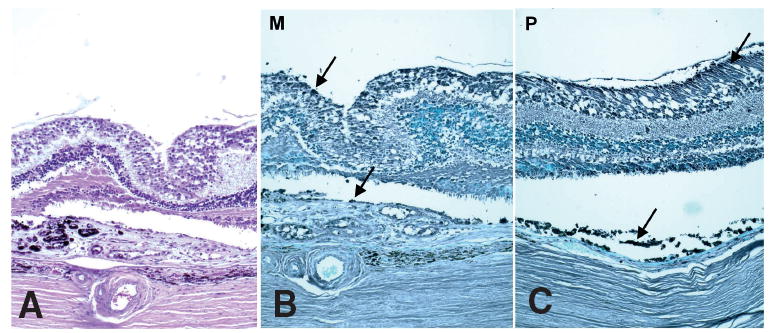
Photomicrograph showing expression of CX3CR1 on Muller cells, photoreceptors and RPE cells. AMD is confirmed in the hematoxylin and eosin slide (A). Lower expression is found in the macula (B) as compared to the perimacula (C) of the same eye with AMD (B and C, avidin-biotin-complex immunoperoxidase, original magnification, × 100).
Discussion
In this study, we have successfully applied a combination of microdissection and PCR-RFLP techniques in order to detect CX3CR1 SNPs on archived, paraffin embedded slides. In addition, we have also successfully detected CX3CR1 transcripts and protein expression in multiple cell types within the retina using microdissection combined with RT-PCR and immunohistochemistry. Due to limitations imposed by the small amount of DNA or RNA yielded via microdissection from the archived slides, we were unable to perform real time quantitative PCR. However, we were able to compare semi-quantitative PCR products to β-actin gene expression. This combination of techniques allows us to more thoroughly analyze the molecular pathology of AMD as well as better understand disease pathophysiology.
This study demonstrated a possible association between the CX3CR1 polymorphism (T280M) and AMD through analysis of archived eye sections. A higher allele frequency of the M280 allele was found in our AMD cases (29.7%) as compared to frequency found in the controls (11%) and general population (10-18%) (Liu et al., 2003; McDermott et al., 2003) reported by LocusLink (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=1524 ). This association was also observed in our recent AMD study, in which we reported an M280 allele frequency of 11.0% (allele, n = 210) in the control population and 19.4% (n = 170) in the AMD case group (Tuo et al., 2004b). In the previous study, we also observed consistent variation in expression levels of the transcripts and protein in matched normal subjects with different genotypes (Tuo et al., 2004b). In the present study, we have further shown a lowered expression of CX3CR1 messenger and protein in the macular area as compared to the peripheral and perimacular retina of the eyes with AMD. These findings support that CX3CR1, a chemokine receptor, may play a role in the development of AMD.
Chemokines are low molecular weight (8-10 kDa) peptides or glycopeptides that mediate leukocyte chemotaxis and increase cellular adhesion (Taub and Oppenheim, 1994; Homey et al., 2002). These essential properties are required in order to efficiently direct cells to sites of infection or inflammation. Chemokine molecules are classified into four distinct families based on the positioning of the first two of four conserved cysteine residues (Zlotnik and Yoshie, 2000). These 4 families are classified as CXC, CC, XC, and CX3C. The receptors for these molecules are seven-transmembrane domain G protein-coupled receptors (Zlotnik et al., 1999). Chemokines are involved in not only inflammatory and immune responses, but in other disease processes as well such as malignancy, angiogenesis, and hematopoiesis.
To date, fractalkine/CX3CL1, also known as neurotactin, is the only identified CX3C chemokine. CX3CL1 is widely expressed in various organs such as the eye (Foxman et al., 2002) brain, lung, heart, kidney and small intestine (Cotter et al., 2002). CX3CR1, the only receptor of CX3CL1, is expressed on different leukocytes such as macrophages as well as on microglia and astrocytes (Mizuno et al., 2003). In this study, we have demonstrated intensive staining of CX3CR1 on the RPE, Müller cells, glial cells, and photoreceptors in the retina of the normal eye.
Strong up-regulation of CX3CR1 in macrophages has been reported in response to experimental ischemic-reperfusion brain injury and glormerulonephritis (Feng et al., 1999; Tarozzo et al., 2002) as well as in the affected skin of patients with atopic dermatitis (Echigo et al., 2004). These findings suggest that CX3CL1 plays a central role in the trafficking of macrophages into tissues with lesions that release CX3CL1. In this context, expression of CX3CR1 on macrophages is important for their recruitment to the appropriate location.
The majority of DNA sequence variation in the human genome is in the form of SNPs (Risch and Merikangas, 1996; Wang et al., 1998; O′Brien et al., 1999). SNPs are defined as persistent substitutions of a single base with a frequency of more than 1%. The screening of common SNPs has become an attractive tool in the exploration of the genetic component of complex diseases such as AMD (Risch, 2000; Wright, 2001; Amouyel, 2002; Hyman and Neborsky, 2002; Stone et al., 2004; Zareparsi et al., 2004).
Recently, a number of SNPs in coding areas have been identified in CX3CR1. These SNPs have been shown to alter susceptibility to both HIV infection and arteriosclerosis (Faure et al., 2000; McDermott et al., 2001; Moatti et al., 2001). The two SNPs that have been implicated include a valine to isoleucine substitution at position 249 (V249I) and a threonine to methionine substitution at position 280 (T280M). These two polymorphisms are in complete linkage disequilibrium, meaning that chromosomes possessing the M280 polymorphism also have the I249 variation. The M280 polymorphism is associated with a decreased binding affinity for the CX3CL1 ligand (Faure et al., 2000). The V249I SNP has been correlated with a 35% decrease in cell surface receptor number (Moatti et al., 2001). In addition, two CX3CR1 promoter SNPs have been found in weak linkage disequilibrium with the T280M and V249I SNPs. Another promoter SNP has also been shown to be in moderately strong linkage disequilibrium with both coding SNPs (DeVries et al., 2003).
In this study, we have found an increase in CX3CR1 M280 allele frequency (29.7%) in the 32 pathologically diagnosed AMD cases as compared to the general control population (10-12%). We have also found a decrease in both CX3CR1 protein and transcript expression in the macula as compared to the peripheral retina of the AMD eyes. There was no significant difference in distribution between the two AMD phenotypes with and without neovascularization. In contrast, CX3CR1 transcription and protein are same in both macular and peripheral retina in normal controls. While discussion of the pathogenic mechanisms associated with the CX3CR1 polymorphisms and AMD development may be still speculative at the present time, the potential involvement of macrophages in AMD appears to be a logical focal point for future studies.
Macrophages have been well recognized as active participants in neovascular AMD development (Kimura et al., 1999; Grossniklaus et al., 2000; Espinosa-Heidmann et al., 2003; Tsutsumi et al., 2003). Macrophages have also been shown to phagocytize pigment and debris and either migrate into the retina or form clumps in the photoreceptor region (Killingsworth et al., 1990). Therefore, macrophages may also accumulate as a result of areolar atrophy without neovascularization.
On the other hand, it has been demonstrated that an increased amount of deposit in Bruch’s membrane is associated with the presence and severity of AMD (van der Schaft et al., 1992; Green, 1999; Spraul et al., 1999). Macrophages, like other cell types such as endothelial cells, aortic smooth muscle cells, neuronal cells, and keratinocytes (Zingg et al., 2000), possess scavenging receptors. Previous studies on animals that completely lack CCL2, a more ubiquitous chemoattractant for macrophages, or its receptor, CCR2, demonstrated a marked reduction in the number of macrophages in the tissue thereby contributing to the development of both drusen and CNV (Ambati et al., 2003b). In other words, in the healthy eye, normal choroidal macrophage activity may contribute to drusen disposal thereby actually preventing AMD formation (Ambati et al., 2003a; Forrester, 2003).
Whether or not CX3CL1, in its function as an adhesion molecule and chemoattractant for macrophages that bear its receptor, CX3CR1, is involved in directing macrophages into Bruch’s membrane is still unknown. However, we hypothesize that aberrant CX3CR1 function may result in inadequate removal of these deposits thereby contributing to the development of AMD (Tuo et al., 2004b). The current pathological study supports this hypothesis. Further studies investigating the functional significance of CX3CR1 in AMD development are currently underway in our laboratory.
References
- AREDS Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: age-related eye disease study report number 3. Age-Related Eye Disease Study Research Group. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003a;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003b;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- Amouyel P. Genetic susceptibility to ageing-associated diseases. CR Biol. 2002;325:741–745. doi: 10.1016/s1631-0691(02)01481-6. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Chemokine biology in cancer. Semin Immunol. 2003;15:49–55. doi: 10.1016/s1044-5323(02)00127-6. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Brennan P. Gene-environment interaction and aetiology of cancer: what does it mean and how can we measure it? Carcinogenesis. 2002;23:381–387. doi: 10.1093/carcin/23.3.381. [DOI] [PubMed] [Google Scholar]
- Cotter R, Williams C, Ryan L, Erichsen D, Lopez A, Peng H, Zheng J. Fractalkine (CX3CL1) and brain inflammation: Implications for HIV-1-associated dementia. J Neurovirol. 2002;8:585–598. doi: 10.1080/13550280290100950. [DOI] [PubMed] [Google Scholar]
- Dastgheib K, Green WR. Granulomatous reaction to Bruch′s membrane in age-related macular degeneration. Arch Ophthalmol. 1994;112:813–818. doi: 10.1001/archopht.1994.01090180111045. [DOI] [PubMed] [Google Scholar]
- De Jong PT, Bergen AA, Klaver CC, Van Duijn CM, Assink JM. Age-related maculopathy: its genetic basis. Eye. 2001;15:396–400. doi: 10.1038/eye.2001.143. [DOI] [PubMed] [Google Scholar]
- DeVries ME, Cao H, Wang J, Xu L, Kelvin AA, Ran L, Chau LA, Madrenas J, Hegele RA, Kelvin DJ. Genomic organization and evolution of the CX3CR1/CCR8 chemokine receptor locus. J Biol Chem. 2003;278:11985–11994. doi: 10.1074/jbc.M211422200. [DOI] [PubMed] [Google Scholar]
- Echigo T, Hasegawa M, Shimada Y, Takehara K, Sato S. Expression of fractalkine and its receptor, CX3CR1, in atopic dermatitis: possible contribution to skin inflammation. J Allergy Clin Immunol. 2004;113:940–948. doi: 10.1016/j.jaci.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- Faure S, Meyer L, Costagliola D, Vaneensberghe C, Genin E, Autran B, Delfraissy JF, McDermott DH, Murphy PM, Debre P. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287:2274–2277. doi: 10.1126/science.287.5461.2274. [DOI] [PubMed] [Google Scholar]
- Feng L, Chen S, Garcia GE, Xia Y, Siani MA, Botti P, Wilson CB, Harrison JK, Bacon KB. Prevention of crescentic glomerulonephritis by immunoneutralization of the fractalkine receptor CX3CR1 rapid communication. Kidney Int. 1999;56:612–620. doi: 10.1046/j.1523-1755.1999.00604.x. [DOI] [PubMed] [Google Scholar]
- Forrester JV. Macrophages eyed in macular degeneration. Nat Med. 2003;9:1350–1351. doi: 10.1038/nm1103-1350. [DOI] [PubMed] [Google Scholar]
- Foxman EF, Zhang M, Hurst SD, Muchamuel T, Shen D, Wawrousek EF, Chan CC, Gery I. Inflammatory mediators in uveitis: differential induction of cytokines and chemokines in Th1- versus Th2-mediated ocular inflammation. J Immunol. 2002;168:2483–2492. doi: 10.4049/jimmunol.168.5.2483. [DOI] [PubMed] [Google Scholar]
- Green WR. Histopathology of age-related macular degeneration. Mol Vis. 1999 ;5:27. [PubMed] [Google Scholar]
- Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Cingle KA, Yoon YD, Ketkar N, L′Hernault N, Brown S. Correlation of histologic 2-dimensional reconstruction and confocal scanning laser microscopic imaging of choroidal neovascularization in eyes with age-related maculopathy. Arch Ophthalmol. 2000;118:625–629. doi: 10.1001/archopht.118.5.625. [DOI] [PubMed] [Google Scholar]
- Gulcher J, Kong A, Stefansson K. The genealogic approach to human genetics of disease. Cancer J. 2001;7:61–68. [PubMed] [Google Scholar]
- Halushka MK, Fan JB, Bentley K, Hsie L, Shen N, Weder A, Cooper R, Lipshutz R, Chakravarti A. Patterns of single-nucleotide polymorphisms in candidate genes for blood-pressure homeostasis. Nat Genet. 1999;22:239–247. doi: 10.1038/10297. [DOI] [PubMed] [Google Scholar]
- Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- Howard OM, Ben-Baruch A, Oppenheim JJ. Chemokines: progress toward identifying molecular targets for therapeutic agents. Trends Biotechnol. 1996;14:46–51. doi: 10.1016/0167-7799(96)80920-6. [DOI] [PubMed] [Google Scholar]
- Husain D, Ambati B, Adamis AP, Miller JW. Mechanisms of age-related macular degeneration. Ophthalmol Clin North Am. 2002;15:87–91. doi: 10.1016/s0896-1549(01)00009-8. [DOI] [PubMed] [Google Scholar]
- Hyman L, Neborsky R. Risk factors for age-related macular degeneration: an update. Curr Opin Ophthalmol. 2002;13:171–175. doi: 10.1097/00055735-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Obayashi H, Hasegawa G, Nakamura N, Yoshikawa T, Imamura Y, Koizumi K, Kinoshita S. Paraoxonase gene polymorphisms and plasma oxidized low-density lipoprotein level as possible risk factors for exudative age-related macular degeneration. Am J Ophthalmol. 2001;132:191–195. doi: 10.1016/s0002-9394(01)00975-8. [DOI] [PubMed] [Google Scholar]
- Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch′s membrane in age-related macular degeneration. Eye. 1990;4(Pt 4):613–621. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- Kimura H, Spee C, Sakamoto T, Hinton DR, Ogura Y, Tabata Y, Ikada Y, Ryan SJ. Cellular response in subretinal neovascularization induced by bFGF-impregnated microspheres. Invest Ophthalmol Vis Sci. 1999;40:524–528. [PubMed] [Google Scholar]
- Kimura K, Isashiki Y, Sonoda S, Kakiuchi-Matsumoto T, Ohba N. Genetic association of manganese superoxide dismutase with exudative age-related macular degeneration. Am J Ophthalmol. 2000;130:769–773. doi: 10.1016/s0002-9394(00)00552-3. [DOI] [PubMed] [Google Scholar]
- Klaver CC, Kliffen M, van Duijn CM, Hofman A, Cruts M, Grobbee DE, van Broeckhoven C, de Jong PT. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet. 1998a;63:200–206. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver CC, Wolfs RC, Vingerling JR, Hofman A, de Jong PT. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998b;116:653–658. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- la Cour M, Kiilgaard JF, Nissen MH. Age-related macular degeneration: epidemiology and optimal treatment. Drugs Aging. 2002;19:101–133. doi: 10.2165/00002512-200219020-00003. [DOI] [PubMed] [Google Scholar]
- Liu MX, Wang FS, Hong WG, Wang CQ, Wang B, Jin L, Hou J, Lei ZY. Distribution of HIV-1 coreceptor CX3CR1 allelic polymorphisms in general population, HIV-1 high-risk group and HIV-1 carriers of Chinese indigenous Han and Uygur people. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24:595–598. in Chinese. [PubMed] [Google Scholar]
- McDermott DH, Colla JS, Kleeberger CA, Plankey M, Rosenberg PS, Smith ED, Zimmerman PA, Combadiere C, Leitman SF, Kaslow RA. Genetic polymorphism in CX3CR1 and risk of HIV disease. Science. 2000;290:2031. doi: 10.1126/science.290.5499.2031a. [DOI] [PubMed] [Google Scholar]
- McDermott DH, Halcox JP, Schenke WH, Waclawiw MA, Merrell MN, Epstein N, Quyyumi AA, Murphy PM. Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ Res. 2001;89:401–407. doi: 10.1161/hh1701.095642. [DOI] [PubMed] [Google Scholar]
- McDermott DH, Fong AM, Yang Q, Sechler JM, Cupples LA, Merrell MN, Wilson PW, D′Agostino RB, O′Donnell CJ, Patel DD, Murphy PM. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest. 2003;111:1241–1250. doi: 10.1172/JCI16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Kawanokuchi J, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979:65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- Moatti D, Faure S, Fumeron F, Amara Mel W, Seknadji P, McDermott DH, Debre P, Aumont MC, Murphy PM, de Prost D, Combadiere C. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97:1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- O′Brien SJ, Menotti-Raymond M, Murphy WJ, Nash WG, Wienberg J, Stanyon R, Copeland NG, Jenkins NA, Womack JE, Marshall Graves JA. The promise of comparative genomics in mammals. Science. 1999;286:458–462. 479–481. doi: 10.1126/science.286.5439.458. [DOI] [PubMed] [Google Scholar]
- Risch NJ. Searching for genetic determinants in the new millennium. Nature. 2000;405:847–856. doi: 10.1038/35015718. [DOI] [PubMed] [Google Scholar]
- Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273:1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Saunders AM, De La Paz MA, Postel EA, Heinis RM, Agarwal A, Scott WK, Gilbert JR, McDowell JG, Bazyk A. Association of the apolipoprotein E gene with age-related macular degeneration: possible effect modification by family history, age, and gender. Mol Vis. 2000;6:287–293. [PubMed] [Google Scholar]
- Seddon JM, Rosner B, Sperduto RD, Yannuzzi L, Haller JA, Blair NP, Willett W. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119:1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- Shen DF, Zhuang Z, LeHoang P, Boni R, Zheng S, Nussenblatt RB, Chan CC. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105:1664–1669. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- Simonelli F, Margaglione M, Testa F, Cappucci G, Manitto MP, Brancato R, Rinaldi E. Apolipoprotein E polymorphisms in age-related macular degeneration in an Italian population. Ophthalmic Res. 2001;33:325–328. doi: 10.1159/000055688. [DOI] [PubMed] [Google Scholar]
- Souied EH, Benlian P, Amouyel P, Feingold J, Lagarde JP, Munnich A, Kaplan J, Coscas G, Soubrane G. The epsilon4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am J Ophthalmol. 1998;125:353–359. doi: 10.1016/s0002-9394(99)80146-9. [DOI] [PubMed] [Google Scholar]
- Spraul CW, Lang GE, Grossniklaus HE, Lang GK. Histologic and morphometric analysis of the choroid, Bruch′s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999;44(Suppl 1):S10–32. doi: 10.1016/s0039-6257(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Stone EM, Braun TA, Russell SR, Kuehn MH, Lotery AJ, Moore PA, Eastman CG, Casavant TL, Sheffield VC. Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med. 2004;351:346–353. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- Tarozzo G, Campanella M, Ghiani M, Bulfone A, Beltramo M. Expression of fractalkine and its recepto CX3CR1, in response to ischaemia-reperfusion brain injury in the rat. Eur J Neurosci. 2002;15:1663–1668. doi: 10.1046/j.1460-9568.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- Taub DD, Oppenheim JJ. Chemokines, inflammation and the immune system. Ther Immunol. 1994;1:229–246. [PubMed] [Google Scholar]
- Tsutsumi C, Sonoda KH, Egashira K, Qiao H, Hisatomi T, Nakao S, Ishibashi M, Charo IF, Sakamoto T, Murata T, Ishibashi T. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74:25–32. doi: 10.1189/jlb.0902436. [DOI] [PubMed] [Google Scholar]
- Tuo J, Bojanowski CM, Chan CC. Genetic factors of age-related macular degeneration. Prog Retin Eye Res. 2004a;23:229–249. doi: 10.1016/j.preteyeres.2004.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Smith BC, Bojanowski CM, Meleth AD, Gery I, Csaky KG, Chew EY, Chan CC. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004b;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaft TL, Mooy CM, de Bruijn WC, Oron FG, Mulder PG, de Jong PT. Histologic features of the early stages of age-related macular degeneration. A statistical analysis Ophthalmology. 1992;99:278–286. doi: 10.1016/s0161-6420(92)31982-7. [DOI] [PubMed] [Google Scholar]
- Wang DG, Fan JB, Siao CJ, Berno A, Young P, Sapolsky R, Ghandour G, Perkins N, Winchester E, Spencer J. Large-scale identification, mapping, and genotyping of single-nucleotide polymorphisms in the human genome. Science. 1998;280:1077–1082. doi: 10.1126/science.280.5366.1077. [DOI] [PubMed] [Google Scholar]
- Wright AF. Strategies for mapping susceptibility genes in age-related maculopathy. Eye. 2001;15:401–406. doi: 10.1038/eye.2001.144. [DOI] [PubMed] [Google Scholar]
- Zareparsi S, Reddick AC, Branham KE, Moore KB, Jessup L, Thoms S, Smith-Wheelock M, Yashar BM, Swaroop A. Association of apolipoprotein E alleles with susceptibility to age-related macular degeneration in a large cohort from a single center. Invest Ophthalmol Vis Sci. 2004;45:1306–1310. doi: 10.1167/iovs.03-1253. [DOI] [PubMed] [Google Scholar]
- Zingg JM, Ricciarelli R, Azzi A. Scavenger receptors and modified lipoproteins: fatal attractions? IUBMB Life. 2000;49:397–403. doi: 10.1080/152165400410245. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]


