Abstract
NgR1, NgR2, and NgR3 which constitute the Nogo-66 receptor family are primarily expressed by neurons in the central nervous system (CNS) and believed to limit axonal growth and sprouting following CNS injury. In an attempt to define the expression and decipher the function of individual members of the Nogo-66 receptor family, we previously reported the generation of selective rabbit polyclonal antibodies. Here we exploit the same immune repertoires by phage display technology to generate rabbit monoclonal antibodies (mAbs) with nanomolar affinity to epitopes that are specific for NgR1 and NgR2, respectively, but at the same time conserved between mouse, rat, and human orthologs. Employing phage display vector pC3C, a newly designed phagemid optimized for the generation and selection of Fab libraries with human constant domains, rabbit mAbs were selected from chimeric rabbit/human Fab libraries, characterized in terms of specificity, affinity, and amino acid sequence, and finally converted to chimeric rabbit/human IgG. Using immunofluorescence microscopy and immunoprecipitation, we demonstrate strong and specific recognition of cell surface bound Nogo-66 receptor family members by chimeric rabbit/human IgG. The rabbit mAbs reported here together with their amino acid sequences constitute a defined panel of species cross-reactive reagents in infinite supply which will aid investigations toward a functional role of the Nogo-66 receptor family in and beyond the CNS.
Keywords: Rabbit monoclonal antibodies, b9 allotype, chimeric rabbit/human Fab, chimeric rabbit/human IgG, phage display, Nogo-66 receptor, NgR1, NgR2, RTN4R, RTN4RL2
1. Introduction
In the CNS of higher vertebrates, myelin impairs axonal regeneration of injured neurons. Several myelin proteins that inhibit axonal growth have been identified, including Nogo-A (Buchli and Schwab, 2005). An extracellular 66-amino acids domain of Nogo-A termed Nogo-66 mediates inhibitory activity by binding to the Nogo-66 receptor (NgR1), a neuronal glycosylphosphatidyl-inositol (GPI)-anchored, leucine-rich repeat (LRR) glycoprotein (Fournier et al., 2001). Two other myelin proteins with documented inhibitory activity, myelin associated glycoprotein (MAG) and oligodendrocyte myelin glycoprotein (OMgp), also bind to NgR1 (McGee and Strittmatter, 2003). NgR1 (gene name RTN4R) and two structurally related glycoproteins, NgR2 (RTN4RL2) and NgR3 (RTN4RL1), constitute the Nogo-66 receptor family (Barton et al., 2003; Lauren et al., 2003; Pignot et al., 2003). NgR2 and NgR3, like NgR1, are neuronal GPI-anchored, LRR glycoproteins widely expressed in the CNS. In spite of structural homology with NgR1 and broadly overlapping expression in the CNS, NgR2 and NgR3 do not bind Nogo-66. MAG, on the other hand, can mediate axonal growth inhibition through binding to NgR2 (Venkatesh et al., 2005). The MAG/NgR2 interaction is of stronger affinity than the MAG/NgR1 interaction, and recombinant soluble NgR2 antagonizes the axonal growth inhibition of MAG and CNS myelin. NgR1 and NgR2 have thus emerged as key coordinators of the inhibitory activity of CNS myelin. To further define the expression and decipher the function of NgR1 and NgR2 in mouse, rat, and human systems, as well as to provide a defined panel of reagents in infinite supply to the research community, we set out to develop species cross-reactive rabbit mAbs with strong affinity and high selectivity for NgR1 and NgR2, respectively.
MAbs have been generated by hybridoma technology since the 1970s. In contrast to mouse mAbs, which were generated through mouse/mouse hybridomas long before the conception of phage display, difficulties in generating rabbit/rabbit hybridomas (Spieker-Polet et al., 1995) gave impetus to developing phage display strategies for the generation of rabbit mAbs (Ridder et al., 1995). The use of display technologies, such as phage display, which physically link mAb phenotype and genotype (Rader, 2001; Hoogenboom, 2005) allows selection for desired specificities from antibody libraries. The generation of rabbit mAbs through phage display has now been established in many laboratories. In addition, rabbit mAbs from an improved fusion cell line for rabbit/rabbit hybridoma generation are now available through an increasing number of vendors. What is the appeal of rabbit mAbs? The rabbit antibody repertoire, which in the form of polyclonal antibodies has been utilized for decades, is an outstanding source for antibodies that feature strong affinity and high specificity (Mage et al., 2006). In addition, rabbits, which belong to the order Lagomorpha, are evolutionarily distant from mice and rats, which belong to the order Rodentia. As a consequence, epitopes conserved between rodent and human antigens that are invisible to rodent mAbs (and also human mAbs generated from transgenic mice with human immunoglobulin genes) can often be recognized by rabbit polyclonal antibodies. In contrast to mAbs, however, polyclonal antibodies are undefined reagents with finite supply. Rabbit mAbs have overcome this limitation, providing access to defined reagents of infinite supply from the rabbit antibody repertoire. Rabbit mAbs generated by phage display offer additional advantages due to the fact that phenotype and genotype are selected at the same time. Knowledge of the rabbit mAb sequence allows the ready generation of a variety of mAb formats, including scFv, Fab, and IgG, and, importantly, humanization and affinity maturation (Rader et al., 2000; Steinberger et al., 2000; Rader, 2001). Consequently, rabbit mAbs generated by phage display have become promising reagents for therapeutic applications in humans. Based on this consideration, we previously compared antibody repertoires from rabbits with different immunoglobulin allotypes and identified the b9 allotype as superior for the generation and selection of chimeric rabbit/human Fab libraries by phage display (Popkov et al., 2003). Chimeric rabbit/human Fab are composed of rabbit variable domains and human constant domains (Fig. 1A). In addition to facilitating expression and purification, the chimeric rabbit/human Fab format can be readily converted to chimeric rabbit/human IgG and also directly channeled into humanization processes. Using this format, we previously reported the generation of rabbit mAbs with nanomolar affinity and mouse/human cross-reactivity to Tie-2 (Popkov et al., 2003) and VEGF-R2 (Popkov et al., 2004), two tyrosine kinase receptors expressed on endothelial cells. Here we go one step further to show that phage display allows the generation of rabbit mAbs that are species cross-reactive but at the same time highly specific for individual members of a protein family. In addition, we present a newly designed phage display vector optimized for the generation and selection of chimeric rabbit/human Fab libraries and suitable for subsequent conversion to chimeric rabbit/human IgG that can be purified in high yield from a transient expression system employing human 293F cells in serum-free medium.
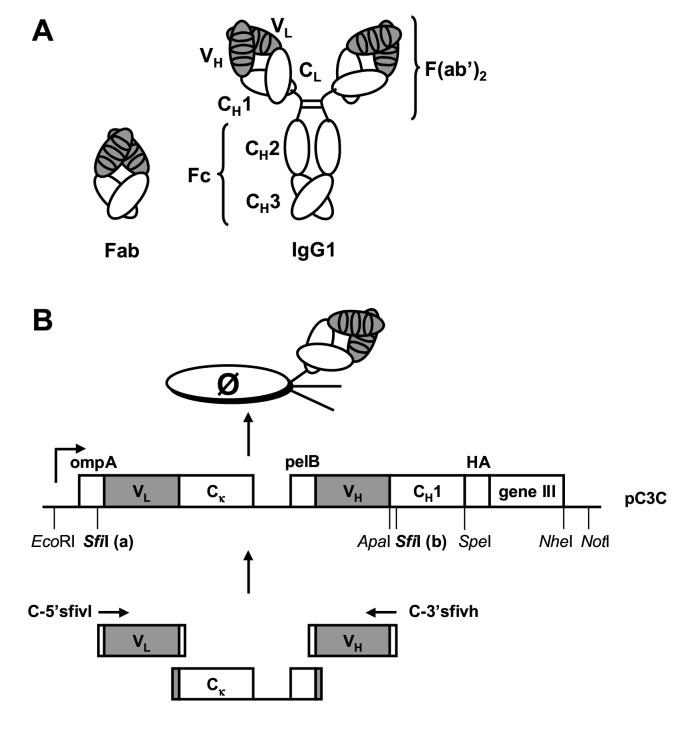
Fig. 1.
(A) Chimeric rabbit/human Fab and IgG. Rabbit variable domains (VL and VH) are shown in gray, human constant domains (Cκ, CH1, CH2 and CH3) in white. The 50-kDa monovalent chimeric rabbit/human Fab fragment (left) contains a chimeric rabbit/human κ light chain associated with a chimeric rabbit/human heavy chain fragment lacking the C-terminal hinge region and constant domains CH2 and CH3. The 150-kDa IgG1 molecule (right) contains two identical chimeric rabbit/human κ light chains and two identical chimeric rabbit/human heavy chains. The antibody binding site results from the convergence of six complementarity determining regions (CDRs), shown as ovals, three provided by each VL and VH. (B) Phage display vector pC3C. The design of pC3C is based on phagemids from the pComb3 series (Barbas et al., 1991). A single lacZ promoter drives the synthesis of a dicistronic transcript. Two ribosome binding sites initiate the translation of two separate polypeptide chains, light chain VL-CL and heavy chain fragment VH-CH1 fused to hemagglutinin (HA) decapeptide, which serves as tag for Fab detection, and the C-terminal domain of the gene III protein. The gene III protein is the minor coat protein of filamentous phage which is displayed in low copy number at one end of the phage. Through the leader peptides ompA and pelB both polypeptides are transported to the periplasm of E. coli, where they associate and form a disulfide bridge. Addition of helper phage leads to the incorporation of the fusion protein into phage particles that display one Fab copy linked to the phage surface by the C-terminal domain of the gene III protein as their phenotype and, as their genotype, contain the corresponding single-stranded phagemid that encodes the Fab. The unique design of pC3C facilitates cloning of Fab libraries through a VL-CL-VH cassette which can be efficiently assembled in one PCR amplification step. SpeI/NheI self ligation of pC3C removes both HA and gene III fragment and results in the expression of soluble Fab.
2. Materials and methods
2.1. Phage display vector pC3C
A sequence encoding the C-terminal domain of the gene III protein was amplified by PCR using pComb3H (Rader and Barbas, 1997) as template and primers C-speg3 and C-notg3, and cloned by SpeI/NotI ligation into pComb3H. The resulting plasmid was digested with SfiI and SpeI. A sequence encoding the CH1 domain of human IgG1 (Cγ11) was amplified by PCR using mammalian expression vector PIGG (Rader et al., 2002) as template and primers C-3'sfich1 and C-spech1, and digested with SfiI and SpeI. A sequence encoding a rabbit Vκ/human Cκ/rabbit VH segment was amplified by PCR using a previously selected chimeric rabbit/human Fab sequence in pComb3H (Rader et al., 2000) as template and primers ompseq and C-3'sfivh, and digested with SfiI. The prepared plasmid and fragments were assembled in one ligation step, yielding pC3C (Fig. 1B) which contained the previously selected chimeric rabbit/human Fab encoding sequence and, thus, allowed a direct comparison of pComb3H and pC3C in terms of Fab expression, affinity, and phage display. Note that the two asymmetric SfiI sites in pC3C, which are marked as SfiI (a) and SfiI (b) in Fig. 1B, are GGCCCAGGCGGCC and GGCCGACGGGGCC, respectively. SfiI (a) is identical in pC3C and pComb3H, whereas SfiI (b) differs in position and sequence. To generate Fab libraries in pC3C, a second plasmid (pCκ) was cloned that served as template for the PCR amplification of the invariable middle fragment (Fig. 1B). For this, a sequence encoding human Cκ and pelB was amplified by PCR using a previously selected Fab sequence in pComb3H as template and primers ecoHKC-F and notLead-B, and cloned by EcoRI/NotI ligation into pC3C. Both pC3C and pCκwere confirmed by restriction analysis and sequencing. As shown in Fig. 1B, rabbit Vκ and Vλ encoding sequences that were amplified by PCR using previously described primers (Barbas, 2001) can be fused to rabbit VH encoding sequences through the human Cκ-pelB fragment in one PCR amplification step using primers C-5'sfivl and C-3'sfivh.
Primer sequences:
C-speg3, gaggaggagactagtggcgcagggtacccgtacgacgttccggactacgcttctgagggtggtggctctgagggtg
C-notg3, caatttcccagatctgcggccgct
C-3'sfich1, gaggaggagggccccgtcggccaagagcacctctgggggcaca
C-spech1, gaggaggagactagttttgtcacaagatttgggctc
C-3'sfivh, gaggaggagggccgacggggccaaggggaagaccgatgggcccttggtggaggctga ompseq, aagacagctatcgcgattgcag
ecoHKC-F, gaggaggaggaattccgaactgtggctgcaccatctgtc
notLead-B, ctgctgctggcggccgcggccatggctggttgggcagc
C-5'sfivl, ctgctgctgggcccaggcggccgagctcg
2.2. Antigens
NgR1
A LRRCT-unique fragment of rat NgR1 (amino acids 278-473) was expressed as (His)6-tagged fusion protein in E. coli and purified by Ni-chelate affinity chromatography as described (Venkatesh et al., 2005). Human and mouse NgR1-Fc fusion proteins were purchased from R&D Systems. Mammalian cell expression vector pMT21 containing the full-length cDNA of rat NgR1 under control of the adenovirus major late promoter was described previously (Venkatesh et al., 2005). A mammalian cell expression vector containing the full-length cDNA of human NgR1 under control of a CMV promoter was purchased from OriGene.
NgR2
A LRRCT-unique fragment of rat NgR2 (amino acids 279-420) was expressed as (His)6-tagged fusion protein in E. coli and purified by Ni-chelate affinity chromatography as described (Venkatesh et al., 2005). A DNA sequence encoding an LRRCT-unique fragment of human NgR2 (amino acids 254-399) and optimized for E. coli expression was custom synthesized (GenScript) and cloned by BamHI/HindIII ligation into plasmid pTrcHis C (Invitrogen). The corresponding (His)6-tagged fusion protein was expressed in E. coli and purified by Ni-chelate affinity chromatography. Mammalian cell expression vector pMT21 containing the full-length cDNA of rat NgR2 under control of the adenovirus major late promoter was described previously (Venkatesh et al., 2005). For the construction of a mammalian cell expression vector containing full-length human NgR2 cDNA, the first two exons of the human NgR2 gene were amplified by RT-PCR from total RNA prepared from human 293F cells using primers hNgR2-5' (agtcggtaccatgctgcccgggctcaggc) and hNgR2-3' (ctgcaagcttaccaggcctcggaagatg), and cloned by KpnI/HindIII ligation into plasmid pCEP4 (Invitrogen). Note that hNgR2-3' was designed to introduce a silent HindIII site at the 3' end of the second exon. A DNA sequence optimized for expression in human cells and corresponding to the 3' end of the second exon, including the silent HindIII site, and the entire third exon of the human NgR2 gene was custom synthesized (GenScript) and cloned by HindIII/BamHI ligation into the above prepared plasmid. The resulting mammalian cell expression vector contained a full-length semi-synthetic cDNA of human NgR2 under control of a CMV promoter.
NgR3
A LRRCT-unique fragment of rat NgR3 (amino acids 274-445) was expressed as (His)6-tagged fusion protein in E. coli and purified by Ni-chelate affinity chromatography as described (Venkatesh et al., 2005). A DNA sequence encoding an LRRCT-unique fragment of human NgR3 (amino acids 248-419) was amplified by RT-PCR from human brain total RNA (BD Biosciences) using primers hNgR3frag-5' (gataaggatccgagcgagttcctccgcctcaatgg) and hNgR3frag-3' (agccaagcttttaggcctgctgcaccccgctgg), and cloned by BamHI/HindIII ligation into plasmid pTrcHis C (Invitrogen). The corresponding (His)6-tagged fusion protein was expressed in E. coli and purified by Ni-chelate affinity chromatography. Mammalian cell expression vector pMT21 containing the full-length cDNA of rat NgR3 under control of the adenovirus major late promoter was described previously (Venkatesh et al., 2005). To generate a mammalian cell expression vector containing full-length human NgR3 cDNA under control of a CMV promoter, the human NgR3 encoding sequence was amplified by PCR from human genomic DNA using primers hNgR3-5' (atgcggtaccccaacatgcttcgcaaagggtg) and hNgR3-3' (atgcggatccttccttggtggacatgtggcag), and cloned by KpnI/BamHI ligation into plasmid pCEP4 (Invitrogen).
2.3. Generation and selection of chimeric rabbit/human Fab libraries
Rabbit immunization and tissue collection protocols were reviewed and approved by the Animal Care and Use Committee of NIAID, NIH (Animal Study Protocol LI6). Spleen and bone marrow from both femurs of two b9 allotype rabbits immunized with the LRRCT-unique fragment of rat NgR1 (amino acids 278-473) and rat NgR2 (amino acids 279-420), respectively (Venkatesh et al., 2005), were collected 5 days after a final boost and processed for total RNA preparation and RT-PCR amplification of rabbit Vκ, Vλ, and VH encoding sequences using established primer combinations and protocols (Barbas, 2001). For each rabbit, rabbit VL/human Cκ/rabbit VH segments compatible with pC3C were assembled in one PCR amplification step. For a 100-μL PCR amplification, 100 ng of each of the three fragments (pooled rabbit VL, human Cκ-pelB, and pooled rabbit VH) were mixed with 60 pmol of each C-5'sfivl and C-3'sfivh in the presence of 75 mM Tris-HCl (pH 8.8), 20 mM (NH4)2SO4, 0.01% (v/v) Tween 20, 2.5 mM MgCl2, 50 μM of each dATP, dCTP, dGTP, and dTTP, as well as 2.5 U recombinant Taq DNA polymerase (Fermentas). Assembly and amplification conditions included an initial 2-min 94°C incubation step followed by 15 cycles, each consisting of a 1-min 94°C denaturation, a 1-min 58°C annealing, and a 2-min 72°C elongation step, followed by a final 10-min 72°C incubation step. A single 1.2-kb product was obtained under these conditions. Using established protocols (Barbas, 2001), this product was purified on a 1% (w/v) agarose gel, digested with SfiI, purified on another 1% (w/v) agarose gel, ligated into SfiI-digested pC3C, and electrotransformed into E. coli strain ER2738 (New England Biolabs), yielding approximately 5 x 107 independent transformants for each of the two libraries. Based on established protocols (Barbas, 2001), the anti-NgR1 library was selected by four rounds of panning against immobilized mouse or, separately, human NgR1-Fc. The anti-NgR2 library was selected by four rounds of panning against the immobilized LRRCT-unique fragment of rat or, separately, human NgR2. All selections yielded a number of clones that were positive in ELISA and expressed different chimeric rabbit/human Fab as revealed by AluI fingerprinting and sequencing (Popkov et al., 2003). The analyses revealed two different repeated clones selected from the anti-NgR1 library and three different repeated clones selected from the anti-NgR2 library. As expected from b9-allotype-derived VL sequences (Popkov et al., 2003), all five clones contained a kappa light chain, without cysteine in position 80 in four out of five clones. From the two anti-NgR1 clones, which both showed species cross-reactivity in ELISA but only shared 76% amino acid sequence identity in the variable domains, clone M5 was chosen based on its stronger signal in ELISA.
Among the three anti-NgR2 clones, two were initially chosen based on their stronger signal in ELISA. From these two clones, which both showed species cross-reactivity in ELISA and shared 91% amino acid sequence identity in the variable domains, clone P14 was chosen based on its higher E. coli expression yields. In summary, anti-NgR1 Fab M5, which had been selected against mouse NgR1-Fc, and anti-NgR2 Fab P14, which had been selected against the LRRCT-unique fragment of rat NgR2, were pursued for further study.
2.4. Expression and purification of chimeric rabbit/human Fab
To remove the gene III fragment of pC3C (Fig. 1B), M5 and P14 phagemids were digested with SpeI/NheI, self-ligated, and transformed into E. coli strain XL1-Blue. Bacterial cultures were inoculated and induced with 2 mM IPTG at an OD600 of 0.8. After shaking at 37°C overnight, the supernatant was isolated by centrifugation, filtered through a 0.45-μm membrane, and tenfold concentrated using an ultrafiltration device with a 10-kDa cutoff membrane (Millipore). The concentrate was diluted 1:1 with PBS and loaded on a 1-mL NHS-activated HiTrap column (GE Healthcare) coated with goat anti-human Fab polyclonal IgG (Bethyl Laboratories). Subsequently, the column was washed with 40 volumes of PBS. Immediately after elution with 0.5 M acetic acid (pH 3.0), the pH was neutralized with 1 M Tris-HCl (pH 8.0). The neutralized eluate was dialyzed at 4°C overnight against PBS using Slide-A-Lyzer cassettes with 10-kDa cutoff (Pierce) and concentrated with 10-kDa cutoff centrifugal filter devices (Millipore). The quality and quantity of purified Fab was monitored by SDS-PAGE and A280 absorbance.
2.5. Expression and purification of chimeric rabbit/human IgG
For the expression of M5 and P14 IgG1κ, the previously described PIGG vector was used (Rader et al., 2002). In this vector, heavy and light chains are expressed by an engineered bidirectional CMV promoter cassette. The light chain encoding sequences of M5 and P14 Fab were PCR amplified using primers M5-light-5' (gaggagaagcttgttgctctggatctctggtgcctacggggaactcgatctgacccagactcca) and P14-light-5' (gaggagaagcttgttgctctggatctctggtgcctacggggaactcgtgatgacccagac), respectively, in combination with primer C-kappa-3' (aattatctagaattaacactctcccctgttgaagctctttgtgacgggcgaactcaggccctg), and cloned by HindIII/XbaI-ligation into PIGG. The VH encoding sequences of M5 and P14 Fab were PCR amplified using primers M5-VH-5' (gaggaggagctcactcccagtcgctggaggagtccgg) and P14-VH-5' (gaggaggagctcactcccaggagcagctggtggagtc) in combination with M5-CH1-3' (ccgatgggcccttggtggaggctgaagagacggtgaccagggtgcctggtccccagatg) and P14-CH1-3' (tgagttccacgacaccgt), respectively, and cloned by ApaI/SacI-ligation into the above prepared PIGG plasmids with the corresponding light chain. The resulting PIGG-M5 and PIGG-P14 plasmids were transiently transfected into human 293F cells (Invitrogen) with 293fectin (Invitrogen) using conditions detailed in the manufacturer's protocol. Transfected 293F cells were cultured in FreeStyle serum-free medium (Invitrogen) in 250-mL Erlenmeyer flasks under constant shaking at 125 rpm in a humidified atmosphere containing 8% CO2 at 37°C. Three days after transfection, the medium was collected after centrifugation, replaced for two additional days, and collected again. The combined supernatants were filtered through a 0.45-μm membrane and tenfold concentrated using an ultrafiltration device with a 30-kDa cutoff membrane (Millipore). The concentrate was 1:1 diluted with PBS and loaded on a 1-mL recombinant Protein A HiTrap column (GE Healthcare). PBS was used for column equilibration and washing, 0.5 M acetic acid (pH 3.0) for elution, and 1 M Tris-HCl (pH 8.0) for immediate neutralization. The neutralized eluate was dialyzed at 4°C overnight against PBS using Slide-A-Lyzer cassettes with 30-kDa cutoff (Pierce) and concentrated with 30-kDa cutoff centrifugal filter devices (Millipore). The quality and quantity of purified IgG1κ was monitored by SDS-PAGE and A280 absorbance.
2.6. ELISA
For coating, each well of a 96-well Costar 3690 plate (Corning) was incubated with 200 ng antigen in 25 μL PBS for 1 h at 37°C. Subsequent incubations were all for 1 h at 37°C. After blocking with 3% (w/v) BSA/PBS, 1 μg/mL Fab (50 ng/well) was added and incubated, washed with H2O (10 × 200 μL/well), and a 1:1,000 dilution of goat anti-human Fab polyclonal antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories) in 1% (w/v) BSA/PBS was added. Washing with H2O was repeated and colorimetric detection was performed using 2,2'-Azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid (Roche) as substrate according to the manufacturer's directions.
2.7. Western blotting
Total cellular lysates were made from 293F cells, three days after transient transfection with human NgR1, NgR2, and NgR3 mammalian cell expression vectors (2.2.), by incubating pelleted cells in 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 150 mM NaCl, 1% (v/v) NP-40, and a protease inhibitor cocktail (Pierce) for 10 min on ice. The lysates were centrifuged, supernatants collected, and their protein concentrations determined using Bradford reagent (Sigma-Aldrich). Samples containing 20 μg protein were electrophoresed on a NuPage 4-12% gradient gel (Invitrogen), blotted on a nitrocellulose membrane (GE Healthcare), and blocked with Western Blocking Reagent (Roche). M5 and P14 Fab were diluted to 1 μg/mL and polyclonal goat anti-human NgR3 antibodies (R&D Systems) to 0.2 μg/mL final concentration in Western Blocking Reagent. For Fab detection, a 1:10,000 dilution of goat anti-human Fab polyclonal antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories) in Western Blocking Reagent was used. For goat polyclonal antibody detection, a 1:10,000 dilution of donkey anti-goat polyclonal antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories) in Western Blocking Reagent was used. Immunoreactive bands were developed using SuperSignal West Pico Chemoluminescent Substrate (Pierce) and visualized using BioMax MR autoradiography film (Kodak).
2.8. Flow cytometry
Three days after transient transfection with human NgR1, NgR2, and NgR3 mammalian cell expression vectors (2.2.), 293F cells were collected by centrifugation and incubated in 10% (v/v) FCS/PBS for 1 hour. Cells were then centrifuged, resuspended in 1% (v/v) FCS/PBS and aliquots of 50 μL containing 5 × 105 cells were distributed into a V-bottom 96-well plate (Corning). M5 and P14 Fab were added to the cells at 4 μg/mL final concentration and incubated for 1 hour. Subsequently, the cells were washed twice in 1% (v/v) FCS/PBS, incubated for 1 hour with a 1:40 dilution of FITC-conjugated goat anti-human Fab (Jackson ImmunoResearch Laboratories) in 1% (v/v) FCS/PBS, washed twice as before, and resuspended in 400 μL 1% (v/v) FCS/PBS. All steps were carried out on ice. Flow cytometry was performed using a FACScan instrument (Becton-Dickinson).
2.9. Surface plasmon resonance
Surface plasmon resonance for the measurement of the affinity of M5 and P14 Fab and the virtual affinity of M5 and P14 IgG1κ to NgR1 and NgR2, respectively, was performed on a BIAcore 2000 instrument. A CM5 sensor chip (BIAcore) was activated for immobilization with N-hydroxysuccinimide and N-ethyl-N'-(3-dimethylaminopropyl)carbodiimide. Human and mouse NgR1-Fc fusion proteins or human and rat NgR2 LRRCT-unique fragments in 15 mM sodium acetate (pH 4) were immobilized at a density between 900 and 1,800 resonance units (RU). Subsequently, the sensor chip was deactivated with 1 M ethanolamine hydrochloride (pH 8.5). Binding of M5 and P14 Fab and IgG1κ was studied by injecting five different concentrations ranging from 100 to 200 nM. The running buffer consisted of 10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA (pH 8.0) and 0.05% (v/v) Tween 20. The sensor chip was regenerated with 25 mM HCl without any loss of binding capacity. Calculation of association (kon) and dissociation (koff) rate constants was based on a 1:1 Langmuir binding model using BIA Evaluation software (BIAcore). The equilibrium dissociation constant (Kd) was calculated from koff/kon.
2.10. Epitope mapping by immunocytochemistry
Sequences encoding chimeric rat Nogo-66 receptors were generated by PCR using rat NgR1, and NgR2 cDNA templates and assembled in the expression vector pMT21 (Venkatesh et al., 2005). A complete list of chimeric receptor constructs generated to map antibody binding sites will be described elsewhere (K.V. et al., in preparation). Chimeric rat Nogo-66 receptors were expressed in transiently transfected COS-7 cells using Lipofectamine 2000 (Invitrogen). The cells were fixed 24 hours after transfection with 4% (w/v) paraformaldehyde in PBS for 20 min, rinsed in PBS, and blocked in 5% (v/v) horse serum in PBS for 1 hour. Cell surface expression was verified using rabbit anti-NgR1 or anti-NgR2 polyclonal antibodies (Venkatesh et al., 2005). M5 and P14 Fab were used at 1 μg/mL, incubated overnight at 4°C, and detected by incubation with goat polyclonal anti-human IgG (H+L) antibodies conjugated to alkaline phosphatase (Promega) for 1 hour at room temperature. Colorimetric detection was performed using BCIP/NBT (5-Bromo-4-chloro-3-indolyl phosphate/Nitro blue tetrazolium) tablets (Sigma-Aldrich) as substrate according to the manufacturer's directions, and the staining was analyzed by microscopy.
2.11. Immunofluorescence microscopy
Rat PC12 cell lines stably expressing full-length rat NgR1 (PC12-NgR1) or NgR2 (PC12-NgR2) (H.L. et al., in preparation) were maintained in 10% (v/v) FBS, 5% (v/v) horse serum, 1 mM L-glutamine in DMEM supplemented with penicillin, streptomycin, and fungizone. Cells were fixed with 4% (w/v) paraformaldehyde in PBS for 20 min, rinsed in PBS, and blocked in 5% (v/v) horse serum in PBS for 1 hour. (Another set of experiments revealed that omitting the fixation step did not change the outcome). Parental PC12 cells and PC12-NgR1 or PC12-NgR2 were incubated with 1 μg/mL M5 or P14 IgG1κ overnight at 4°C in 2% (v/v) horse serum/PBS followed by 1 hour in a 1:1,000 dilution of goat anti-human IgG polyclonal antibodies conjugated to Alexa Fluor® 594 (Invitrogen) in 2% (v/v) horse serum/PBS. Nuclei were stained with 0.5 μg/mL Hoechst 33258 for 5 min. Pictures were taken with a DP70 digital camera attached to an IX71 Olympus inverted microscope.
2.12. Immunoprecipitation
Exogenous NgR1
Rat PC12 cell lines stably expressing full-length rat NgR1 or NgR2 were maintained in culture as described above (2.11.). Approximately 5 × 106 cells plated on 10-cm dishes were lysed in modified RIPA buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% (v/v) NP-40, 1 mM MgCl2, 0.25% (v/v) sodium deoxycholate, 25 μg/ml protease inhibitor cocktail (Sigma)] for 1 hour at 4°C. Cell lysates were tumbled for 4 hours at 4°C in the presence of 1.6 μgM5 IgG1κ and precipitated with 20 μL of Protein G Plus/Protein A-agarose beads (Calbiochem) after incubation for 1 hour at 4°C. Precipitated beads were rinsed three times with a buffer containing 1 mM EDTA, 20 mM Tris-HCl (pH 8.0), and 150 mM NaCl. Bound proteins were eluted with 2 × SDS sample buffer and loaded on a 7% SDS-PAGE gel. The supernatant of immunoprecipitated cell lysates was loaded as well. Western blotting was carried out essentially as described above (2.7.) using polyclonal goat anti-human NgR1 antibodies (R&D Systems).
Endogenous NgR1
A membrane preparation from adult mouse cortex and hippocampus was prepared by subcellular fractionation as follows. Adult mouse cortex and hippocampus were homogenized in a low sucrose buffer containing 250 mM sucrose, 10 mM HEPES (pH 7.4), 3 mM DTT, 5 mM EDTA, and 1 mL/50 g weight tissue protease inhibitor (Sigma-Aldrich). The nuclei and cell debris were removed from the homogenate by centrifugation at 1,100 g for 10 min at 4°C. The pellet was resuspended in the low sucrose buffer and centrifuged at 1,000 g for 5 min at 4°C. The supernatants were pooled and diluted 3.2-fold in high sucrose buffer containing 1.8 M sucrose, 10 mM HEPES (pH 7.4), 3 mM DTT, 5 mM EDTA and 1 mL/50 g weight tissue protease inhibitor. The samples were then over layered with 0.85 M sucrose and centrifuged at 20,000 g for 1 h. Membranes enriched at the 0.85 M/1.32 M sucrose interphase were collected with a Pasteur pipette, washed in PBS, and resuspended in 5 mL 10 mM HEPES (pH 7.4). The total protein concentration of the membrane samples was 6.92 mg/mL, as determined by a BCA (bicinchoninic acid) assay (Pierce). For the immunoprecipitation of NgR1, 3.46 mg of membranes in 0.5 mL 10 mM HEPES were lysed in 1:1 (v/v) modified RIPA buffer as described for PC12 cells above. Lysates were centrifuged at 18,000 g for 15 min at 4°C, and 350 μL of the supernatant were used for immunoprecipitation as described for PC12 cells above using both M5 and P14 IgG1κ.
3. Results and discussion
3.1. Design and generation of phage display vector pC3C
A new phage display vector, phagemid pC3C, was designed to facilitate the generation and selection of Fab libraries with human constant domains. A key feature that distinguishes pC3C from its predecessors pComb3H (Rader and Barbas, 1997) and pComb3X (Andris-Widhopf et al., 2000) is its ability to accommodate human and chimeric nonhuman/human Fab libraries assembled in two rather than three PCR amplification steps (Fig. 1B). For the generation of Fab libraries in pComb3H and pComb3X, amplified VL and VH encoding sequences are fused by overlap extension PCR to CL and CH1, respectively, and subsequently assembled by another amplification step (Barbas, 2001). The resulting VL-CL-VH-CH1 cassette is then cloned directionally into the phagemid using two asymmetric sites of the restriction enzyme SfiI with the rare recognition sequence 5′-GGCCNNNNNGGCC-3′. In pC3C, the downstream SfiI (b) site was moved upstream to a location close to the border of VH and CH1 encoding sequences. This unique design facilitates cloning of Fab libraries through the shorter VL-CL-VH cassette, which in contrast to the VL-CL-VH-CH1 cassette can be efficiently assembled in one PCR amplification step (Fig. 1B). The elimination of one out of three PCR amplification steps is more likely to preserve the initial sequence diversity of VL and VH and, thus, improve the Fab library complexity and ultimately the number, diversity, and affinity of selected Fab. As a consequence, human CH1 (we chose human Cγ11) is an invariable part of pC3C. Introduction of the SfiI (b) site led to an A15S amino acid mutation in human Cγ11. Pilot experiments revealed that this mutation does not have an impact on Fab expression, affinity, and phage display (data not shown). Notably, the conversion of Fab to IgG utilizes an ApaI site upstream from SfiI (b), thereby restoring the native human Cγ11 amino acid sequence. The generation and selection of chimeric rabbit/human Fab libraries for the identification of rabbit mAbs with species cross-reactivity to NgR1 and NgR2 constituted the first test of pC3C.
3.2. Generation and selection of chimeric rabbit/human Fab libraries
We recently reported the generation of rabbit polyclonal antibodies selective for all three members of the Nogo-66 receptor family by immunizing b9 allotype rabbits with a recombinant C-terminal fragment of rat NgR1, NgR2, and NgR3 (Venkatesh et al., 2005). In the Nogo-66 receptor family, this C-terminal fragment, which consists of the LRRCT domain plus a stalk sequence referred to as unique domain, has less amino acid sequence similarity than the conserved N-terminal LRR cluster composed of LRRNT domain and eight LRR domains (Pignot et al., 2003) and therefore was chosen as immunogen (Venkatesh et al., 2005). Spleen and bone marrow from the anti-NgR1 and anti-NgR2 rabbits were collected and separately processed for total RNA preparation and RT-PCR amplification of rabbit VL (Vκ and Vλ) and VH encoding sequences as described (Rader et al., 2000; Barbas, 2001). For each specificity (anti-NgR1 and anti-NgR2), VL-CL-VH cassettes consisting of rabbit VL, human Cκ, and rabbit VH were assembled in one PCR amplification step and cloned by asymmetric SfiI ligation into pC3C (Fig. 1B). The resulting two libraries each consisted of approximately 5 × 107 independently transformed chimeric rabbit/human Fab clones. The anti-NgR1 library was selected by phage display on recombinant human and mouse NgR1-Fc fusion proteins that contained the entire LRRNT-LRR-LRRCT-unique extracellular domain of NgR1. The anti-NgR2 library was selected by phage display on the recombinant C-terminal fragments of rat and human NgR2. A number of selected clones encoding chimeric rabbit/human Fab were subjected to an initial characterization by ELISA, AluI fingerprinting, and sequencing (Popkov et al., 2003). Based on criteria outlined in the materials and methods section, we focused on anti-NgR1 chimeric rabbit/human Fab M5 and the anti-NgR2 chimeric rabbit/human Fab P14.
3.3. Characterization of selected chimeric rabbit/human Fab
Sequencing revealed that M5 and P14 Fab contained unique rabbit Vκ and VH sequences with the highest amino acid sequence similarity to antibody sequences from other b9 allotype rabbits we previously studied and submitted to GenBank (Popkov et al., 2003; Popkov et al., 2004) (Fig. 2 and data not shown). As expected from b9-allotype-derived Vκ sequences (Popkov et al., 2003) neither contained a cysteine in position 80. We have previously provided evidence that an unpaired cysteine in position 80, which forms an intrachain disulfide bridge with rabbit but not human Cκ, reduces the selectable diversity in chimeric rabbit/human Fab libraries. The specificity and species cross-reactivity of purified M5 and P14 Fab was analyzed by ELISA and Western blotting (Fig. 3). In ELISA, M5 Fab revealed strong binding to both human and mouse NgR1 but no binding to human NgR2, rat NgR2, human NgR3, and rat NgR3, whereas P14 Fab revealed strong binding to both human and rat NgR2 but no binding to human NgR1, mouse NgR1, human NgR3, and rat NgR3 (Fig. 3A). Western blotting of lysates of 293F cells transiently transfected with full-length human NgR1, NgR2, and NgR3 constructs confirmed the specificity of M5 and P14 Fab (Fig. 3B). We next analyzed the same transient transfectants by flow cytometry (Fig. 4). Strong and specific staining revealed the binding of M5 and P14 Fab to cell surface human NgR1 and NgR2, respectively. A similar experiment was conducted to map the epitopes of M5 and P14 Fab on NgR1 and NgR2, respectively. For this, a panel of engineered chimeric rat NgR1/NgR2 receptors was transiently expressed in COS-7 cells and probed with M5 and P14 Fab by immunocytochemistry. The distinctive epitopes of M5 and P14 Fab were pinpointed to a C-terminal segment of the unique domain (Fig. 5). Finally, the affinities of M5 and P14 Fab was determined by surface plasmon resonance based on immobilized NgR1 and NgR2 antigens (Table I). M5 Fab, which was selected on mouse NgR1, was found to bind human NgR1 with 104-nM and mouse NgR1 with 61-nM affinity. A unique feature of M5 Fab revealed in the kinetic analysis of antigen binding was a combination of fast association with fast dissociation (Table I). P14 Fab, which was selected on rat NgR2, was found to bind human NgR2 with 80-nM and rat NgR2 with 19-nM affinity. These numbers revealed that despite their strong cross-reactivity, both M5 and P14 Fab maintained a moderate binding preference toward the antigen they were selected on. As discussed in the next paragraph, however, any binding preference virtually disappeared after conversion from Fab to IgG.
Fig. 2.
Amino acid sequence alignment of rabbit variable domains of selected chimeric rabbit/human Fab clones M5 (anti-NgR1) and P14 (anti-NgR2). Shown are framework regions (FR) and complementary determining regions (CDR) of Vκ and VH using Kabat numbering (Kabat, 1991). GenBank accession numbers for the corresponding nucleotide sequences are DQ648593 (M5 Vκ), DQ648594 (M5 VH), DQ648595 (P14 Vκ), and DQ648596 (P14 VH).
Fig. 3.
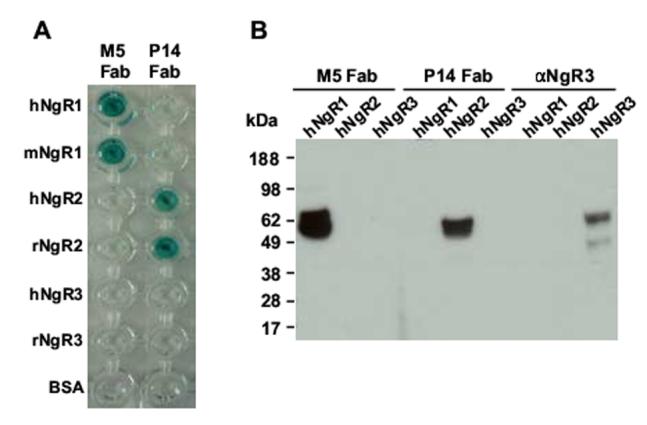
Analysis of the specificity and species cross-reactivity of M5 and P14 Fab by ELISA and Western Blotting. (A) Binding of M5 and P14 Fab to immobilized human (h) and mouse (m) NgR1, human and rat (r) NgR2, human and rat NgR3, and BSA was analyzed by ELISA using goat anti-human Fab polyclonal antibodies conjugated to horseradish peroxidase. (B) For Western blotting, lysates of 293F cells transfected with vectors for mammalian cell expression of human NgR1, NgR2, and NgR3, respectively, were separated by SDS-PAGE, blotted onto nitrocellulose, and probed with M5 and P14 Fab followed by goat anti-human Fab polyclonal antibodies conjugated to horseradish peroxidase. Goat anti-human NgR3 polyclonal antibodies followed by donkey anti-goat polyclonal antibodies conjugated to horseradish peroxidase were used as positive control for the expression of human NgR3.
Fig. 4.
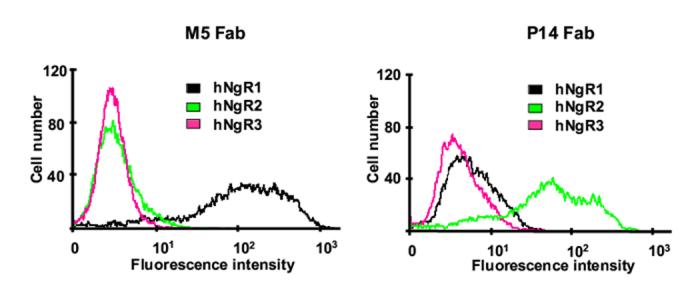
Flow cytometry analysis of M5 and P14 Fab binding to transfected human 293F cells. Flow cytometry histograms showing the binding of M5 Fab (left) and P14 Fab (right) to 293F cells transfected with vectors for mammalian cell expression of human (h) NgR1, NgR2, and NgR3, respectively. FITC-conjugated goat anti-human Fab polyclonal antibodies were used for detection.
Fig. 5.
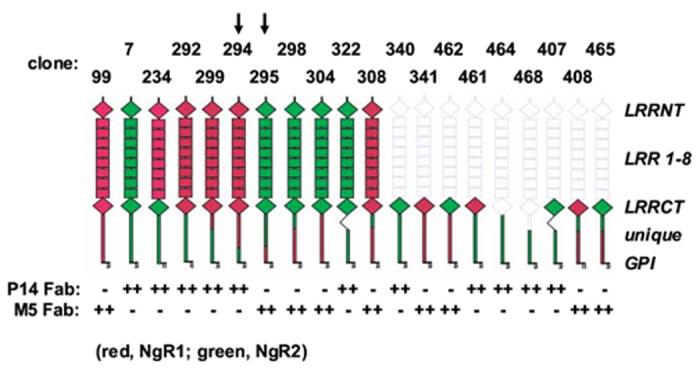
Epitope mapping of M5 and P14 Fab based on engineered chimeric rat NgR1/NgR2 constructs. Chimeric rat Nogo-66 receptors were expressed in transiently transfected COS-7 cells and probed with M5 and P14 Fab by immunocytochemistry using goat polyclonal anti-human IgG antibodies conjugated to alkaline phosphatase for detection. Based on clones 294 and 295 (highlighted by arrows), the epitopes of M5 and P14 Fab were pinpointed to a C-terminal segment of the unique domain encompassing amino acids S379-G448 of NgR1 and L351-G399 of NgR2, respectively.
Table I.
Analysis of M5 and P14 Fab and IgG1κ binding to NgR1 and NgR2, respectively, by surface plasmon resonance using a BIAcore 2000 instrument.
| Antibody | Antigen | kon (104) (M-1s-1) | koff (10-4) (s-1) | Kd (nM) |
|---|---|---|---|---|
| M5 Fab | hNgR1 | 22.1 | 230 | 104 |
| mNgR1 | 27.2 | 165 | 61 | |
| M5 IgG | hNgR1 | 7.75 | 2.25 | “2.9” |
| mNgR1 | 7.75 | 2.25 | “2.9” | |
| P14 Fab | hNgR2 | 4.91 | 39.2 | 80 |
| rNgR2 | 8.1 | 15.4 | 19 | |
| P14 IgG | hNgR2 | 7.98 | 1.83 | “2.3” |
| rNgR2 | 13.6 | 0.65 | “0.48” |
Human (h), rat (r), and mouse (m) NgR1 and NgR2 were immobilized on the sensor chip. Association (kon) and dissociation (koff) rate constants were calculated using BIA Evaluationsoftware. The equilibrium dissociation constant (Kd) was calculated from koff/kon. The “Kd” values for M5 and P14 IgG1κ reflect bivalent rather than monovalent binding and are shown in quotes to emphasize the contribution of the avidity effect to this virtual affinity.
3.3. Conversion of selected chimeric rabbit/human Fab to IgG
The rabbit variable domains of M5 and P14 Fab were cloned into mammalian expression vector PIGG (Rader et al., 2002) to form a chimeric rabbit/human kappa light chain cassette and a chimeric rabbit/human heavy chain cassette under control of a bidirectional CMV promoter. The corresponding chimeric rabbit/human M5 and P14 IgG1κ were expressed through transiently transfected 293F cells in serum-free medium and spin flasks and purified by Protein A affinity chromatography. Whereas P14 IgG1κ yielded approximately 2 mg/L in this system, M5 IgG1κ consistently gave 5 times less, underscoring the strong impact of variable domain sequences on antibody expression yields that has been analyzed systematically in other systems (Ewert et al., 2003). When analyzed by surface plasmon resonance on the same immobilized antigen surfaces that were used to determine the affinity of the Fab, both M5 and P14 IgG1κ revealed a strong avidity effect with a virtual affinity in the 1-nM range (Table I). On average, the conversion of monovalent Fab to bivalent IgG (Fig. 1A) resulted in a 30 times stronger interaction, well within avidity gains obtained in a comparable study (Dmitriev et al., 2001). At the same time, specificity and cross-reactivity of M5 and P14 Fab and IgG1κ were maintained. In fact, the virtual affinity of M5 IgG1κ to human and mouse NgR1 was indistinguishable (Table I). We next investigated whether the specificity of M5 and P14 was conserved in the conversion from Fab to IgG. For this, rat Nogo-66 receptor family members were exogenously expressed on the surface of stably transfected rat neuronal cell line PC12 and probed for M5 and P14 IgG1κ recognition by immunofluorescence microscopy. As shown in Fig. 6, M5 and P14 IgG1κ selectively recognized NgR1 and NgR2, respectively. Notably, despite the larger size of the IgG molecule (Fig. 1A) and the membrane proximal locations of the M5 and P14 epitopes (Fig. 5), recognition at the cell surface remained strong and specific. Given that IgG is a preferred format for immunoprecipitations, we examined the applicability of chimeric rabbit/human IgG for the detection of both exogenously and endogenously expressed antigens by a combination of immunoprecipitation and Western blotting. Both exogenously expressed rat NgR1 from stably transfected PC12 cell lysates (Fig. 7A) and endogenously expressed mouse NgR1 from adult mouse cortex/hippocampus membrane lysates (Fig. 7B) were successfully and selectively immunoprecipitated with M5 IgG1κ as revealed by subsequent Western blotting with independent polyclonal antibodies to NgR1. Likewise, endogenously expressed mouse NgR2 from adult mouse cortex/hippocampus membrane lysates was selectively immunoprecipitated with P14 IgG1κ (data not shown).
Fig. 6.
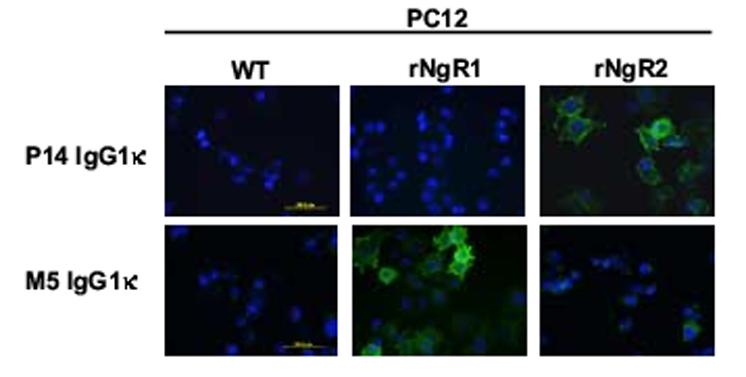
Immunofluorescence microscopy analysis of M5 and P14 IgG1κ binding to stably transfected rat PC12 cells. Binding of M5 and P14 IgG1κ to rat PC12 cells stably transfected with vectors for mammalian cell expression of rat (r) NgR1 or NgR2 was detected with Alexa Fluor® 594-conjugated goat anti-human IgG polyclonal antibodies.
Fig. 7.
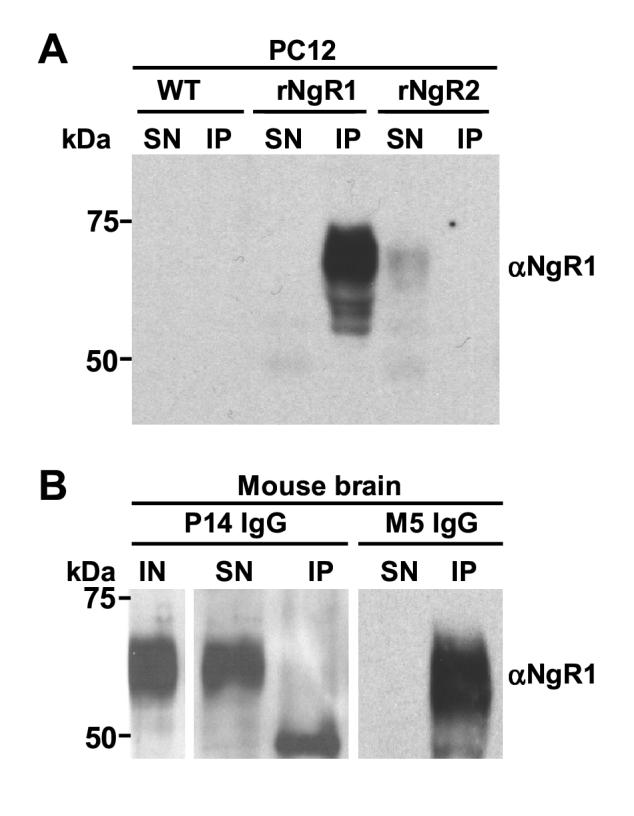
Immunoprecipitation of exogenously and endogenously expressed rat and mouse NgR1. Lysates from (A) rat PC12 cells stably transfected with vectors for mammalian cell expression of rat (r) NgR1 or NgR2 and (B) lysates from adult mouse cortex/hippocampus membranes were subjected to immunoprecipitation using M5 IgG1κ and Protein A/G beads. P14 IgG1κ were used as negative control. Input (IN), supernatants (SN), and immunoprecipitates (IP) were separated by SDS-PAGE, blotted onto nitrocellulose, and probed with goat anti-human NgR1 polyclonal antibodies conjugated to horseradish peroxidase.
Conclusions
The versatility of rabbit mAbs in general, and chimeric rabbit/human Fab and IgG in particular, was shown by exploiting several classical assays for antigen definition and characterization, including ELISA, Western blotting, surface plasmon resonance, flow cytometry, immunocytochemistry, immunofluorescence microscopy, and immunoprecipitation. The combination of rabbit variable domains, which facilitate recognition of a broad epitope repertoire of rodent antigens, and human constant domains, which permit the use of anti-human polyclonal antibodies for low background detection and immunoprecipitation in rodent systems, make chimeric rabbit/human mAbs particularly appealing for defining and characterizing mouse and rat antigens in vitro and in vivo. For example, as revealed in a parallel study (K.V. et al., in preparation), the chimeric rabbit/human Fab and IgG described here were essential reagents for a structure-to-function analysis of the NgR1/MAG and NgR2/MAG interactions which may open new avenues to a targeted therapy of CNS injuries. In addition, cross-reactivity with human orthologs, full antibody effector functions through human constant domains, and feasibility of further humanization may allow chimeric rabbit/human mAbs a faster transition from rodent models to nonhuman primate models to clinical trials where warranted.
Acknowledgements
This research was supported by (i) the Intramural Research Program of the Center for Cancer Research, NCI, NIH (C. R.), (ii) the Intramural Research Program of NIAID, NIH (R. G. M.), (iii) NINDS, NIH grant NS047333 (R. J. G.), and (iv) the New York State Spinal Cord Injury Research Program (R. J. G. and C. R.). T. H. was the recipient of a postdoctoral fellowship from the Schweizerische Stiftung für medizinisch-biologische Stipendien. W. T. was a visiting scientist funded by the Royal Thai Government. We thank Dr. Barbara A. Newman and Cornelius B. Alexander for rabbit immunization and tissue collection, Reema R. Sikka for assisting in library generation and selection, Dr. Sivasubramanian Baskar for supporting flow cytometry studies, Lisa Boyd and Dr. David H. Margulies for supporting BIAcore studies, and Dr. Stephen J. Stahl for reading the manuscript.
Glossary
Abbreviations:
- CNS
central nervous system
- mAb
monoclonal antibody
- GPI
glycosylphosphatidyl-inositol
- LRR
leucine-rich repeat
- MAG
myelin associated glycoprotein
- OMgp
oligodendrocyte myelin glycoprotein
- CDR
complementarity determining region
- FR
framework region
- HA
hemagglutinin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andris-Widhopf J, Rader C, Steinberger P, Fuller R, Barbas CF., 3rd. Methods for the generation of chicken monoclonal antibody fragments by phage display. J Immunol Methods. 2000;242:159–81. doi: 10.1016/s0022-1759(00)00221-0. [DOI] [PubMed] [Google Scholar]
- Barbas CF, 3rd, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991;88:7978–82. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas CF, 3rd, Burton DR, Scott JK, Silverman GJ. Phage Display: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- Barton WA, Liu BP, Tzvetkova D, Jeffrey PD, Fournier AE, Sah D, Cate R, Strittmatter SM, Nikolov DB. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. Embo J. 2003;22:3291–302. doi: 10.1093/emboj/cdg325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchli AD, Schwab ME. Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann Med. 2005;37:556–67. doi: 10.1080/07853890500407520. [DOI] [PubMed] [Google Scholar]
- Dmitriev DA, Massino YS, Segal OL, Smirnova MB, Kolyaskina GI, Pavlova EV, Osipov AP, Egorov AM, Dmitriev AD. The comparison of the ability of monoclonal antibodies directed to different proteins (human IgG, human myoglobin and HRP) and bispecific antibodies derived thereof to bind antigens immobilized on a surface of a solid phase. Clin Chim Acta. 2001;309:57–71. doi: 10.1016/s0009-8981(01)00531-9. [DOI] [PubMed] [Google Scholar]
- Ewert S, Huber T, Honegger A, Pluckthun A. Biophysical properties of human antibody variable domains. J Mol Biol. 2003;325:531–53. doi: 10.1016/s0022-2836(02)01237-8. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–6. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23:1105–16. doi: 10.1038/nbt1126. [DOI] [PubMed] [Google Scholar]
- Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of Proteins of Immunological Interest. Public Health Service, National Institutes of Health; Bethesda, MD: 1991. [Google Scholar]
- Lauren J, Airaksinen MS, Saarma M, Timmusk T. Two novel mammalian Nogo receptor homologs differentially expressed in the central and peripheral nervous systems. Mol Cell Neurosci. 2003;24:581–94. doi: 10.1016/s1044-7431(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Mage RG, Lanning D, Knight KL. B cell and antibody repertoire development in rabbits: the requirement of gut-associated lymphoid tissues. Dev Comp Immunol. 2006;30:137–53. doi: 10.1016/j.dci.2005.06.017. [DOI] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–8. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Pignot V, Hein AE, Barske C, Wiessner C, Walmsley AR, Kaupmann K, Mayeur H, Sommer B, Mir AK, Frentzel S. Characterization of two novel proteins, NgRH1 and NgRH2, structurally and biochemically homologous to the Nogo-66 receptor. J Neurochem. 2003;85:717–28. doi: 10.1046/j.1471-4159.2003.01710.x. [DOI] [PubMed] [Google Scholar]
- Popkov M, Jendreyko N, Gonzalez-Sapienza G, Mage RG, Rader C, Barbas CF., 3rd. Human/mouse cross-reactive anti-VEGF receptor 2 recombinant antibodies selected from an immune b9 allotype rabbit antibody library. J Immunol Methods. 2004;288:149–64. doi: 10.1016/j.jim.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Popkov M, Mage RG, Alexander CB, Thundivalappil S, Barbas CF, 3rd, Rader C. Rabbit immune repertoires as sources for therapeutic monoclonal antibodies: the impact of kappa allotype-correlated variation in cysteine content on antibody libraries selected by phage display. J Mol Biol. 2003;325:325–35. doi: 10.1016/s0022-2836(02)01232-9. [DOI] [PubMed] [Google Scholar]
- Rader C. Antibody libraries in drug and target discovery. Drug Discov Today. 2001;6:36–43. doi: 10.1016/s1359-6446(00)01595-6. [DOI] [PubMed] [Google Scholar]
- Rader C, Barbas CF., 3rd. Phage display of combinatorial antibody libraries. Curr Opin Biotechnol. 1997;8:503–8. doi: 10.1016/s0958-1669(97)80075-4. [DOI] [PubMed] [Google Scholar]
- Rader C, Popkov M, Neves JA, Barbas CF., 3rd. Integrin alpha(v)beta3 targeted therapy for Kaposi′s sarcoma with an in vitro evolved antibody. Faseb J. 2002;16:2000–2. doi: 10.1096/fj.02-0281fje. [DOI] [PubMed] [Google Scholar]
- Rader C, Ritter G, Nathan S, Elia M, Gout I, Jungbluth AA, Cohen LS, Welt S, Old LJ, Barbas CF., 3rd. The rabbit antibody repertoire as a novel source for the generation of therapeutic human antibodies. J Biol Chem. 2000;275:13668–76. doi: 10.1074/jbc.275.18.13668. [DOI] [PubMed] [Google Scholar]
- Ridder R, Schmitz R, Legay F, Gram H. Generation of rabbit monoclonal antibody fragments from a combinatorial phage display library and their production in the yeast Pichia pastoris. Biotechnology (N Y) 1995;13:255–60. doi: 10.1038/nbt0395-255. [DOI] [PubMed] [Google Scholar]
- Spieker-Polet H, Sethupathi P, Yam PC, Knight KL. Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas. Proc Natl Acad Sci U S A. 1995;92:9348–52. doi: 10.1073/pnas.92.20.9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger P, Sutton JK, Rader C, Elia M, Barbas CF., 3rd. Generation and characterization of a recombinant human CCR5-specific antibody. A phage display approach for rabbit antibody humanization. J Biol Chem. 2000;275:36073–8. doi: 10.1074/jbc.M002765200. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005;25:808–22. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]