Summary
The small GTPase Arf6 regulates multiple cellular processes including endocytosis, secretion, phagocytosis, cell adhesion and cell migration [1, 2]. ACAP1 is a GTPase activating protein specific for Arf6 and functions as an important negative regulator of Arf6-mediated signals [3–7]. However, how other cellular proteins regulate ACAP1 and Arf6 mediated signaling is not well understood. GULP/CED-6 is an evolutionarily conserved PTB-domain containing adapter protein initially identified for its role in engulfment of apoptotic cells [8–13], and subsequently shown to function in cholesterol homeostasis in cells [14]. Here, we identify a novel role for GULP as a positive regulator of Arf6-mediated signaling. Knockdown of endogenous GULP resulted in decreased cellular Arf6-GTP level, while overexpression of GULP increased cellular Arf6-GTP. At the mechanistic level, GULP was found to influence Arf6 at four levels. First, GULP binds directly to GDP-bound Arf6 via its PTB domain in vitro and in cells. Second, GULP was found in a complex with ACAP1, an Arf6 specific GAP, at endogenous levels of the two proteins. Third, GULP countered the actions of ACAP1 at a biochemical level by reversing the decrease in Arf6-GTP induced by ACAP1, and at a functional level by reversing the ACAP1-mediated inhibition of cell migration. Fourth, GULP, ACAP1 and GDP-bound Arf6 were part of a trimeric complex, with evidence for cooperative binding, suggesting the sequestration of ACAP1 as one mechanism of GULP action. Taken together, these data identify GULP as a novel and important regulator of cellular Arf6-GTP, as well as a regulator of ACAP1. Since multiple PTB domain containing adapter proteins have been shown to regulate endocytosis/trafficking of membrane proteins/cell migration [15, 16], our data support a model wherein PTB domain containing adapter proteins regulate Arf family proteins.
Results and Discussion
Direct binding of GULP to Arf6
Our previous studies on GULP and its potential role in endocytosis [14] prompted us to examine a link between GULP and Arf6 mediated signaling. When we tested a potential GULP:Arf6 interaction, bacterially produced full length GULP was able to precipitate GFP-tagged Arf6 from cell lysates. GULP bound two mutant forms, Arf6T27N and Arf6T44N that preferentially bind GDP. No detectable binding was observed toward Arf6Q67L that is preferentially GTP bound [17] (Figure 1A). GULP did not bind other small GTPases such as Rac1, RhoG or Cdc42 either in the preferentially GTP bound (QL mutants) or GDP bound (TN mutants) forms (Figure 1B). GULP also precipitated endogenous Arf6 from cell lysates (Figure 1C, lane 1).
Figure 1. GULP binds to Arf6 and regulates endogenous Arf6-GTP level.
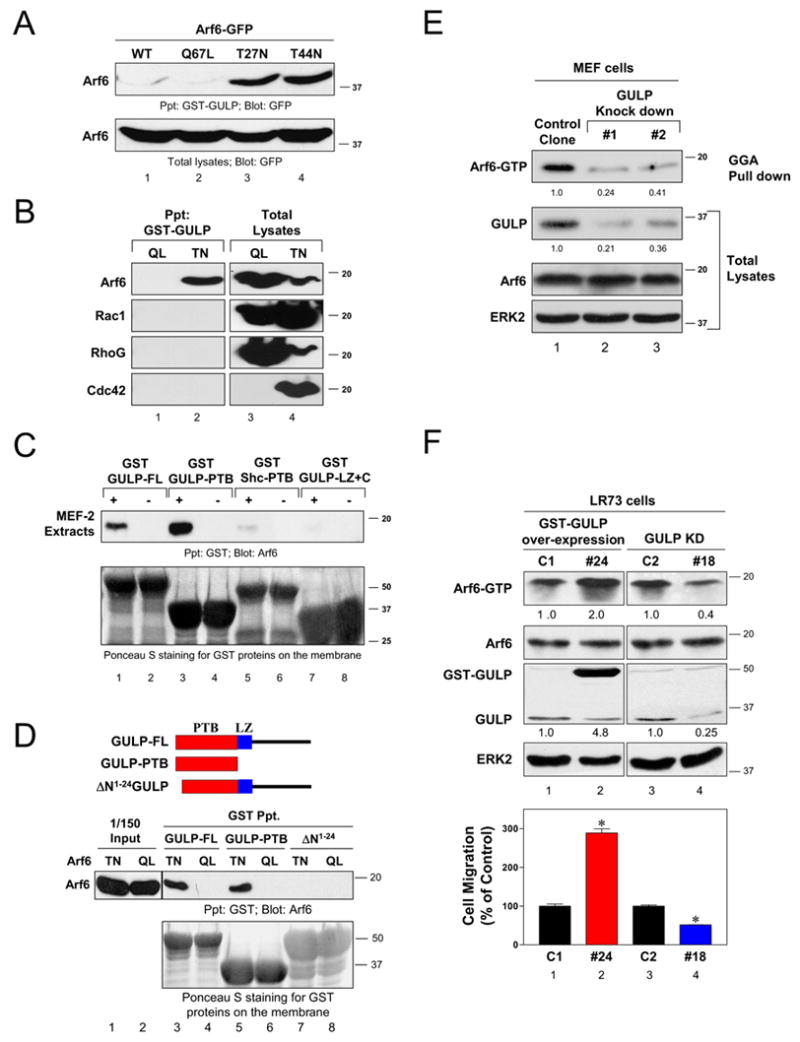
(A) GULP binds GDP-Arf6. GFP-tagged wild-type Arf6 or the mutants expressed in HeLa cells, were precipitated with bacterially produced GST-GULP and assessed for binding. Comparable expression of the Arf6 proteins in total lysates is shown (bottom). (B) GULP binds specifically to Arf6. Q → L or T → N mutants of Arf6-HA, Flag-Rac1, Flag-RhoG or Myc-Cdc42 were expressed in HeLa cells and their precipitation with GST-GULP was assessed by immunoblotting for the individual epitope-tagged GTPases. (C) GULP interacts with endogenous Arf6. GST-tagged full length GULP (GULP-FL), GULP-PTB, GULP-LZ+C, or the Shc-PTB domain were incubated with MEF-2 cell lysates and Arf6 binding was detected by immunoblotting. The GST fusion proteins were visualized by Ponceau S staining. (D) GULP directly binds Arf6 via the PTB domain. Schematic diagram of GULP and the mutants (top). Arf6T27N and Arf6Q67L mutants were incubated with GULP (all bacterially produced), and binding assessed by anti-Arf6 immunoblotting. GST-GULP was visualized by Ponceau S staining. ΔN1–24 mutant lacks the first 24 amino acids GULP-PTB domain. A line indicates the lanes from the same gel that were not run contiguously, but spliced together for presentation. (E) Knockdown of GULP expression decreases endogenous Arf6-GTP in cells. Arf6-GTP levels in two of the GULP knockdown MEF-1 clones and a control clone were assessed by a GST-GGA pull down assay. Comparable Arf6 expression in the cell lines was revealed by immunoblotting. ERK2 immunoblotting revealed equal protein loading. Arf6-GTP levels were compared after setting the ratio of the Arf6-GTP signal to total Arf6 signal in the control clone as 1.0. Relative GULP expression was compared after setting the ratio of GULP signal to ERK2 signal in the control clone as 1.0. (F) Knockdown or overexpression of GULP in LR73 cells affects cellular Arf6-GTP and cellular migration. An arf6-GTP level in cells with GULP overexpression or knockdown was assessed as in E. Cell migration to fibronectin was don in a Transwell assay. * indicates P<0.05 (n=3) compared to the respective control clone.
GULP is composed of an N-terminal phosphotyrosine-binding domain followed immediately by a leucine zipper (LZ) domain, and a C-terminal region of 100 amino acids with no obvious domains (Figure 1D, top) [18]. The GULP-PTB domain was able to precipitate endogenous Arf6 (Figure 1C, lane 3). Under these conditions, the LZ+C region of GULP or the PTB domain of another adapter Shc did not appreciably precipitate endogenous Arf6 (Figure 1C). To further examine whether the GULP:Arf6 interaction is direct, we produced recombinant Arf6T27N or Arf6Q67L versions in bacteria. We first confirmed that Arf6Q67L was GTP-bound by a GST-GGA pull down assay, and Arf6T27N was not precipitated by GGA (Supplemental Figure S1). Both full length GULP and isolated PTB domain of GULP bound specifically to the Arf6T27N, but not the Arf6Q67L. Thus, the binding of GULP to Arf6 occurred preferentially to the GDP bound form of Arf6. A mutant of GULP that lacks the first 24 amino acids of the PTB domain severely impaired GULP interaction with Arf6 (Figure 1D). These data suggested a novel interaction between GULP-PTB and Arf6 and a possible role for GULP in regulating Arf6 function.
GULP regulates Arf6-GTP level in cells
We then asked whether GULP would regulate Arf6-GTP level in cells. We knockdown GULP expression in MEF-1 cells or LR73 cells and assessed the effect of GULP depletion on endogenous Arf6-GTP. Compared to control MEF-1 cells, cells depleted of endogenous GULP showed a dramatic reduction in cellular Arf6-GTP (as determined by GGA-mediated precipitation of Arf6-GTP) (Figure 1E). No reduction was observed in total Arf6 protein or a control protein, ERK2, in the same cell lysates (Figure 1E). Similar reduction in Arf6-GTP was also observed in LR73 cells after knockdown of GULP. As a corollary, overexpression of full length GULP in LR73 cells led to increased Arf6-GTP. These data suggested a key role for GULP in regulating endogenous Arf6 activation. Since Arf6 can regulate cell migration, we assessed whether GULP-mediated regulation of Arf6-GTP level would influence migration of LR73 cells. Compared to control LR73 cells, GULP overexpression promoted cell migration toward fibronectin, while knockdown of GULP reduced cell migration (Figure 1F).
GULP binds the Arf6-GAP ACAP1 and influences cellular Arf6-GTP level
The effect of GULP on cellular Arf6-GTP level could be either direct or indirect. We tested whether GULP itself might directly promote GTP loading of Arf6. However, neither full length GULP nor the GULP-PTB domain affected the rate or magnitude of GTP bound to Arf6, suggesting no direct effect of GULP on GTP loading of Arf6 (Supplemental Figure S2).
Another mechanism by which GULP could positively influence the Arf6-GTP level in cells might be through its association with guanine nucleotide exchange factors (GEFs) for Arf6. However, we failed to detect an interaction of GULP either with ARNO or EFA6, two known Arf6-specific GEFs [19, 20] (Figure 2A). Since GULP itself did not promote GTP loading of Arf6 and did not appear to bind an Arf6-GEF, we considered whether GULP might bind an Arf6-GAP; in such a case, through negative regulation of the Arf-GAP activity GULP could indirectly increase the Arf6-GTP level. When we tested several known GAPs, GULP specifically bound ACAP1, but not other Arf6-GAPs such as ACAP2, Git1 or Git2 (Figure 2A). We then tested whether GULP and ACAP1 could form a complex at endogenous level of expression. Endogenous ACAP1 was coprecipitated with endogenous GULP from MEF-1 cells, but not from cells in which GULP was depleted by RNAi (Figure 2B). To further test whether GULP and ACAP1 bind directly, we expressed a His-tagged ACAP1 fragment encoding amino acids 264–741 in bacteria. This ACAP1 fragment associated with bacterially produced GULP (Figure 2C, lane3), suggesting a direct interaction between these two proteins.
Figure 2. GULP binds to the Arf6-GAP ACAP1 and reverses ACAP1 mediated downregulation of Arf6-GTP level in cells.
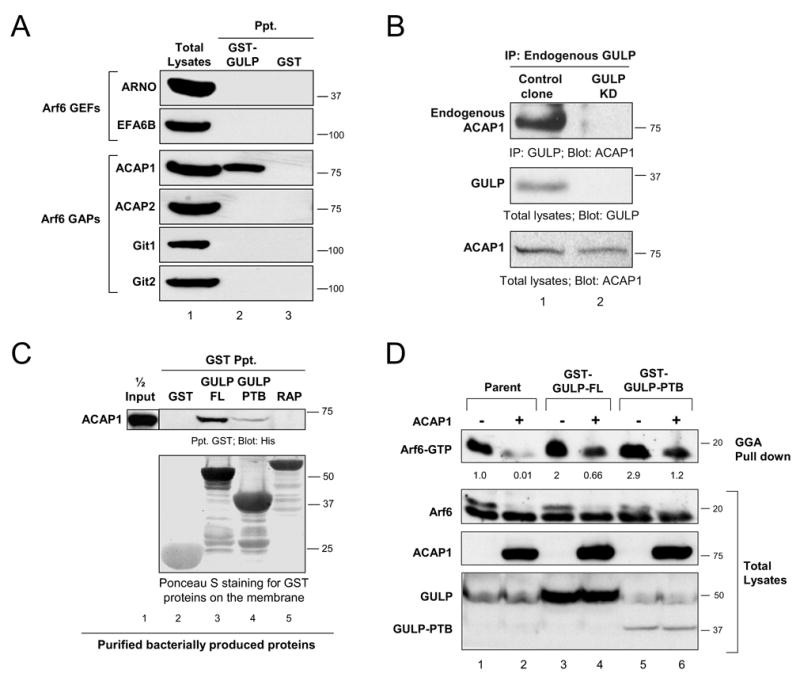
(A) GULP binds specifically to ACAP1 but not other Arf6-GAPs or Arf6-GEFs. Epitope-tagged versions of Arf6-GEFs or the Arf6-GAPs were expressed in HeLa cells and precipitated with bacterial GST-GULP or GST alone and the binding assessed by immunoblotting. (B) Interaction between endogenous GULP and ACAP1. Lysates from control or GULP-knockdown MEF-1 cells were immunoprecipitated with anti-GULP and immunoblotted for ACAP-1. GULP knockdown and comparable ACAP1 in total cell lysates were confirmed by immunoblotting. (C)GULP directly binds ACAP1 via the PTB domain. Recombinant, bacterially produced His-tagged ACAP1 fragment was incubated with bacterially produced GST-GULP, GST-alone or an unrelated protein GST-RAP. The bound ACAP1 was detected by anti-His immunoblotting. (D) Partial reversal of the ACAP1 mediated inhibition of endogenous Arf6-GTP by GULP and GULP-PTB. Parental LR73 cells or LR73 cells stably expressing GST-tagged full length GULP or GULP-PTB domain were transiently transfected with Flag-ACAP1 or empty vector. The endogenous Arf6-GTP levels were assessed as in 1E.
We then asked whether the GULP:ACAP1 interaction influences Arf6-GTP level in cells. For this, we used parental LR73 cells or LR73 cells stably expressing full length GULP (denoted GULP-FL). Expression of GULP-FL enhanced the Arf6-GTP level by two-fold relative to parental cells (Figure 2D, lane 3) as seen previously (Figure 1F). To test the effect of ACAP1 in regulating Arf6-GTP and how this might be regulated by GULP, we transfected ACAP1 into different LR73 cell lines. Overexpression of ACAP1 in parental LR73 cells strongly reduced the basal level of Arf6-GTP. However, this effect of ACAP1 was partially reversed by overexpressing the GULP-FL (Figure 2D, compare lane 2 versus 4). The effect of GULP on ACAP1 appeared specific, as coexpression of GULP with other Arf-GAPs ACAP2 or AGAP1 did not affect the cellular Arf6-GTP level (Supplemental Figure S3). Thus, GULP could counter the effect of ACAP1 and thereby increase the level of Arf6-GTP in cells.
GULP reverses the inhibition of cell migration induced by ACAP1
We then tested a functional link between GULP and ACAP1 in a cell migration assay. Migration of HeLa cells toward serum in a Boyden chamber assay was severely inhibited by expression of ACAP1 and this inhibition was dose-dependent (Figure 3A, lanes 3, 5 and 7). Although overexpression of GULP alone did not enhance migration (lane 2), GULP coexpression partially reversed the inhibition due to ACAP1 at all three doses of ACAP1 tested (Figure 3A, lanes 4, 6 and 8). The expression levels of the transfected ACAP1 correlated with the dose of plasmids cotransfected, and GULP was comparably expressed in the different conditions (Figure 3A, bottom panels).
Figure 3. GULP regulates ACAP1 mediated inhibition of cell migration.
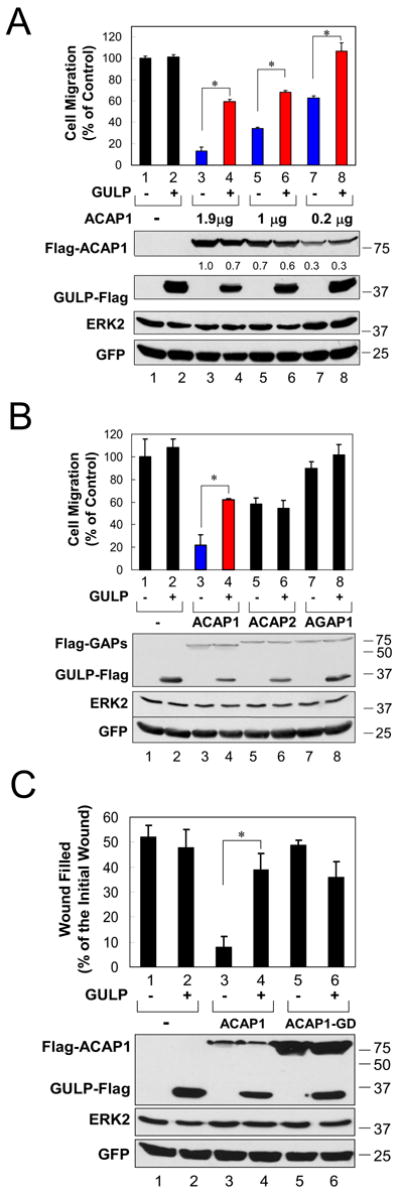
(A) Reversal of ACAP1 mediated inhibition of cellular migration by GULP is dose dependent. HeLa cells were co-transfected with a GULP plasmid and decreasing amounts of ACAP1 plasmid. A control GFP expressing plasmid was included in all of the transfections. The migration of cells in a Transwell assay toward 20% FBS was assessed. Results were standardized against the condition in which only GFP and a control plasmid were transfected. Expression of transfected ACAP1 and GULP, as well as the GFP and ERK2 levels (to ensure equal loading of cells into the upper chamber of the Transwell) was assessed by immunoblotting. Relative ACAP1 expression levels were compared after setting the ratio of the ACAP1 signal to GFP signal in lane 3 only as 1.0. * P<0.05 (unpaired t test, n=3). (B) GULP specifically regulates ACAP1 inhibition of cellular migration. HeLa cells were co-transfected with the indicated plasmids and cell migration was assessed as in Figure 3A. * P<0.05 (unpaired t test, n=3). (C) GULP regulates ACAP1-mediated inhibition of wound healing. HeLa cells transfected with a GULP plasmid and wild-type ACAP1 or GAP-deficient mutant (ACAP1-GD) were examined in the wound-healing assay. * P<0.05 (unpaired t test, n=3).
We then assessed whether this effect of GULP on ACAP1 was specific. Inhibition of cell migration by another Arf6-GAP ACAP2 [3], which does not bind GULP, was not affected by GULP coexpression (Figure 3B, lanes 5 and 6). Moreover, overexpression of AGAP1, an Arf1-specific GAP [21], did not significantly alter cell migration and was also not affected by coexpression of GULP (Figure 3B, lanes 7 and 8). Moreover, the ability of GULP to reverse the effect of ACAP1 was inhibited by coexpression of a dominant negative Arf6T27N (data not shown).
We also tested the effect of GULP to reverse the ACAP1 mediated inhibition of cell migration using a wound-healing assay. Overexpression of ACAP1 severely inhibited the ability of cells to fill the wound; coexpression of GULP reversed the effect of ACAP1 and restored wound closure to near control levels (Figure 3C, compare lane 3 vs. 4). This effect of ACAP1 was dependent on its Arf6-GAP activity, as a GAP-deficient ACAP1 mutant did not affect migration, and coexpression of GULP did not alter wound closure (Figure 3C, lanes 5 and 6). Taken together, these data reveal that GULP can functionally counter the effects of ACAP1 that rely on its GAP activity.
The PTB domain of GULP regulates ACAP1 via binding to its GAP and Ankyrin repeats
We then addressed the region of GULP required for binding to ACAP1. Endogenous ACAP1 was precipitated with the recombinant PTB domain of GULP, but not the LZ+C region of GULP or a control protein, RAP (Figure 4A). Thus, the PTB domain of GULP appeared both necessary and sufficient for binding to ACAP1.
Figure 4. Defining GULP:ACAP1 interaction and the formation of trimeric complex between GULP, ACAP1 and Arf6.
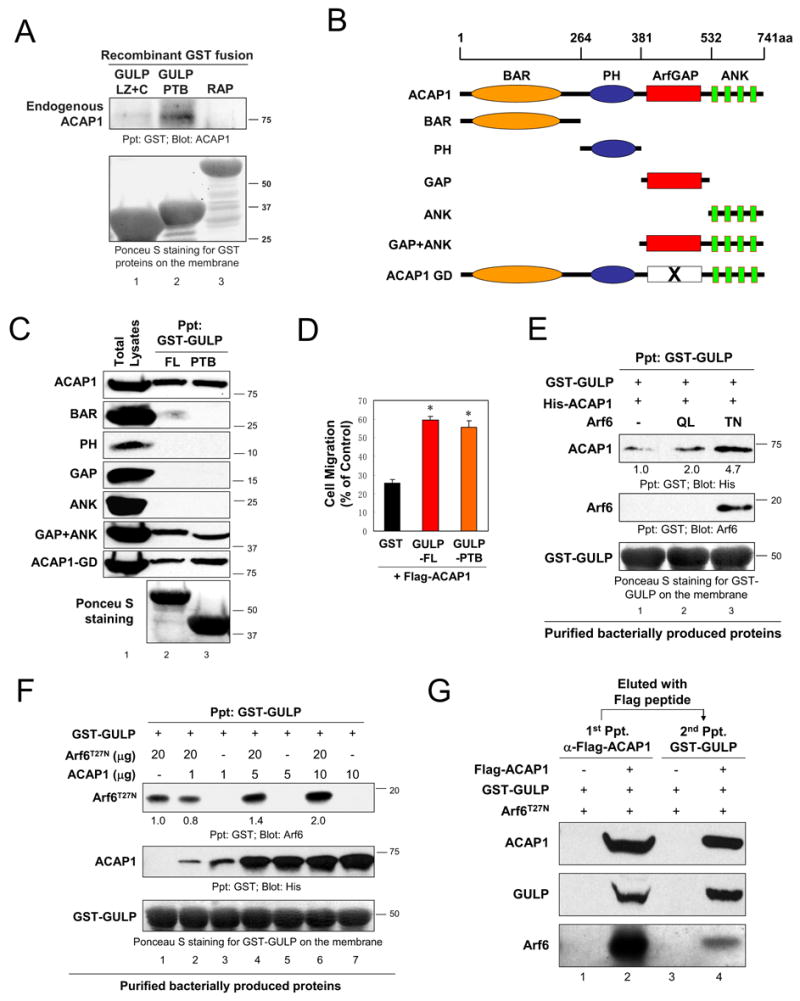
(A) GULP-PTB is necessary and sufficient for binding to ACAP1. GST-tagged GULP PTB, GULP LZ+C or an unrelated protein RAP was incubated with HeLa cell lysates and the binding of endogenous ACAP1 was assessed by immunoblotting. (B) Schematic diagrams of ACAP1 mutants with an N-terminal Flag tag. (C) GULP binds to the GAP+Ankyrin repeats of ACAP1. Full length ACAP1 or the mutants expressed in HeLa cells were tested for binding to GULP or GULP-PTB. The GST-GULP proteins on the membranes were visualized by Ponceau S staining. (D) GULP-PTB partially rescues ACAP1 mediated inhibition of cell migration. GST alone, GST-tagged GULP or GULP-PTB was co-transfected with ACAP1 into HeLa cells. Cell migration across the Transwell membrane was assessed as in Figure 3A. * indicate statistical significance (unpaired t test, P<0.05, n=4). (E, F, G) Trimeric complex formation among GULP, Arf6 and ACAP1. (E) Arf6 mutants were incubated with GST-GULP bacterially produced bound to glutathione beads. Recombinant His-ACAP1 was then added and the bound ACAP1 assessed. Precipitated ACAP1 levels were compared after setting the ACAP1 signal in the condition of GST-GULP +ACAP1 as 1.0 (lane 1). (F) Indicated amounts of bacterially produced recombinant Arf6T27N and increasing amount of His-ACAP1 proteins were mixed with bacterially produced GST-GULP bound to glutathione beads. Arf6 levels were compared after setting the Arf6 signal in the GST-GULP +Arf6 T27N only as 1.0 (lane 1). (G) Trimeric complex formation between GULP, ACAP1 and Arf6. Flag-tagged ACAP1 was incubated with GST-GULP and Arf6T27N. After washing and elution with Flag peptide, a second precipitation using glutathione beads was performed, and the ACAP1 and Arf6 proteins precipitated with GST-GULP were assessed by immunoblotting.
We then examined which region(s) of ACAP1 was required for GULP binding. ACAP1 possesses sequentially a BAR domain, PH domain, Arf-GAP domain and a set of Ankyrin repeats (Figure 4B). We generated plasmid constructs encoding the various domains of ACAP1. When the binding of the individual domains was assessed, we found that a construct encoding both the GAP domain and Ankyrin repeats of ACAP1 bound to both GULP-FL and the PTB domain (Figure 4C). Since neither the isolated GAP domain nor the Ankyrin repeats detectably bound GULP, it is possible that GULP might bind both domains simultaneously, or the Ankyrin repeats might confer a conformation on the GAP domain that favours its binding to GULP. In fact, previous crystal structure studies of other Arf-GAPs revealed extensive interface between the GAP domain and Ankyrin repeats [22]. Similarly, bacterially produced GULP-PTB was also able to bind bacterially produced fragment of ACAP1 containing the GAP+Ankyrin repeats (Figure 2C, lane 4), although this interaction was less efficient than binding of ACAP1 from mammalian cells. Notably, GULP-FL and the GULP-PTB bound comparably to both wild type ACAP1 and the GAP-deficient mutant of ACAP1 (ACAP1-GD) (Figure 4C), suggesting that GAP activity is not a requirement for binding of GULP to ACAP1.
Since the PTB domain alone is necessary and sufficient to bind the GAP +Ankyrin repeats of ACAP1, we tested the effect of GULP-PTB on Arf6-GTP level in LR73 cells. Stable expression of GULP-PTB resulted in a 2.9 fold increase in Arf6-GTP compared to parental LR73 cells (Figure 2D, lane 5). Moreover, the PTB domain alone reversed the effect of ACAP1 and increased the level of Arf6-GTP in these cells (Figure 2D, lane 6). Functionally, PTB domain of GULP was able to reverse the inhibition of cell migration due to ACAP1, comparable to full length GULP (Figure 4D). These data suggest that the PTB domain of GULP is important for regulation of ACAP1 function in vivo.
Trimeric complex formation between GULP, ACAP1 and GDP-Arf6
The binding of both Arf6 and ACAP1 to the PTB domain of GULP raised the possibility that Arf6 and ACAP1 might compete with each other for binding to GULP, or alternatively, all three proteins could be part of the same complex. Using the purified bacterial versions of GULP, ACAP1 and Arf6T27N, we tested the complex formation by mixing all three proteins simultaneously, or by sequential addition. We could detect a complex between GULP, ACAP1 and Arf6T27N (Figure 4E and 4F). The GTP-bound Arf6Q67L had no effect on the trimeric complex formation. When we increased the amount of ACAP1 added, the binding of Arf6T27N to GULP was enhanced in a dose-dependent fashion (Figure 4F). Moreover, the amount of ACAP1 binding to GULP is significantly enhanced by initial formation of a GULP:Arf6T27N complex (Figure 4E). Taken together, the binding of ACAP1 or Arf6 to GULP does not inhibit the binding of the other, and that there is a cooperative effect in the formation of the GULP:ACAP1:Arf6 complex.
We also tested the tripartite complex formation by sequential precipitations of FLAG-ACAP followed by elution of the complex and re-precipitation with GULP; this definitively demonstrated the GULP:ACAP1:Arf6 complex formation (Figure 4G). Moreover, when we coexpressed all three proteins in cells, the level of GULP coprecipitated with ACAP1 was increased three fold in the presence of exogenous Arf6T27N (data not shown). This result together with the in vitro data using the purified proteins, suggest that the complex formation between GULP:ACAP1:GDP-bound Arf6 could serve as one mechanism by which GULP could sequester ACAP1 and thereby regulate the function of ACAP1.
While downregulating Arf6 signaling via Arf6-GAPs is important for regulation of various Arf6-dependent cellular processes [1, 5], how Arf6-GAPs are regulated is not well understood. The data presented here identifies GULP as a novel regulator of the Arf6-GAP ACAP1 at the endogenous level of these proteins, and that removal of this GULP-mediated regulation of ACAP1 can adversely affect the cellular Arf6-GTP. This, in turn correlates with the effect of GULP in countering ACAP1 mediated inhibition of cell migration. One possible interpretation of the binding data between GULP and ACAP1 is that the GULP PTB domain binds to the GAP and Ankyrin repeats and inhibits GAP activity. Our in vitro assays to test this possibility have been inconclusive, either when we added recombinant GULP to ACAP1 isolated from eukaryotic cells or the bacterially produced fragment of ACAP1 (data not shown). Since GULP can clearly reverse the ACAP1 mediated decrease in endogenous Arf6-GTP level in cells, it is possible that the GULP regulation of ACAP1 function might be more complex, or might require better in vitro reagents that are yet to be developed. Nevertheless, the trimeric complex formation between GULP, ACAP1 and Arf6T27N suggests that the sequestration of ACAP1 (as part of a complex with GDP-bound Arf6) is one mechanism for GULP-mediated regulation of ACAP1, although a direct inhibition of ACAP1 GAP activity is still formally possible.
A large class of PTB domain containing adapters including Dab1, Dab2, ARH, Numb and GULP modulate endocytosis of cell surface receptors as well as intracellular movement of lipids such as cholesterol [15]; yet, whether these adapters couple to Arf family proteins that also regulate endocytosis/trafficking has not been explored. Our data provide the first evidence for a biochemical and functional link between these two classes of proteins. Although a short fragment of X11/MINT PTB domain was part of the clone isolated in a yeast two-hybrid screen with GTP-Arf3/4, this lacked much of the PTB domain, and the interaction was ascribed to the PDZ domains [23]. While the PTB domains were initially named for their binding to phosphotyrosine-containing NPXY motifs, PTB domains can bind other types of ligands [24, 25]. We have shown that GULP-PTB can recognize non-phosphorylated ψXNPXY motifs [18]; however, the precise motifs recognized by GULP for binding to ACAP1 and Arf6 are unclear. Moreover the ability of GULP to engage Arf6 and ACAP1 simultaneously via its PTB domain suggests a more complicated type of recognition that remains to be established. However, given the large number of PTB domain containing proteins that have been shown to play a role in trafficking of receptors and their bound cargos, our identification of the PTB-mediated association of GULP with Arf6 and a key intracellular regulator of Arf6 (ACAP1), have broad implications for multiple cellular processes.
Supplementary Material
Supplemental Figure S1. Characterization of the recombinant Arf6T27N and Q67L mutants. Bacterially produced and purified Arf6T27N and Arf6Q67L mutant proteins were incubated with GST or GST-GGA bound to glutathione beads. The bound Arf6 proteins were detected by rabbit anti-Arf6 immunoblotting. The GST and the GST-GGA on the membrane were visualized by Ponceau S staining. A line indicates the lanes from the same gel that were not run contiguously, but spliced together for presentation.
Supplemental Figure S2. GULP does not affect GTP binding to Arf6 in vitro. To test whether GULP might directly regulate binding of GTP to Arf6, purified myristoylated wild-type Arf6 was incubated with [32P]GTPγS in the presence or absence of recombinant GST-GULP or GST-GULP-PTB domain. The reaction was continued for 10 or 30 min. GTPγS bound to Arf6 protein was determined by a nitrocellulose filter binding assay.
Supplemental Figure S3. GULP specifically regulates ACAP1, but not ACAP2-mediated inhibition of endogenous Arf6-GTP level. HeLa cells were co-transfected with equal amount of plasmids encoding Flag-tagged Arf GAPs (ACAP1, ACAP2 or AGAP1) and GULP-Flag or a control vector. The endogenous Arf6-GTP levels were assessed by GST-GGA pull down and immunoblotting for Arf6. Comparable levels of endogenous Arf6, transfected GULP and Arf GAPs in the total lysates were confirmed by immunoblotting. Arf6-GTP levels determined by densitometry were compared after setting the ratio of the Arf6-GTP signal to total Arf6 signal in the control cells as 1.0 (lane 1).
Acknowledgments
The authors thank the members of the Ravichandran and Casanova labs for helpful discussions and advice, and for comments on the manuscript. The authors also thank Lorraine Santy and Scott Walk for technical advice. This work was supported by grants from the NIH (GM069998 to K.S.R.). Z. M. was supported by a postdoctoral fellowship from the Immunology Training Grant and Infectious Diseases/Biodefense Training Grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson JG, Honda A. Localization and function of Arf family GTPases. Biochem Soc Trans. 2005;33:639–642. doi: 10.1042/BST0330639. [DOI] [PubMed] [Google Scholar]
- 3.Jackson TR, Brown FD, Nie Z, Miura K, Foroni L, Sun J, Hsu VW, Donaldson JG, Randazzo PA. ACAPs are arf6 GTPase-activating proteins that function in the cell periphery. J Cell Biol. 2000;151:627–638. doi: 10.1083/jcb.151.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai J, Li J, Bos E, Porcionatto M, Premont RT, Bourgoin S, Peters PJ, Hsu VW. ACAP1 promotes endocytic recycling by recognizing recycling sorting signals. Dev Cell. 2004;7:771–776. doi: 10.1016/j.devcel.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Randazzo PA, Hirsch DS. Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal. 2004;16:401–413. doi: 10.1016/j.cellsig.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Ballif BA, Powelka AM, Dai J, Gygi SP, Hsu VW. Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta1 to control cell migration. Dev Cell. 2005;9:663–673. doi: 10.1016/j.devcel.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Nie Z, Randazzo PA. Arf GAPs and membrane traffic. J Cell Sci. 2006;119:1203–1211. doi: 10.1242/jcs.02924. [DOI] [PubMed] [Google Scholar]
- 8.Liu QA, Hengartner MO. Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans. Cell. 1998;93:961–972. doi: 10.1016/s0092-8674(00)81202-7. [DOI] [PubMed] [Google Scholar]
- 9.Liu QA, Hengartner MO. Human CED-6 encodes a functional homologue of the Caenorhabditis elegans engulfment protein CED-6. Curr Biol. 1999;9:1347–1350. doi: 10.1016/s0960-9822(00)80061-5. [DOI] [PubMed] [Google Scholar]
- 10.Smits E, Van Criekinge W, Plaetinck G, Bogaert T. The human homologue of Caenorhabditis elegans CED-6 specifically promotes phagocytosis of apoptotic cells. Curr Biol. 1999;9:1351–1354. doi: 10.1016/s0960-9822(00)80062-7. [DOI] [PubMed] [Google Scholar]
- 11.Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–867. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Kinchen JM, Cabello J, Klingele D, Wong K, Feichtinger R, Schnabel H, Schnabel R, Hengartner MO. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature. 2005;434:93–99. doi: 10.1038/nature03263. [DOI] [PubMed] [Google Scholar]
- 13.Su HP, Brugnera E, Van Criekinge W, Smits E, Hengartner M, Bogaert T, Ravichandran KS. Identification and characterization of a dimerization domain in CED-6, an adapter protein involved in engulfment of apoptotic cells. J Biol Chem. 2000;275:9542–9549. doi: 10.1074/jbc.275.13.9542. [DOI] [PubMed] [Google Scholar]
- 14.Kiss RS, Ma Z, Nakada-Tsukui K, Brugnera E, Vassiliou G, McBride HM, Ravichandran KS, Marcel YL. The lipoprotein receptor-related protein-1 (LRP) adapter protein GULP mediates trafficking of the LRP ligand prosaposin, leading to sphingolipid and free cholesterol accumulation in late endosomes and impaired efflux. J Biol Chem. 2006;281:12081–12092. doi: 10.1074/jbc.M600621200. [DOI] [PubMed] [Google Scholar]
- 15.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uhlik MT, Temple B, Bencharit S, Kimple AJ, Siderovski DP, Johnson GL. Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J Mol Biol. 2005;345:1–20. doi: 10.1016/j.jmb.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Macia E, Luton F, Partisani M, Cherfils J, Chardin P, Franco M. The GDP-bound form of Arf6 is located at the plasma membrane. J Cell Sci. 2004;117:2389–2398. doi: 10.1242/jcs.01090. [DOI] [PubMed] [Google Scholar]
- 18.Su HP, Nakada-Tsukui K, Tosello-Trampont AC, Li Y, Bu G, Henson PM, Ravichandran KS. Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP) J Biol Chem. 2002;277:11772–11779. doi: 10.1074/jbc.M109336200. [DOI] [PubMed] [Google Scholar]
- 19.Franco M, Peters PJ, Boretto J, van Donselaar E, Neri A, D'Souza-Schorey C, Chavrier P. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. Embo J. 1999;18:1480–1491. doi: 10.1093/emboj/18.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank S, Upender S, Hansen SH, Casanova JE. ARNO is a guanine nucleotide exchange factor for ADP-ribosylation factor 6. J Biol Chem. 1998;273:23–27. doi: 10.1074/jbc.273.1.23. [DOI] [PubMed] [Google Scholar]
- 21.Nie Z, Stanley KT, Stauffer S, Jacques KM, Hirsch DS, Takei J, Randazzo PA. AGAP1, an endosome-associated, phosphoinositide-dependent ADP-ribosylation factor GTPase-activating protein that affects actin cytoskeleton. J Biol Chem. 2002;277:48965–48975. doi: 10.1074/jbc.M202969200. [DOI] [PubMed] [Google Scholar]
- 22.Mandiyan V, Andreev J, Schlessinger J, Hubbard SR. Crystal structure of the ARF-GAP domain and ankyrin repeats of PYK2-associated protein beta. Embo J. 1999;18:6890–6898. doi: 10.1093/emboj/18.24.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill K, Li Y, Bennett M, McKay M, Zhu X, Shern J, Torre E, Lah JJ, Levey AI, Kahn RA. Munc18 interacting proteins: ADP-ribosylation factor-dependent coat proteins that regulate the traffic of beta-Alzheimer's precursor protein. J Biol Chem. 2003;278:36032–36040. doi: 10.1074/jbc.M301632200. [DOI] [PubMed] [Google Scholar]
- 24.Farooq A, Zhou MM. PTB or not to be: promiscuous, tolerant and Bizarro domains come of age. IUBMB Life. 2004;56:547–557. doi: 10.1080/15216540400013895. [DOI] [PubMed] [Google Scholar]
- 25.Seet BT, Dikic I, Zhou MM, Pawson T. Reading protein modifications with interaction domains. Nat Rev Mol Cell Biol. 2006;7:473–483. doi: 10.1038/nrm1960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Characterization of the recombinant Arf6T27N and Q67L mutants. Bacterially produced and purified Arf6T27N and Arf6Q67L mutant proteins were incubated with GST or GST-GGA bound to glutathione beads. The bound Arf6 proteins were detected by rabbit anti-Arf6 immunoblotting. The GST and the GST-GGA on the membrane were visualized by Ponceau S staining. A line indicates the lanes from the same gel that were not run contiguously, but spliced together for presentation.
Supplemental Figure S2. GULP does not affect GTP binding to Arf6 in vitro. To test whether GULP might directly regulate binding of GTP to Arf6, purified myristoylated wild-type Arf6 was incubated with [32P]GTPγS in the presence or absence of recombinant GST-GULP or GST-GULP-PTB domain. The reaction was continued for 10 or 30 min. GTPγS bound to Arf6 protein was determined by a nitrocellulose filter binding assay.
Supplemental Figure S3. GULP specifically regulates ACAP1, but not ACAP2-mediated inhibition of endogenous Arf6-GTP level. HeLa cells were co-transfected with equal amount of plasmids encoding Flag-tagged Arf GAPs (ACAP1, ACAP2 or AGAP1) and GULP-Flag or a control vector. The endogenous Arf6-GTP levels were assessed by GST-GGA pull down and immunoblotting for Arf6. Comparable levels of endogenous Arf6, transfected GULP and Arf GAPs in the total lysates were confirmed by immunoblotting. Arf6-GTP levels determined by densitometry were compared after setting the ratio of the Arf6-GTP signal to total Arf6 signal in the control cells as 1.0 (lane 1).


