Abstract
Testicular lymphoma is a rare neoplasm of the testis that is most commonly seen in older patients. It metastasizes preferentially to extranodal sites, including the skin, central nervous system, Waldeyer ring, contralateral testis, and lung. Two case reports of patients with a history of testicular lymphoma who developed involvement of the vitreous and retina are presented. These are interesting cases as the testis, central nervous system, and eye are all immune privileged organs, which may account for occurrence of disease in these sites. Histopathologic examination of diagnostic vitrectomy specimens from both cases showed atypical lymphoid cells with immunoglobulin heavy chain (IgH) gene rearrangements, consistent with the diagnosis of intraocular B-cell lymphoma. The results of a literature review of all reports of ocular involvement with testicular lymphoma are discussed. Patients with testicular lymphoma are at risk for relapse, particularly in the central nervous system. Clinicians should be suspicious for intraocular lymphoma in patients with a history of testicular lymphoma who present with vitritis or retinal lesions.
Keywords: immune privileged organ, immunohistochemistry, intraocular lymphoma, microdissection, PCR, retina, testicular lymphoma, vitreous
Introduction
Testicular lymphoma is a rare disease entity that accounts for only 1–9% of all testicular neoplasms and 1% of all non-Hodgkin lymphomas. Although it is the most common testicular tumor in men over the age of 60, testicular lymphoma is rarely seen in younger males (< 30 years).31 Testicular lymphoma has a marked tendency to metastasize, particularly to extranodal sites, including the skin, central nervous system (CNS), Waldeyer ring, contralateral testis, and lung. Clinical reports of ocular involvement in testicular lymphoma are rare.24 We report two patients with testicular lymphoma who developed ocular involvement in the vitreous and retina, clinically mimicking primary intraocular lymphoma (PIOL). Based on a review of the literature, these cases were compared with all known reports of ocular involvement with testicular lymphoma.
Case Reports
CASE 1
A 75-year-old white man with a previous history of testicular lymphoma presented with a chief complaint of decreased vision OU in March 1998. Ocular examination revealed bilateral vitritis that was greater in the right eye. Systemic evaluation included normal blood work and a bone marrow biopsy that revealed monoclonal gammopathy of undetermined significance. Imaging studies of the brain, chest, and abdomen were all negative. Cytologic examination of a diagnostic vitrectomy specimen from the right eye was negative for malignancy in May 1999. The patient had a past medical history significant for coronary artery disease status post myocardial infarctions in 1968 and 1994, congestive heart failure, chronic obstructive pulmonary disease, and Parkinson disease. In March 1985 he was diagnosed with prostate carcinoma that was successfully treated with resection and pelvic radiation therapy. He was diagnosed with right testicular lymphoma that was treated with an orchiectomy in July 1992 followed by prophylactic orchiectomy of the left testis in August 1992 and an unknown regimen of chemotherapy. His medications included simvastatin, metoprolol, cimetidine, carbidopa/levidopa, amitriptyline, aspirin, diclofenac, vitamin E, ipratropium, albuterol, beclomethasone, and nitroglycerine as needed.
Ocular examination in March 2000 revealed a visual acuity of 20/50 OD and 20/80 OS with intraocular pressures of 16.5 mm Hg OD and 14 mm Hg OS and no afferent pupillary defect. He scored 15/16 OD and 0/16 OS on the Ishihara color plates. Anterior segment examination was normal except for the presence of 2+ nuclear sclerosis OU and a fine dusting of cells on the inferior aspect of the posterior capsule OD. Posterior segment examination of the right eye revealed trace vitreous cells without haze and no apparent retinal lesions. The optic nerve cup-to-disk ratio was 0.4. Examination of the posterior segment of the left eye revealed marked vitritis with 3+ vitreous cells and 2+ haze. Mottling of the retinal pigment epithelium and subretinal infiltrates in the posterior pole were noted in the left eye although the vitritis obscured the view of the macula (Fig. 1A). The cup-to-disk ratio of the optic nerve was 0.4.
Fig. 1.
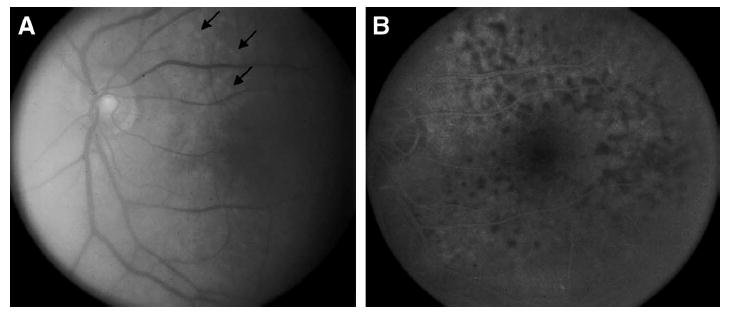
A: Fundus photograph from case 1 showing vitreous haze and subretinal infiltrates (arrows). B: Fluorescein angiogram from Case 1 showing multiple small hypofluorescent lesions.
Fluorescein angiogram of the right eye revealed no cystoid macular edema. Angiography of the left eye provided a limited view. A repeat angiogram post vitrectomy in the left eye in late March 2000, however, revealed multiple blocking defects typical of those described in patients with PIOL (Fig. 1B).6,64 Imaging studies included an MRI of the brain which revealed periventricular white matter changes consistent with small vessel ischemia, but there was no evidence of tumor. Computerized tomography of the chest, abdomen, and pelvis was unremarkable with no evidence of tumor. Examination of the cerebrospinal fluid (CSF) revealed the absence of malignant cells and no erythrocytes, although there were 25 white blood cells present, 95% of which were lymphocytes. Cytokine analysis of the CSF revealed an IL-6 level < 7.8 pg/ml (normal IL-6 level < 15.6 pg/ ml) and an IL-10 = 15.4 pg/ml (normal IL-10 level < 11.7 pg/ml) with an IL-10: IL-6 ratio of 1.97.
Histopathologic examination of the diagnostic pars plana vitrectomy specimen from the left eye revealed malignant large B lymphoid cells. Consequently, the patient returned to his referring ophthalmologist and oncologist for treatment.
CASE 2
The second case was a 61-year-old white man with a history of a stage IIa, intermediate grade, polymorphic, centroblastic non-Hodgkin lymphoma involving the left epididymis of the testis and the para-aortic lymph nodes. He received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) therapy six times, achieving complete remission after two courses, although there was persistence of the M component in the serum. A year later a hypothalamic lesion was noted on MRI. Although a specimen of the CSF was clear of malignant cells, the patient was treated with 40 Gy to the brain and eyes and six courses of intrathecal methotrexate. He was in remission for 4 years until vitreous haze was noted in his right eye. Although his bone marrow was negative for recurrence, he was treated with six cycles of chlorambucil and prednisone achieving clinical remission in the eye in February 1998. In May 1998, a recurrence was noted in the thalamus, although the CSF again was clear of malignant lymphoid cells. He again achieved remission following treatment with six cycles of methotrexate, procarbazine, CCNU, and prednisone. In January 1999, however, worsening vitritis was noted in the right eye. In February 1999, a subretinal lesion was noted in the temporal midperiphery on funduscopic examination and fluorescein angiogram (Fig. 2A and B). A pars plana vitrectomy performed in the right eye was diagnostic for a large B-cell lymphoma, as was an aspirate of the subretinal cells. The subretinal mass lesion subsided with the use of a combination of intravitreal methotrexate and dexamethasone. The patient passed away after three months from complications of radiotherapy and CNS lymphoma.
Fig. 2.
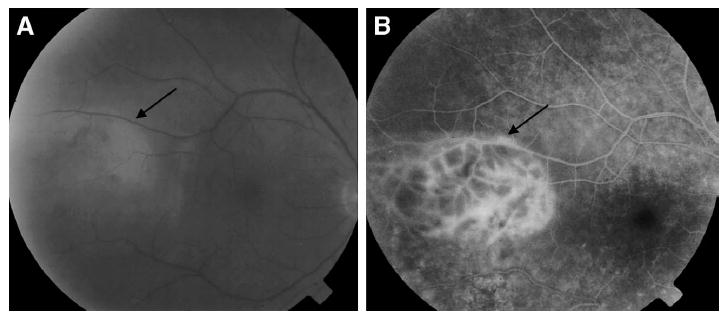
A: Fundus photograph from case 2 showing vitreous haze and a subretinal infiltrate (arrow). B: Fluorescein angiogram from case 2 showing a hyperfluorescent subretinal infiltrate (arrow).
Histocytology and Molecular Pathology
Vitrectomy specimens from both cases were submitted for cytological analysis as described previously.38 Briefly, the specimen was cytospun immediately after collection. The supernatant was submitted for cytokine analysis by ELISA technique. The cells were collected on coated slides and stained with Giemsa and conducted immunohistochemistry for CD3, CD20, kappa, and lambda using the avidin-biotin-complex staining method.
The atypical lymphoid cells were manually microdissected under light microscope as described previously.56 The microdissected cells were subjected to molecular analysis for detection of IgH gene rearrangements and the bcl-2 t(14;18) translocation. DNA was extracted and used for PCR amplification. The following three primer pairs were used for detection of immunoglobulin (IgH) gene rearrangements at the third complementarity determining region (CDR3) of the IgH region: the FR3A, sense (upstream), 5′-ACA CGG CYS TGT ATT ACT GT-3′ and antisense (downstream), 5′-GGA TGG TAT CAA GCT TTG AGG AGA CGG TGA CCA-3′; the FR2A, sense, 5′-TGG RTC CGM CAG CAG SCV YCN GG-3′ and antisense, 5′-ACC TGA GGA GAC GGT GAC C-3′; and the CDR3, sense, 5′-GR CTG CAG GCY YCC GGR AAR RGT CTG GAG TGG-3′ and antisense, 5′-TAC AGG ATC CG AGG AGA CGG TGA CC-3′ (mixed base: R = A/G, M = A/C, S = G/C, V = G/A/C, Y = C/T, N = A/T/C/G). Conditions were used as described previously.8 The primers used for detection of the bcl-2 t(14;18) translocation at the major break point region were as follows: sense: 5′–TTA GAG AGT TGC TTT ACG TGG CCT-3′ and antisense primer CFW1: 5′–ACC TGA GGA GAC GGT GAC CAG GGT–3′. The PCR amplifiable mixture contained microdissected DNA, 4 pmol 32P-labeled sense primer, 5 pmol nonlabeled antisense primer CFW1, 10 nmol dNTP, 15 nmol MgCl2, and 0.5 U AmpliTaq Gold Enzyme in a final volume of 10 μl. The cycling parameters were as follows: initial incubation at 94°C for 9 minutes; 35 cycles of 94°C for 1 minute, 55°C for 2 minutes, and 72°C for 2 minutes; and a final incubation at 72°C for 7 minutes. The polymerase chain reaction (PCR) products were separated on 3% agarose gels and visualized with autoradiography.
The results of these two cases are the following.
CASE 1
Cytopathology of the testicular biopsy specimen revealed densely packed, classical atypical lymphoid cells with mitotic figures (Fig. 3A). Cytopathology of the vitrectomy specimen revealed many atypical lymphoid cells with enlarged hyperchromatic nuclei and scant cytoplasm (Fig. 3B). Reactive lymphocytes and giant cells were also present. Immunohistochemistry of the atypical cells was positive for B cell marker CD20. These cells were negative for CD3, kappa, and lambda light chain staining.
Fig. 3.
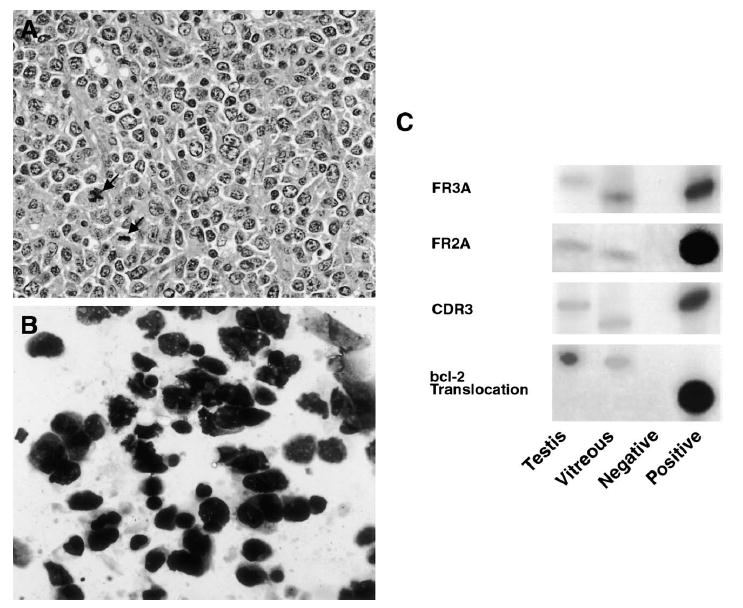
Case 1. A: Histopathology of the testicular biopsy specimen showing atypical lymphoid cells with multiple mitotic figures (arrows) (hematoxylin & eosin, original magnification, ×400). B: Cytology of the vitreous specimen showing atypical lymphoid cells with enlarged hyperchromatic nuclei and scant cytoplasm (giemsa, original magnification, ×640). C: Polymerase chain reaction amplification of microdissected cells from the testicular and vitreous specimens showing IgH gene rearrangements of the CDR3 region with three different primer sets. There are different patterns of IgH gene rearrangements at the CDR3 sites between the testicular and intraocular lymphomas. The testicular and vitreous samples were also positive for the bcl-2 t(14;18) translocation.
Cytokine analysis of the vitreous sample showed the IL-10 level to be 292 pg/ml (normal IL-10 level < 11.7 pg/ml) and that of IL-6 to be less than 15.6 pg/ml (normal IL-6 level < 15.6 pg/ml) for an IL-10:IL-6 ratio > 1, consistent with PIOL in the vitreous.7,10,69,70 Polymerase chain reaction analysis of microdissected cells from the vitrectomy specimen and of a sample from the previous testicular biopsy revealed positivity for immunoglobulin heavy chain (IgH) gene rearrangements (Fig. 3C). Different patterns of IgH gene rearrangements at the CDR3 sites suggest that the ocular and testicular clones could be distinct. The testicular and vitreous samples were also positive for the bcl-2 t(14;18) translocation (Fig. 3C).
CASE 2
Cytopathology of the second case demonstrated lymphocytes and occasional large atypical lymphoid cells with scanty cytoplasm (Fig. 4A). In addition, microdissection and PCR revealed a positive IgH gene rearrangement at the CDR3 site with the FR3A and CDR3 primers, confirming that this was an intraocular lymphoma. The vitreous sample was negative for the bcl-2 t(14;18) translocation (Fig. 4C).
Fig. 4.
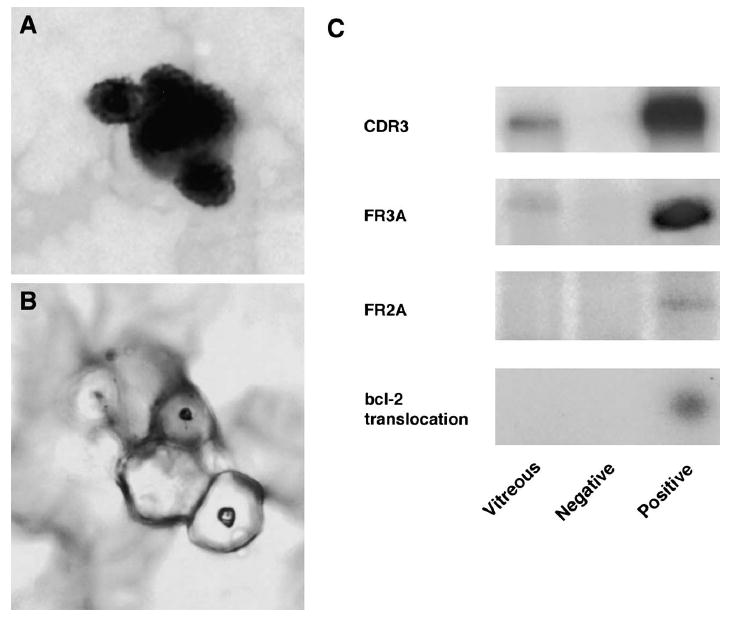
Case 2. A: Histopathology of the vitreous specimen showing large lymphoid cells with scanty cytoplasm, prior to microdissection (giemsa, original magnification, ×400). B: Histopathology of the same vitreous specimen post microdissection (giemsa, original magnification, ×400). C: Polymerase chain reaction amplification of microdissected cells from the vitreous specimen showing a positive IgH gene rearrangement at the CDR3 site with the FR3A primers. The vitreous sample was negative for the bcl-2 t(14;18) translocation.
Discussion
The finding of malignant lymphoid cells in the eyes of patients with testicular lymphoma is interesting, especially given the observation that the testis, like the brain and eye, is considered an immunoprivileged site.59 In these sites, strong blood–tissue barriers and an altered immune response allow cells including certain malignant cells expressing non-self antigens to escape destruction by the immune system.25,27,59 Immunoprivilege in these specific sites may account for the appearance and metastasis of primary B-cell lymphoma in the eye, brain, and testis (Table 1).
TABLE 1.
Summary of Characteristics of Primary Testicular Lymphoma and Primary Intraocular Lymphoma
| Primary Lymphoma | ||
|---|---|---|
| Testis | Eye (Retina/Vitreous) | |
| Immunoprivileged Site | Yes | Yes |
| Incidence among all non-Hodgkin Lymphomas | 1% | 0.2% |
| Clinical presentation | Painless mass in older man (usually unilateral) | Masquerade uveitis in elder people (frequent bilateral) |
| Morphology | Predominantly (68%) diffuse large B-cell; 30% small non-cleaved cell26,63,71 | Mostly diffuse large B-cell lympoma48 |
| Metastases | ||
| CNS | 6–16.5% | 60–80% |
| Vitreous | One reported case19 | — |
| Uvea | One reported case26 | Rare15,50,57,65,66 |
| Orbit | Three reported cases26,37,44 | Extremely rare* |
| Testis | — | None reported |
From our experience at the NEI, only 1 in 72 cases involved the orbit; this case is an AIDS-associated PIOL.
Primary malignant lymphoma of the testis was first described in 1877 and later recognized as a separate clinical entity in 1878.17,40 Primary testicular lymphoma has a proclivity to spread to unusual extranodal sites. In addition, it is also likely to involve both testicles; to relapse after clinical remission occurs; and to have a general poor prognosis.24
Testicular lymphoma is rare. As noted previously, it accounts for only 1–8% of all testicular tumors and 1% of all non-Hodgkin lymphoma.12,24,29 However, this is the most common type of testicular tumor in men older than 60 and is the most frequent secondary neoplasm affecting the testis among all age groups. On the other hand, this disease is rare in men less than 30 years of age.32 This phenomenon may be attributed to a general loss of tumor surveillance, which is hypothesized to occur with aging.16 Primary testicular lymphoma must be distinguished from secondary involvement of the testis during metastasis of systemic non-Hodgkin lymphoma, which is more common. Testicular lymphoma is usually a B-cell lymphoma, of which 68% are of the intermediate-grade diffuse large B-cell subtype. The next most frequent histology (30%) is high-grade diffuse small-noncleaved cell (Burkitt and Burkitt-like) subtypes.26,63,71
There is no predisposing cause for testicular lymphoma, although there have been reports of associations with trauma, chronic orchitis, cryptorchidism, and filiariasis.31,53,60,62 There is, however, an increased incidence in immunosuppressed patients, mainly in those positive for the human immunodeficiency virus (HIV). These patients usually present with disease at an early age (median of 37 years); have aggressive histologies (immunoblastic or small-non cleaved cell subtype); and have a worse prognosis.4,5,32
Primary lymphoma of the testis usually presents as a painless or slightly uncomfortable unilateral testicular mass in an older man. Other urologic symptoms are absent.24,55 Constitutional symptoms, including fever, weight loss, anorexia, night sweats, and weakness, are present in 25–41% of patients.44,47 On examination, a non-tender firm mass is present and testicular size may vary from normal to 16 cm.24,53 At presentation, 60–70% of patients have disease localized to the testicle alone or the testicle and pelvic or abdominal lymph nodes.13 Spread to the epididymus, spermatic cord, and scrotal skin is also common.26,31 Bilateral testicular lymphomas occur in 19.5% of patients.49
Metastasis to unusual extranodal sites is quite common, and these metastases may be present at initial disease presentation or develop later in the disease course. These sites include the CNS, Waldeyer ring, skin, and lung. The prostate, kidney, liver, bone marrow, pleura, scrotal skin, and bone are also involved less commonly.55 The CNS is the most common site of metastasis with an incidence ranging from 6% to 16.5%. As in primary CNS lymphomas, patients with CNS involvement may present with headache, cranial nerve palsy, focal motor weakness, sensory deficits, and gait abnormalities. Lymphomas that progress to invade the CNS are usually of the lymphoblastic and diffuse undifferentiated subtypes.51,63,71 Thus, examination of the CSF should be performed due to the high incidence of CNS involvement.24,55 Patients with skin involvement, which usually manifests as purplish to reddish brown firm papules, nodules, tumors, or plaques, also have a poor prognosis.3 Patients with involvement of Waldeyer ring and surrounding oropharynx and nasopharynx usually present with nasal obstruction and frequent ear infections. Involvement of Waldeyer ring is not associated with a worse prognosis.55
Treatment of testicular lymphoma is complex and dependent on the initial characteristics of the patient. An orchiectomy should be performed, especially in stage I and stage II disease. An orchiectomy is advantageous because it provides tissue for pathological evaluation and removes a sanctuary site, as the blood–testis barrier makes testicular tumors inaccessible to systemic chemotherapy.55 Although long-term survival can be achieved in stage IE disease with orchiectomy alone, most patients relapse within the first 2 years, therefore an adjunctive chemotherapy or radiotherapy regimen is necessary. Adjunctive radiotherapy alone leads to relapse rates of 50%.55 On the other hand, the benefits of combination chemotherapy regimen is supported by the Danish Lymphoma Study group which found a relapse rate of 15% and a median relapse free survival of 28 months for Stage IE or IIE patients who received combination chemotherapy after orchiectomy compared to a relapse rate of 64% and a median survival of 14 months for patients treated with orchiectomy with or without adjuvant RT.43 The benefits of adjuvant chemotherapy have been corroborated by other studies.14,61,73 A multiagent chemotherapy regimen that includes doxorubicin, cyclophosphamide, vincristine, and a corticosteroid is usually administered.13 Prophylactic regimens of radiotherapy to the scrotum and intrathecal chemotherapy have also been considered.55
There have been five documented cases of primary testicular lymphoma relapsing in the eye.19,27,37,45 The first reported cases detailing ocular involvement in patients with malignant lymphoma of the testis were included by Ferry et al in a case series of 69 patients. He briefly mentioned two patients whose stage IV testicular lymphomas presented to the orbit and two patients who relapsed in the eye, one in the orbit and one in the uveal tract of both eyes.26 There were no further details provided about these patients’ clinical presentations or specifics of their immunopathologies. Inui et al presented a case of a Japanese male who presented with uveitis and hypopyon who upon evaluation had a serum IgD monoclonal gammopathy which led to a diagnosis of diffuse poorly differentiated lymphocytic lymphoma of the testis and orbit.37 Mullaney described a 66-year-old man who presented with a conjunctival nodule in July 1988 after a history of orchiectomy in April 1988 for seminoma. Conjunctival biopsy revealed small B-cell lymphoma. He presented a month later with orbital lymphoma. Because of this unusual finding, the patient’s testicular biopsy was reviewed and the diagnosis was revised to lymphoma.45 The orbital and uveal involvement in these four cases most likely resulted from hematogenous metastasis, thus distinguishing these cases from PIOL that presents in the retina and vitreous and usually does not involve the choroid.
Davis et al presented a case report of a 60-year-old white man whose bilateral testicular lymphoma, initially involving retroperitoneal lymph nodes, metastasized to the vitreous.19 He was initially diagnosed with a bilateral malignant testicular lymphoma, high-grade, B-cell type, diffuse large cell, and clinical stage IIE. He refused orchiectomy and was treated with 6 cycles of CHOP therapy and 40 Gy to both testes and the retroperitoneal lymph nodes. The patient achieved clinical remission for 52 months, until he noted changes in his visual acuity. Right vitreous washings showed intermediate to high-grade B-cell lymphoma with kappa light chain restriction and positive staining for CD19 and CD20. Imaging studies, scrotal ultrasound, bone marrow biopsy, and lumbar puncture were all negative. The patient chose to be treated with radiotherapy of 38 Gy to both eyes. He had no evidence of disease progression at 3 months of follow-up.19 Although this represents an interesting case as it is the first description of intraocular relapse of malignant testicular lymphoma, the authors also noted that this was a rare case where low-grade testicular lymphoma was treated with multiagent chemotherapy and radiation therapy as opposed to orchiectomy, the usual recommended treatment.19
Like testicular lymphoma, PIOL is also a rare disease (Table 1). Primary intraocular lymphoma is a subset of primary central nervous system lymphoma (PCNSL), a diffuse large B-cell non-Hodgkin lymphoma that can originate in the brain, spinal cord, leptomeninges, or eye. Both PCNSL and PIOL may rarely present as T-cell lymphomas.35,48 In PIOL, malignant lymphoid cells initially invade the retina, vitreous, or optic nerve head, with or without concomitant CNS involvement.9,36 Primary intraocular lymphoma is a distinct disease entity from primary orbital lymphoma (MALT lymphoma) and from systemic non-Hodgkin lymphomas that usually metastasize to the uveal tissues in the eye and thus are commonly seen in the choroid.
The incidence of PIOL has increased greatly over the past 15 years due to the concurrent rise in the incidence of PCNSL. primary central nervous system lymphoma’s incidence has increased in both immunocompetent and immunocompromised people from 0.027/100,000 in 1973 to 1/100,000 in the early 1990s.54 Ocular involvement is seen in 15–25% of patients with PCNSL, and 60–80% of patients who are initially diagnosed with PIOL go on to develop CNS disease within a mean of 29 months.2,22,30,33,68 Ocular disease is bilateral in 80% of cases.30
Because of the nonspecific findings on examination, the diagnosis of PIOL is difficult and often requires a median of 4.3 diagnostic procedures.35,52,68 Due to the link to CNS disease, patients with suspected PIOL should receive a thorough medical and neurological examination, neuroimaging of the brain and orbits, and a lumbar puncture. Cerebrospinal fluid should be sent for routine cytological, chemistry, and cytokine analysis. If no evidence of disease is found by neuroimaging or CSF examination, then a diagnostic vitrectomy should be performed to identify the lymphoma cells.
Because of the difficulty in making a pathologic diagnosis of PIOL based on the morphology of the often rare tumor cells, other diagnostic methods have been developed. Immunohistochemistry and flow cytometry have revealed that intraocular lymphomas are monoclonal populations of B-lymphocytes that stain positively for B-cell markers (CD19, CD20, CD22) and have restricted kappa and lambda light chain expression.18,39,68 In addition, microdissection and PCR have allowed for isolation of a relatively pure cell population from tissue samples. These techniques have been used to reveal rearranged IgH gene sequences in the CDR3 of the IgH variable region, which serves as a molecular marker of clonal expansion of lymphocytes and an indication of malignancy.1,11 Microdissection and PCR have also been used to detect the bcl-2 t(14;18) translocation. Bcl-2, a gene involved in the regulation of apoptosis, is located on chromosome 18, whereas IgH is located on chromosome 14.34 The t(14;18) translocation brings the bcl-2 gene under the control of the IgH promoter, thus deregulating the bcl-2 gene and resulting in Bcl-2 expression.46 This translocation is found in 85% of follicular non-Hodgkin lymphomas and 28% of DLBCLs.67,72
Cytokine analysis can also be used as an adjunctive tool to diagnose PIOL. IL-10 is produced by malignant B-lymphocytes in PIOL and PCNSL, whereas IL-6 is mainly produced by inflammatory cells in uveitis. Thus PIOL is associated with an elevated IL-10 level and an increased IL-10 to IL-6 ratio (< 1.0).7,10,42,69,70
The best method of treatment for patients with PIOL or PCNSL with ocular involvement is yet to be determined. Radiation once was the most commonly used treatment for PIOL, however, delayed complications have made this a less favorable mode of treatment.30,41 Currently, more patients are being treated with combined chemotherapy/radiotherapy or chemotherapy-alone regimens using high-dose methotrexate. High-dose methotrexate regimens lead to complete response rates of 50–80%, while combination chemotherapy/radiotherapy regimens lead to a median survival of 40 months, with 25% surviving 5 months or more.21,23 Intravitreal methotrexate for isolated and recurrent ocular disease has also been shown to have favorable results.20,28,58
We report two additional patients with testicular lymphoma involving the eye whose clinical presentation was similar to that of PIOL. Both of these patients presented with vitreous cells and subretinal infiltrates. With the use of similar pathologic and molecular techniques to those used in the diagnosis of PIOL, we are able to show that both of these patients had lymphoma in their vitreous and retina. In both patients, microscopic examination of vitrectomy specimens revealed malignant lymphoid cells. In addition, IgH gene rearrangements were documented in both cases. In case 1, where the testicular specimen was available we were able to perform microdissection and PCR on this sample as well. Both had differing IgH gene rearrangement patterns indicating that these may be distinct clonal populations of cells. Because of the small quantity of cells obtained by this technique we were unable to perform sequencing analysis on these specimens to confirm this finding. Of note, the bcl-2 t(14;18) translocation was detected in case one although it was not present in the lymphoma cells of the second patient. Finally, in case 1, cytokine analysis of the CSF revealed an elevated level of IL-10 and an IL-10: IL-6 ratio greater than 1 similar to that found in patients with PIOL and PCNSL.
Summary
Testicular lymphoma is a rare disease that typically presents in older patients. Although treatment is helpful in controlling disease initially, there is a strong risk of relapse, particularly in the CNS. There has been one previous case report documenting vitreal involvement in this disease, and we have presented two additional cases of metastases to the retina and vitreous. Because of the immunoprivileged status of testis and the eye, these two cases may suggest that primary lymphoma develops in multiple immune privileged organs at different times or kinetics; however, further studies are needed to prove this hypothesis. Clinicians should be suspicious for intraocular relapse of testicular lymphoma in patients with a history of testicular lymphoma who present with vitritis or retinal lesions.
Method of Literature Search
The search engine PubMed was used to search the database Medline of the National Library of Medicine from 1966 to 2004. The search engine Science Direct was used to search EMBASE from 1974 to 2004. The search engine Web of Science was used to search Science Citation Index from 1980 to the present. Earlier sources were obtained by cross referencing. Subject searches were conducted for testicular lymphoma, intraocular lymphoma, CNS lymphoma, diffuse large B cell lymphoma, ocular, intraocular, testis, and eye. All articles judged to be of clinical importance and additional references of key articles were included.
Footnotes
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Abdel-Reheim FA, Edwards E, Arber DA. Utility of a rapid polymerase chain reaction panel for the detection of molecular changes in B-cell lymphoma. Arch Pathol Lab Med. 1996;120:357–63. [PubMed] [Google Scholar]
- 2.Akpek EK, Ahmed I, Hochberg FH, et al. Intraocular-central nervous system lymphoma: clinical features, diagnosis, and outcomes. Ophthalmology. 1999;106:1805–10. doi: 10.1016/S0161-6420(99)90341-X. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Winkelmann RK. Lymphosarcoma of the skin and testis. Arch Dermatol. 1960;82:943–7. doi: 10.1001/archderm.1960.01580060097014. [DOI] [PubMed] [Google Scholar]
- 4.Armenakas NA, Schevchuk MM, Brodherson M, et al. AIDS presenting as primary testicular lymphoma. Urology. 1992;40:162–4. doi: 10.1016/0090-4295(92)90519-3. [DOI] [PubMed] [Google Scholar]
- 5.Buzelin F, Karam G, Moreau A, et al. Testicular tumor and the acquired immunodeficiency syndrome. Eur Urol. 1994;26:71–6. doi: 10.1159/000475346. [DOI] [PubMed] [Google Scholar]
- 6.Cassoux N, Merle-Beral H, Leblond V, et al. Ocular and central nervous system lymphoma: clinical features and diagnosis. Ocul Immunol Inflamm. 2000;8:243–50. doi: 10.1076/ocii.8.4.243.6463. [DOI] [PubMed] [Google Scholar]
- 7.Cassoux N, Merle-Beral H, Lehoang P, et al. Interleukin-10 and intraocular-central nervous system lymphoma. Ophthalmology. 2001;108:426–7. doi: 10.1016/s0161-6420(00)00401-2. [DOI] [PubMed] [Google Scholar]
- 8.Chan CC. Molecular pathology of primary intraocular lymphomas. Trans Am Ophthalmol Soc. 2003;101:43–60. [PMC free article] [PubMed] [Google Scholar]
- 9.Chan CC, Buggage RR, Nussenblatt RB. Intraocular lymphoma. Curr Opin Ophthalmol. 2002;13:411–8. doi: 10.1097/00055735-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Chan CC, Whitcup SM, Solomon D, et al. Interleukin-10 in the vitreous of patients with primary intraocular lymphoma. Am J Ophthalmol. 1995;120:671–3. doi: 10.1016/s0002-9394(14)72217-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen YT, Whitney KD, Chen Y. Clonality analysis of B-cell lymphoma in fresh-frozen and paraffin-embedded tissues: the effects of variable polymerase chain reaction parameters. Mod Pathol. 1994;7:429–34. [PubMed] [Google Scholar]
- 12.Collins DH, Pugh RCB. Classification and frequency of testicular tumors. Br J Urol. 1964;36:1–11. [PubMed] [Google Scholar]
- 13.Connors JM. Problems in lymphoma management: special sites of presentation. Oncology. 1998;12:185–91. discussion 192–5. [PubMed] [Google Scholar]
- 14.Connors JM, Klimo P, Voss N, et al. Testicular lymphoma: improved outcome with early brief chemotherapy. J Clin Oncol. 1988;6:776–81. doi: 10.1200/JCO.1988.6.5.776. [DOI] [PubMed] [Google Scholar]
- 15.Corriveau C, Easterbrook M, Payne D. Lymphoma simulating uveitis (masquerade syndrome) Can J Ophthalmol. 1986;21:144–9. [PubMed] [Google Scholar]
- 16.Cui Z, Willingham MC. The effect of aging on cellular immunity against cancer in SR/CR mice. Cancer Immunol Immunother. 2004;53:473–8. doi: 10.1007/s00262-003-0488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curling TB, editor. A practical treatise on diseases of the testis . 4 London: Churchill; 1878. [Google Scholar]
- 18.Davis JL, Solomon D, Nussenblatt RB, et al. Immunocytochemical staining of vitreous cells. Indications, techniques, and results. Ophthalmology. 1992;99:250–6. doi: 10.1016/s0161-6420(92)31984-0. [DOI] [PubMed] [Google Scholar]
- 19.Davis JW, Moriarty RP, Schlossberg SM, et al. Bilateral testicular lymphoma treated with chemotherapy and radiation without orchiectomy: complete response relapsed at 52 months in the vitreous humor. Urology. 2001;57:555. doi: 10.1016/s0090-4295(00)01022-0. [DOI] [PubMed] [Google Scholar]
- 20.de Smet MD, Vancs VS, Kohler D, et al. Intravitreal chemotherapy for the treatment of recurrent intraocular lymphoma. Br J Ophthalmol. 1999;83:448–51. doi: 10.1136/bjo.83.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeAngelis LM. Primary CNS lymphoma: treatment with combined chemotherapy and radiotherapy. J Neurooncol. 1999;43:249–57. doi: 10.1023/a:1006258619757. [DOI] [PubMed] [Google Scholar]
- 22.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–23. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 23.DeAngelis LM, Yahalom J, Thaler HT, et al. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10:635–43. doi: 10.1200/JCO.1992.10.4.635. [DOI] [PubMed] [Google Scholar]
- 24.Doll DC, Weiss RB. Malignant lymphoma of the testis. Am J Med. 1986;81:515–24. doi: 10.1016/0002-9343(86)90308-6. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson TA, Green DR, Griffith TS. Cell death and immune privilege. Int Rev Immunol. 2002;21:153–72. doi: 10.1080/08830180212058. [DOI] [PubMed] [Google Scholar]
- 26.Ferry JA, Harris NL, Young RH, et al. Malignant lymphoma of the testis, epididymis, and spermatic cord. A clinicopathologic study of 69 cases with immunophenotypic analysis. Am J Surg Pathol. 1994;18:376–90. doi: 10.1097/00000478-199404000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Filippini A, Riccioli A, Padula F, et al. Control and impairment of immune privilege in the testis and in semen. Hum Reprod Update. 2001;7:444–9. doi: 10.1093/humupd/7.5.444. [DOI] [PubMed] [Google Scholar]
- 28.Fishburne BC, Wilson DJ, Rosenbaum JT, et al. Intravitreal methotrexate as an adjunctive treatment of intraocular lymphoma. Arch Ophthalmol. 1997;115:1152–6. doi: 10.1001/archopht.1997.01100160322009. [DOI] [PubMed] [Google Scholar]
- 29.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–60. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Freeman LN, Schachat AP, Knox DL, et al. Clinical features, laboratory investigations, and survival in ocular reticulum cell sarcoma. Ophthalmology. 1987;94:1631–9. doi: 10.1016/s0161-6420(87)33256-7. [DOI] [PubMed] [Google Scholar]
- 31.Gowing NFC. Malignant lymphoma of the testis . In: Pugh RCB, editor. Pathology of the Testis. London: Blackwell Scientific; 1976. pp. 334–55. [Google Scholar]
- 32.Haddy TB, Sandlund JT, Magrath IT. Testicular involvement in young patients with non-Hodgkin’s lymphoma. Am J Pediatr Hematol Oncol. 1988;10:224–9. doi: 10.1097/00043426-198823000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg. 1988;68:835–53. doi: 10.3171/jns.1988.68.6.0835. [DOI] [PubMed] [Google Scholar]
- 34.Hockenbery D, Nuñez G, Milliman C, et al. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–6. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman PM, McKelvie P, Hall AJ, et al. Intraocular lymphoma: a series of 14 patients with clinicopathological features and treatment outcomes. Eye. 2003;17:513–21. doi: 10.1038/sj.eye.6700378. [DOI] [PubMed] [Google Scholar]
- 36.Hormigo A, DeAngelis LM. Primary ocular lymphoma: clinical features, diagnosis, and treatment. Clin Lymphoma. 2003;4:22–9. doi: 10.3816/clm.2003.n.010. [DOI] [PubMed] [Google Scholar]
- 37.Inui J, Nakamine H, Nishihara T, et al. Extranodal lymphoma terminating in acute leukemia associated with IgD monoclonal gammopathy. A case report. Cancer. 1984;53:2487–90. doi: 10.1002/1097-0142(19840601)53:11<2487::aid-cncr2820531121>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 38.Levy-Clarke GA, Byrnes GA, Buggage RR, et al. Primary intraocular lymphoma diagnosed by fine needle aspiration biopsy of a subretinal lesion. Retina. 2001;21:281–4. doi: 10.1097/00006982-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 39.Lopez JS, Chan CC, Burnier M, et al. Immunohistochemistry findings in primary intraocular lymphoma. Am J Ophthalmol. 1991;112:472–4. doi: 10.1016/s0002-9394(14)76269-5. [DOI] [PubMed] [Google Scholar]
- 40.Malassez M. Lymphadenome Due Testicule. Bull Soc Anta. 1877;52:176–8. [Google Scholar]
- 41.Margolis L, Fraser R, Lichter A, et al. The role of radiation therapy in the management of ocular reticulum cell sarcoma. Cancer. 1980;45:688–92. doi: 10.1002/1097-0142(19800215)45:4<688::aid-cncr2820450412>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 42.Merle-Béral H, Davi F, Cassoux N, et al. Biological diagnosis of primary intraocular lymphoma. Br J Haematol. 2004;124:469–73. doi: 10.1046/j.1365-2141.2003.04800.x. [DOI] [PubMed] [Google Scholar]
- 43.Møller MB, d’Amore F, Christensen BE. Testicular lymphoma: a population-based study of incidence, clinicopathological correlations and prognosis. The Danish Lymphoma Study Group, LYFO. Eur J Cancer. 1994;30A:1760–4. doi: 10.1016/0959-8049(94)00311-r. [DOI] [PubMed] [Google Scholar]
- 44.Mostofi FK. Secondary tumors initially manifest as testicular neoplasms. In: Mostofi FK, Price EB Jr, editors. Tumors of the Male Genital System. Washington, DC: Armed Forces Institute of Pathology; 1973. pp. 131–6. [Google Scholar]
- 45.Mullaney J. Peculiar ophthalmic proliferations. Eye. 1990;4(Pt 1):79–88. doi: 10.1038/eye.1990.9. [DOI] [PubMed] [Google Scholar]
- 46.Ngan BY, Chen-Levy Z, Weiss LM, et al. Expression in non-Hodgkin’s lymphoma of the bcl-2 protein associated with the t(14;18) chromosomal translocation. N Engl J Med. 1988;318:1638–44. doi: 10.1056/NEJM198806233182502. [DOI] [PubMed] [Google Scholar]
- 47.Paladugu RR, Bearman RM, Rappaport H. Malignant lymphoma with primary manifestation in the gonad: a clinicopathologic study of 38 patients. Cancer. 1980;45:561–71. doi: 10.1002/1097-0142(19800201)45:3<561::aid-cncr2820450324>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 48.Paulus W. Classification, pathogenesis and molecular pathology of primary CNS lymphomas. J Neurooncol. 1999;43:203–8. doi: 10.1023/a:1006242116122. [DOI] [PubMed] [Google Scholar]
- 49.Poulsen MG, Roberts SJ, Taylor K. Testicular lymphoma—the need for a new approach. Australas Radiol. 1991;35:257–60. doi: 10.1111/j.1440-1673.1991.tb03019.x. [DOI] [PubMed] [Google Scholar]
- 50.Raju VK, Green WR. Reticulum cell sarcoma of the uvea. Ann Ophthalmol. 1982;14:555–60. [PubMed] [Google Scholar]
- 51.Read G. Lymphomas of the testis-results of treatment 1960–77. Clin Radiol. 1981;32:687–92. doi: 10.1016/s0009-9260(81)80340-6. [DOI] [PubMed] [Google Scholar]
- 52.Rothova A, Ooijman F, Kerkhoff F, et al. Uveitis masquerade syndromes. Ophthalmology. 2001;108:386–99. doi: 10.1016/s0161-6420(00)00499-1. [DOI] [PubMed] [Google Scholar]
- 53.Sampat MB, Sirsat MV, Kamat MR. Malignant lymphoma of the testis in Indians. Br J Urol. 1974;46:569–75. doi: 10.1111/j.1464-410x.1974.tb03858.x. [DOI] [PubMed] [Google Scholar]
- 54.Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol. 1999;43:199–201. doi: 10.1023/a:1006290032052. [DOI] [PubMed] [Google Scholar]
- 55.Shahab N, Doll DC. Testicular lymphoma. Semin Oncol. 1999;26:259–69. [PubMed] [Google Scholar]
- 56.Shen DF, Zhuang Z, LeHoang P, et al. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105:1664–9. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- 57.Siegel MJ, Dalton J, Friedman AH, et al. Ten-year experience with primary ocular ‘reticulum cell sarcoma’ (large cell non-Hodgkin’s lymphoma) Br J Ophthalmol. 1989;73:342–6. doi: 10.1136/bjo.73.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith JR, Rosenbaum JT, Wilson DJ, et al. Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmology. 2002;109:1709–16. doi: 10.1016/s0161-6420(02)01125-9. [DOI] [PubMed] [Google Scholar]
- 59.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–89. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 60.Sussman EB, Hajdu SI, Lieberman PH, et al. Malignant lymphoma of the testis: a clinicopathologic study of 37 cases. J Urol. 1977;118:1004–7. doi: 10.1016/s0022-5347(17)58277-4. [DOI] [PubMed] [Google Scholar]
- 61.Touroutoglou N, Dimopoulos MA, Younes A, et al. Testicular lymphoma: late relapses and poor outcome despite doxorubicin-based therapy. J Clin Oncol. 1995;13:1361–7. doi: 10.1200/JCO.1995.13.6.1361. [DOI] [PubMed] [Google Scholar]
- 62.Turley HK, Moore TD. Malignant lymphoma primarily manifested as a testicular tumor. J Urol. 1952;68:744–6. doi: 10.1016/S0022-5347(17)68275-2. [DOI] [PubMed] [Google Scholar]
- 63.Turner RR, Colby TV, MacKintosh FR. Testicular lymphomas: a clinicopathologic study of 35 cases. Cancer. 1981;48:2095–102. doi: 10.1002/1097-0142(19811101)48:9<2095::aid-cncr2820480930>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 64.Velez G, Chan CC, Csaky KG. Fluorescein angiographic findings in primary intraocular lymphoma. Retina. 2002;22:37–43. doi: 10.1097/00006982-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Velez G, de Smet MD, Whitcup SM, et al. Iris involvement in primary intraocular lymphoma: report of two cases and review of the literature. Surv Ophthalmol. 2000;44:518–26. doi: 10.1016/s0039-6257(00)00118-1. [DOI] [PubMed] [Google Scholar]
- 66.Vogel MH, Font RL, Zimmerman LE, et al. Reticulum cell sarcoma of the retina and uvea. Report of six cases and review of the literature. Am J Ophthalmol. 1968;66:205–15. doi: 10.1016/0002-9394(68)92065-5. [DOI] [PubMed] [Google Scholar]
- 67.Weiss LM, Warnke RA, Sklar J, et al. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987;317:1185–9. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- 68.Whitcup SM, de Smet MD, Rubin BI, et al. Intraocular lymphoma. Clinical and histopathologic diagnosis. Ophthalmology. 1993;100:1399–406. doi: 10.1016/s0161-6420(93)31469-7. [DOI] [PubMed] [Google Scholar]
- 69.Whitcup SM, Stark-Vancs V, Wittes RE, et al. Association of interleukin 10 in the vitreous and cerebrospinal fluid and primary central nervous system lymphoma. Arch Ophthalmol. 1997;115:1157–60. doi: 10.1001/archopht.1997.01100160327010. [DOI] [PubMed] [Google Scholar]
- 70.Wolf LA, Reed GF, Buggage RR, et al. Vitreous cytokine levels. Ophthalmology. 2003;110:1671–2. doi: 10.1016/S0161-6420(03)00811-X. [DOI] [PubMed] [Google Scholar]
- 71.Woolley PV, Osborne CK, Levi JA, et al. Extranodal presentation of non-Hodgkin’s lymphomas in the testis. Cancer. 1976;38:1026–35. doi: 10.1002/1097-0142(197608)38:2<1026::aid-cncr2820380256>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 72.Yunis JJ, Oken MM, Kaplan ME, et al. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin’s lymphoma. N Engl J Med. 1982;307:1231–6. doi: 10.1056/NEJM198211113072002. [DOI] [PubMed] [Google Scholar]
- 73.Zietman AL, Coen JJ, Ferry JA, et al. The management and outcome of stage IAE nonHodgkin’s lymphoma of the testis. J Urol. 1996;155:943–6. [PubMed] [Google Scholar]


