Abstract
Pro-inflammatory cytokines, such as IL-1, IL-6, and TNF, are considered to be major mediators of osteolysis and ultimately aseptic loosening. This study demonstrated that synergistic interactions among these cytokines are required for the in vitro stimulation of osteoclast differentiation by titanium particles. In contrast, genetic knock out of these cytokines or their receptors does not protect murine calvaria from osteolysis induced by titanium particles. Thus, the extent of osteolysis was not substantially altered in single knock out mice lacking either the IL-1 receptor or IL-6. Osteolysis also was not substantially altered in double knock out mice lacking both the IL-1 receptor and IL-6 or in double knock out mice lacking both TNF receptor-1 and TNF receptor-2. The differences between the in vivo and the cell culture results make it difficult to conclude whether the pro-inflammatory cytokines contribute to aseptic loosening. One alternative is that in vivo experiments are more physiological and that therefore the current results do not support a role for the pro-inflammatory cytokines in aseptic loosening. We however favor the alternative that, in this case, the cell culture experiments can be more informative. We favor this alternative because the role of the pro-inflammatory cytokines may be obscured in vivo by compensation by other cytokines or by the low signal to noise ratio found in measurements of particle-induced osteolysis.
Introduction
Total joint arthroplasty is a widely successful approach that reduces pain, restores mobility, and allows arthritis patients to return to varied activities of daily living. Nonetheless, aseptic loosening, the major cause of failure of total joint arthroplasties, causes approximately 50,000 revision surgeries per year in the Unites Sates [1]. Aseptic loosening is thought to be due to a cascade of events, including production of wear particles from the bearing surfaces and other implant interfaces, secretion of pro-inflammatory cytokines by macrophages, production of pro-resorptive cytokines, such as RANKL, by osteoblasts and fibroblasts, stimulation of osteoclast differentiation, induction of osteolysis or local bone loss, and loosening of the implant [1–3].
Substantial progress has been made in recent years elucidating the mechanisms responsible for aseptic loosening [1–3]. One area of investigation that has advanced rapidly is the demonstration that specific pro-inflammatory cytokines not only are produced in response to wear particles but are responsible for the downstream processes leading to osteolysis. TNFα is the best studied of these. Thus, TNFα production is up-regulated during aseptic loosening [4–7] and by wear particles in vitro [1–3] and in vivo [8, 9]. It has been reported that TNFα receptor knock out mice are partially protected from particle-induced osteolysis in the murine calvaria model [10, 11]. Moreover, blockage of TNFα activity inhibits both osteoclast differentiation and osteolysis induced by wear particles in the murine calvaria model [12, 13]. This cytokine also likely contributes to osteolysis in patients since a polymorphism in the TNFα promoter is associated with an increased frequency of aseptic loosening [14].
IL-1 is the second best studied pro-inflammatory cytokine in aseptic loosening. It is up-regulated in aseptic loosening [4, 6, 7, 15, 16] and by wear particles in vitro [1–3] and in vivo [8, 9]. Blockage of IL-1 activity inhibits particle-induced inflammation and osteoclast differentiation, respectively, in the murine femoral and air pouch models [17, 18]. Moreover, a polymorphism in the gene that encodes the IL-1 receptor antagonist is also associated with an increased frequency of aseptic loosening [19].
Although IL-6 is the third major pro-inflammatory cytokine, much less is known about it’s role in aseptic loosening compared with TNFα and IL-1. However, IL-6 is up-regulated in aseptic loosening [4, 6, 7, 20] and by wear particles in vitro [1–3] and in vivo [8]. Unlike TNFα which primarily acts directly on the osteoclast precursors, IL-1 and IL-6 both primarily stimulate bone resorption indirectly by increasing RANKL production by osteoblasts, other mesenchymal cells, and lymphocytes [21–23]. This indirect, pro-osteoclastogenic, effect of IL-6 is substantially larger, and therefore is more important physiologically and pathophysiologically, than the anti-osteoclastogenic effect that IL-6 exerts directly on osteoclast precursors [21, 23, 24].
Despite the abundant evidence described in the previous paragraphs suggesting an association between aseptic loosening and the pro-inflammatory cytokines, there is little experimental evidence directly demonstrating a role for IL-1 or IL-6 in either in vitro or in vivo models of aseptic loosening. For example in the murine femoral model, knock out of the IL-1 receptor blocked particle-induced inflammation but not particle-induced osteolysis [17]. Similarly, neutralizing antibodies to IL-1 did not block osteolysis in an organ culture model of aseptic loosening [25]. The current study was therefore designed to compare the roles of IL-1, IL-6, and TNFα during induction, by orthopaedic wear particles, of osteoclast differentiation in vitro and osteolysis in vivo.
Methods
All experiments were in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals and were approved by our Institutional Animal Care and Use Committee.
Commercially pure titanium particles (Lot G11G04, Catalog #00681, Johnson Matthey, Ward Hill, MA, 90% <3.6 um, Beckman Coulter Particle Characterization Laboratory, Miami, FL) were sterilized in 100% ethanol and stored at 4°C in phosphate buffered saline (PBS) with 100U/ml penicillin and 100ug/ml streptomycin at a concentration of 2.0 × 1010 particles/ml. These particles have high levels of adherent endotoxin (20–40 Endotoxin Units/109 particles) [26].
Statistical analyses were performed by analysis of variance with Fisher’s Least Significant Difference post hoc tests (SigmaStat, version 3, SPSS, Chicago, IL). A one-sided power analysis was performed using the equation: Required n = 2Kα,βσ2/Δ2, where α = 0.05, β = 0.2, Kα,β = 6.2, Δ = 0.5, and σ = the measured standard deviation.
In vitro studies
Particle-induced osteoclast differentiation was measured in vitro as we have previously described [27]. Briefly, nucleated marrow cells from 6–12 week old female C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were cultured for 24 hours in the presence or absence of sterile titanium particles with adherent endotoxin (8 × 107/cm2). Conditioned media were harvested and stored at −80°C. Osteoclast differentiation was induced by adding conditioned media at a concentration of 25% to co-cultures of osteoclast precursors (nucleated murine spleen cells) and CIMC-4 mesenchymal support cells in the presence of 0.2 nM 1α,25-dihydroxyvitamin D3, 100 nM dexamethasone, and 50 ug/ml ascorbic acid. The CIMC-4 cells support osteoclast differentiation in response to all cytokines that have been tested, including IL-1, IL-6, IL-11, and TNFα [22]. The role of specific cytokines was assessed by supplementing the conditioned media with neutralizing antibodies (goat anti-murine IL-1α, goat anti-murine IL-1β, rat anti-murine IL-6, and goat anti-murine TNFα, all from R&D Systems, Minneapolis, MN), control immunoglobulins (goat IgG or rat IgG1, both from R&D Systems), or recombinant murine IL-1 receptor antagonist (R&D Systems). The indicated concentrations of these reagents are the final concentrations after addition of the conditioned media to the osteoclast differentiation cell cultures. Osteoclast differentiation was quantitated after 9 days of culture by counting the number of tartrate-resistant acid phosphatase-positive multinucleated cells [27]. We have previously shown that these cells create resorption pits when cultured on dentin slices and, therefore, represent bona fide osteoclasts [27]. Reported results represent data from representative experiments presented as means ±SEM.
In vivo studies
Particle-induced osteolysis was measured in vivo using our previously described murine calvarial model of titanium-induced osteolysis [8, 28]. All experiments compared cytokine knock out mice and wild-type mice with matched age, gender, and genetic background (Jackson Labs, Bar Harbor, Maine).
The following knock out mice were examined: IL-1 receptor single knock out mice (B6.129S7-Il1r1tm1Imx/J, Jackson Labs), IL-6 single knock out mice (B6.129S2-Il6tm1Kopf/J, Jackson Labs), IL-1 receptor/IL-6 double knock out mice (see below for derivation), and TNF receptor-1/TNF receptor-2 double knock out mice (B6;129S-Tnfrsf1atm1Imx Tnfrsf1btm1Imx/J, Jackson Labs). All mice were 6–8 week old females and were age matched within each series of experiments.
IL-1 receptor/IL-6 double knock out mice were derived by crossing the IL-1 receptor single knock out mice and the IL-6 single knock out mice. F1 generation mice were bred to obtain double knock out mice, which were used to initiate a breeding colony. PCR-based genotyping was performed on tail DNA using the protocols and primers recommended by Jackson Labs, except that the IL-1 receptor primers were designed using Oligo 6 software (Molecular Biology Insights, Cascade, CO) and were 5′-TGAAATTGATATTCGCAAGTG-3′ and 5′-GGACCTCGGGTAACTCAT-3′.
As previously described, 40 ul of the particle suspensions were implanted on the surface of bilateral parietal bones after removal of the periosteum [8, 28]. Each experiment also included sham surgeries in which 40 ul of the phosphate buffered saline were implanted without particles. After 7 days, the parietal bones were harvested and air-dried after removal of all particles and soft tissues. Microradiographs were prepared using 110kVp for 4 minutes (model 8050–10; Faxitron X-ray Corporation, Buffalo Grove, IL). The percentage of each parietal bone that demonstrated osteolysis was determined from the microradiographs, in a blinded fashion, using computer-assisted histomorphometry (Scion Image, Scion Corporation, Frederick, MD) as previously described [28]. Osteolysis was also measured in a region 300 pixels wide centered over the central suture and extending the entire length of the parietal bones. Reported results represent data pooled from all experiments in each series and are represented by box plots showing the 10th, 25th, 50th, 75th, and 90th, percentiles of the measured amounts of osteolysis.
Results
In vitro studies
We have previously shown that titanium particles with adherent endotoxin substantially increase production of IL-1β, IL-6, and TNFα by murine marrow cells as well as the ability of conditioned media from these cells to increase osteoclast differentiation [27, 29]. We therefore determined whether the increased cytokine production contributes to the increased osteoclast differentiation. Neutralizing antibodies directed against either IL-1α or IL-1β dose-dependently inhibited osteoclast differentiation (Fig. 1A). Neither antibody completely blocked osteoclast differentiation; maximal inhibition (p<0.0001) was 77% by the anti-IL-1α antibody and 71% by the anti-IL-1β antibody (open circles and open triangles in Fig. 1A). Addition of both antibodies together virtually completely blocked osteoclast differentiation (97% inhibition, p<0.0001, open squares in Fig. 1A). This result was confirmed by showing that IL-1 receptor antagonist, which inhibits activity of both IL-1α and IL-1β [30], also virtually completely blocks osteoclast differentiation (94% inhibition, p<0.0001, Fig. 2). Neutralizing antibodies directed against either IL-6 or TNFα also dose-dependently inhibited osteoclast differentiation. Maximal inhibition was 66% by the anti-IL-6 antibody (p<0.0001, open circles in Fig. 1B) and 84% by the anti-TNFα antibody (p<0.0001, open circles in Fig. 1C). Addition of goat or rat immunoglobulins as negative controls had no detectable effect on osteoclast differentiation (filled circles in Fig. 1A–B). The effects of the neutralizing antibodies and IL-1 receptor antagonist were not tested in the absence of conditioned media from the bone marrow cells or in the presence of conditioned media from bone marrow cells incubated without titanium particles since the number of osteoclasts that differentiate, in either case, are too low to determine whether the reagents further inhibit the differentiation [27].
Figure 1.
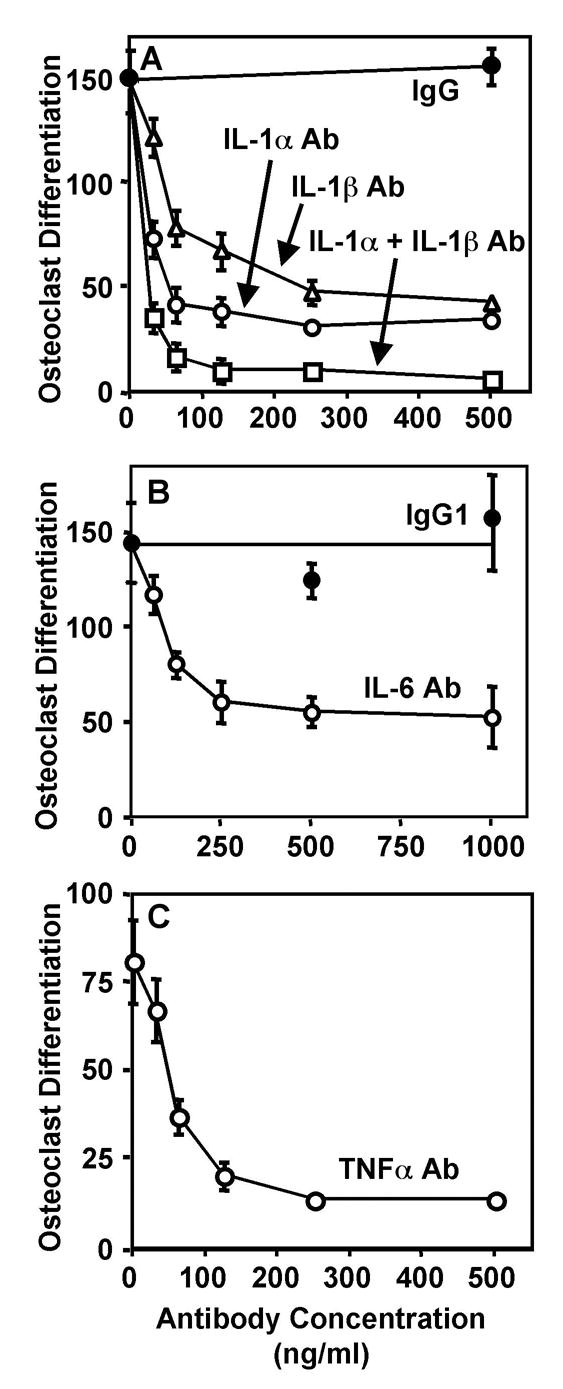
Neutralizing antibodies that target IL-1α (A), IL-1β (B), IL-6 (C), and TNFα (D) significantly inhibit osteoclast differentiation induced in vitro by titanium particles. Control cultures were incubated with non-immune goat IgG (A) or non-immune rat IgG1 (B). Osteoclast differentiation was assessed by measuring the number of TRAP-positive multinuclear cells/cm2. N = 5–6 for all groups.
Figure 2.
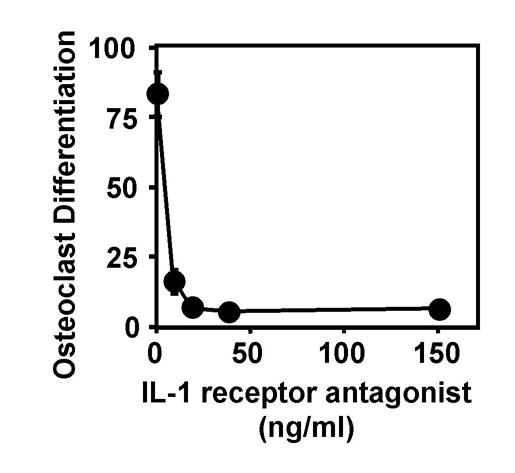
Recombinant IL-1 receptor antagonist significantly inhibits osteoclast differentiation induced in vitro by titanium particles. Osteoclast differentiation was assessed by measuring the number of TRAP-positive multinuclear cells/cm2. N = 5–6 for all groups.
In vivo studies
We have previously measured particle-induced osteolysis over the entire parietal bone [8, 28, 29], whereas most other investigators have restricted their analysis to the area surrounding the sagittal suture [10–13]). This study therefore compared measurements over the entire parietal bones (Figs. 3A, 4A, 5A, and 6A) with measurements within a rectangular region centered on the sagittal suture (Figs. 3B, 4B, 5B, and 6B). Moreover, each region of interest was assessed in two different ways, as we have done previously [29]. Thus, the extent of osteolysis induced by titanium particles was compared before (third and fourth bars in Figs. 3–6) and after (fifth and sixth bars in Figs. 3–6) subtraction of the apparent osteolysis in the sham controls. As expected from qualitative examination of the radiographs [28], the extent of titanium-induced osteolysis is much greater in the region surrounding the suture than in the entire parietal bone (note the difference in scale between the y-axes of panel A and the y-axes of panel B in Figs. 3–6). Despite this, the relative differences between groups of mice are generally similar in the two types of measurements (compare panels A and B in Figs. 3–6). It is difficult to conclude that one method is preferable to the other since the entire parietal bone method captures more information and provides twice the number of measurements per group but the suture region method shows larger effects of the particles. We therefore recommend that both regions be analyzed in studies of particle-induced osteolysis. As an example of the utility of analyzing both regions, it has recently been reported that osteopontin knock out mice are protected from titanium-induced osteolysis only in the region distant from the sagittal suture [31]. To allow analysis of both regions, it is necessary to remove the periosteum prior to implanting the particles, since particle-induced osteolysis is restricted to the suture region if the periosteum is not removed (data not shown).
Figure 3.
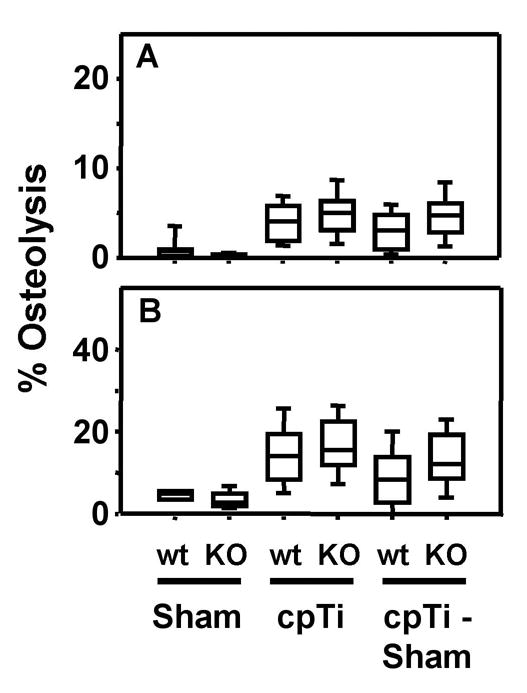
IL-1 receptor single knock out mice are not protected from titanium particle-induced osteolysis. Osteolysis was measured in the entire parietal bones (A) and in the suture regions (B). Data are presented as box plots showing the 10th, 25th, 50th, 75th, and 90th, percentiles. In A, n = 16–18 for first and second groups, n = 48 for the third and fifth groups, and n = 34 for the fourth and sixth groups. In B, n = 8–9 for the first and second groups, n = 24 for the third and fifth groups, and n = 17 for the fourth and sixth groups.
Figure 4.
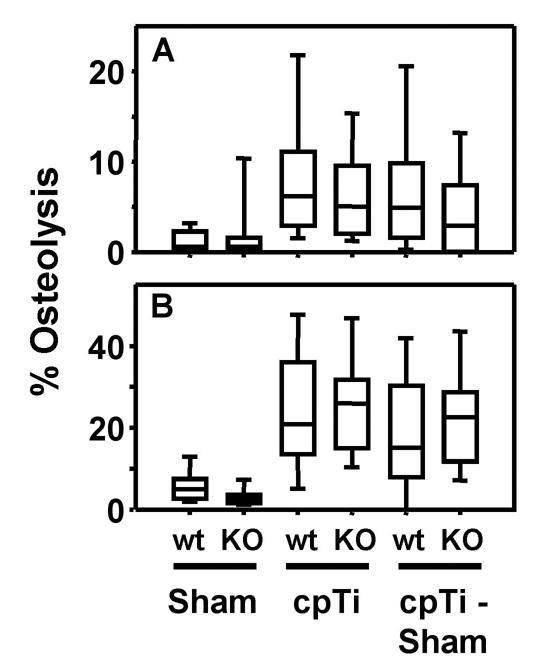
IL-6 single knock out mice are not protected from titanium particle-induced osteolysis. Osteolysis was measured in the entire parietal bones (A) and in the suture regions (B). Data are presented as box plots showing the 10th, 25th, 50th, 75th, and 90th, percentiles. In A, n = 22–28 for all groups. In B, n = 11–14 for all groups.
Figure 5.
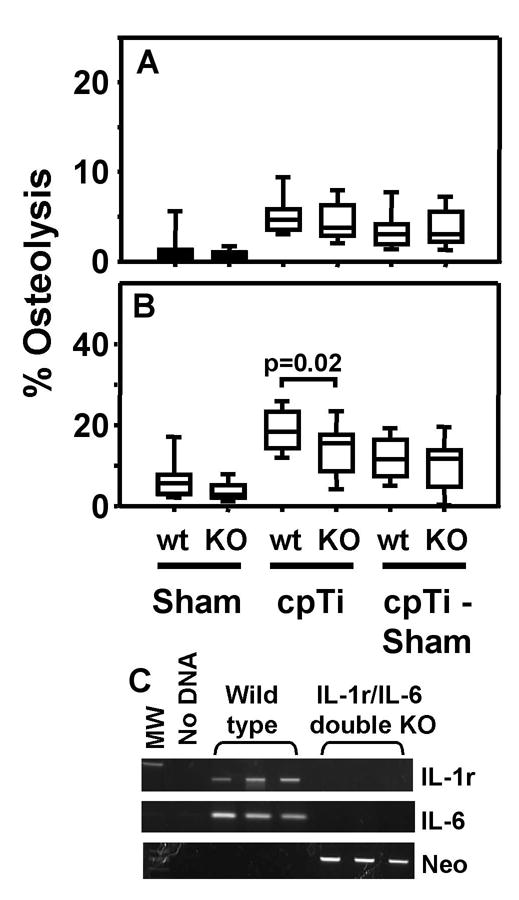
IL-1 receptor/IL-6 double knock out mice are not protected from titanium particle-induced osteolysis. Osteolysis was measured in the entire parietal bones (A) and in the suture regions (B). Data are presented as box plots showing the 10th, 25th, 50th, 75th, and 90th, percentiles. In A, n = 26–36 for all groups. In B, n = 13–18 for all groups. Panel C shows genotyping of the IL-1 receptor/IL-6 double knock out mice. PCR amplicons from three representative wild type and three representative double knock mice are shown. Tail clip DNA was amplified with primers specific for the IL-1 receptor gene, the IL-6 gene, and the neomycin cassette, respectively.
Figure 6.
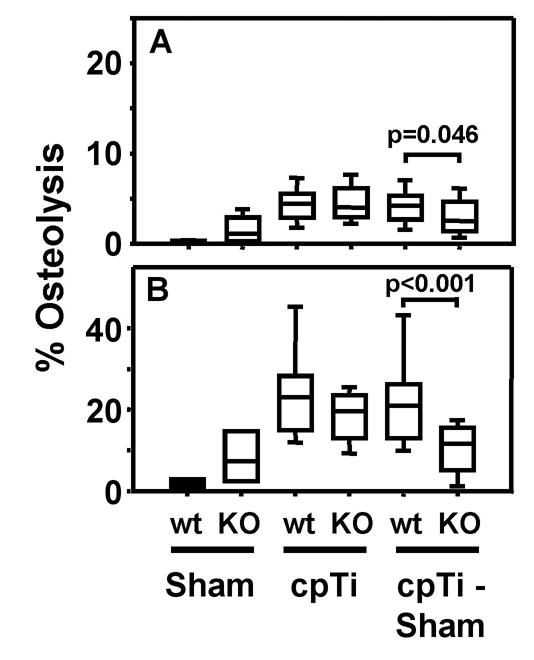
TNF receptor 1/TNF receptor 2 double knock out mice are not protected from titanium particle-induced osteolysis. Osteolysis was measured in the entire parietal bones (A) and in the suture regions (B). Data are presented as box plots showing the 10th, 25th, 50th, 75th, and 90th, percentiles. In A, n = 12 for the first and second groups and n = 24–26 for the third, fourth, fifth, and sixth groups. In B, n = 6 for the first and second groups and n = 12–13 for the third, fourth, fifth, and sixth groups.
Titanium-induced osteolysis in the knock out mice was compared with osteolysis in wild type mice, matched for age, gender, and genetic background. Neither the IL-1 receptor single knock out (Fig. 3) nor the IL-6 single knock out (Fig. 4) significantly reduced particle-induced osteolysis in any of the four methods of analysis. The IL-1 receptor results are similar to those obtained in the murine femoral model of particle-induced osteolysis [17].
To determine whether the lack of major effects in the IL-1 receptor knock out and IL-6 knock out mice were due to compensation between the two cytokines [3], we generated double knock out mice lacking both the IL-1 receptor and IL-6. Genotyping of these mice showed the expected pattern (Fig. 5C). Nonetheless, the only significant difference observed between the double knock out and their wild type controls was in the analysis of the suture region that did not include subtraction of the data from the sham operated mice (compare third and fourth groups in Figure 5B). Moreover, the difference between the means of the two groups was modest and the level of significance was borderline (p = 0.02). Represenatative examples of osteolysis induced by titanium particles in the wild type and double knock mice are shown in Figure 7. Taken together, only one of the 12 analyses of the IL-1 receptor/IL-6 single and double knock out mice showed significant inhibition of titanium-induced osteolysis (Figs. 3–5).
Figure 7.
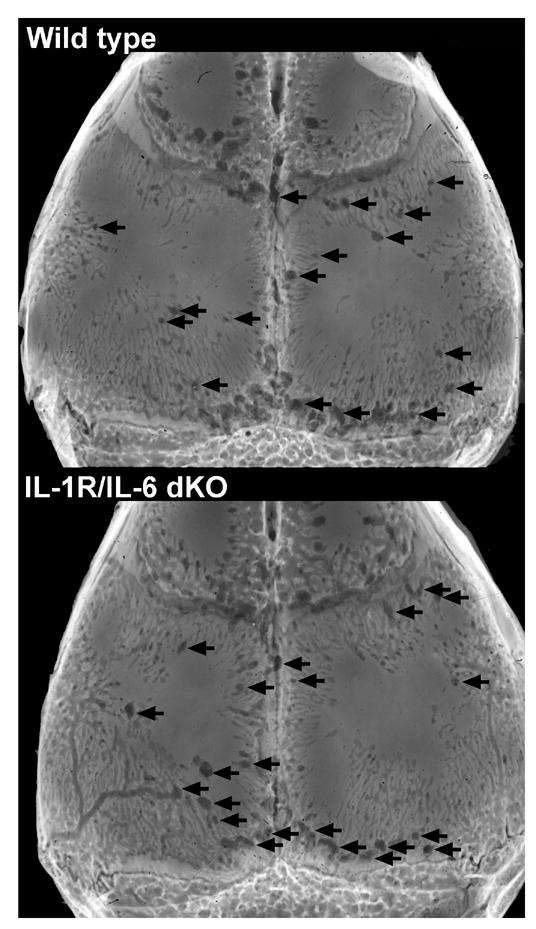
Represenative examples of titanium-induced osteolysis in wild type and IL-1 receptor/IL-6 double knock out. The images are of the skull with the per cent osteolysis in the suture region that is closest to the median for each group. Arrows indicate some of the osteolytic areas.
To compare these results to the findings of other investigators using the calvarial model [10, 11], we measured titanium-induced osteolysis in TNF receptor double knock out mice. The only significant differences observed between these mice and their wild type controls was in the analyses after subtraction of the data from the sham operated mice (Fig. 6A–B). However, these apparent differences were due to an unusually large difference in the level of apparent osteolysis between the wild type and knock out mice following sham surgeries (compare first and second bars in Figs. 6A–B with first and second bars in Figs. 3–5), which may be due to the smaller number of measurements obtained in the sham groups in the TNF receptor experiments. Moreover, the apparent difference in the analysis of the entire parietal bones was of borderline significance (p = 0.046, Fig. 6B). These results are consistent with findings that TNFα is not required for induction of inflammatory arthritis and other autoimmune diseases [32].
Discussion
The major conclusion of this study is that osteoclast differentiation induced in vitro by titanium particles depends on IL-1α, IL-1β, IL-6, and TNFα. Similar results to those in the current study were recently published by other workers examining the effects of polystyrene particles on osteoclast differentiation in vitro [33]. In our study, IL-1 receptor antagonist or neutralizing antibodies to any of the cytokines significantly inhibit the ability of titantium particles to increase osteoclast differentiation.
The sum of the inhibitions induced by the four antibodies (77% + 71% + 66% + 84%) substantially exceeds 100%. We conclude that the cytokines act synergistically to induce osteoclast differentiation in response to titanium particles. In agreement with this, we have previously shown that IL-1β, IL-6, and TNFα synergistically stimulate osteoclast differentiation in the absence of wear particles [22]. Moreover, we have shown that osteoprotegerin blocks stimulation of osteoclast differentiation induced by IL-1, IL-6, the synergistic combination of cytokines, or the conditioned media produced by bone marrow cells incubated with titanium particles [22, 34]. These results are consistent with the conclusion that all of these agents stimulate osteoclast differentiation indirectly by up-regulating RANKL production [35].
A limitation of this study is that titanium particles were used rather than ultrahigh molecular weight polyethylene, which are the most common type of orthopaedic wear particle in patients [1]. However, we have recently shown that these titanium particles induce osteolysis in the murine calvarial model by mechanisms that are indistinguishable from polyethylene particles [8]. Moreover, polyethylene particles are difficult to study in cell culture since they float away from the cells. A related limitation is that the titanium particles were produced by a commercial milling process and therefore are not authentic orthopaedic wear particles. However, they have been used by many investigators as a model system (reviewed in [26, 27]) and have similar biological activities as authentic wear particles [36–38]. For example, we have shown that the commercially-available titanium particles stimulate cytokine production, osteoclast differentiation, and osteolysis [8, 27–29]. Finally, it should be pointed out that substantial amounts of endotoxin adhere to the commercially-available titanium particles [26]. Although the clinical relevance of adherent endotoxin in aseptic loosening is controversial, we and others have shown that it increases the biological effects of orthopaedic wear particles both in cell culture and in vivo [2, 39, 40].
The murine model of wear particle-induced osteolysis is thought to faithfully reproduce the mechanisms responsible for aseptic loosening in patients. Thus, the animal model recapitulates the formation of a surrounding fibrous layer containing abundant macrophages that have phagocytosed the wear particles, elevated production of pro-inflammatory cytokines, increased osteoclast differentiation, and dependence on RANKL [8, 28, 41–43]. Nonetheless and in contrast to the in vitro results, genetic knock out of IL-6 or the receptors for IL-1 or TNFα does not substantially reduce particle-induced osteolysis in vivo. These in vivo results are reminiscent of the lack of effect of neutralizing antibodies to IL-1 and TNFα, either singly or in combination, in an organ culture model of aseptic loosening [25]. Similarly, calvarial osteolysis induced by low dose lipopolysaccharide is not affected by IL-1 receptor knockout, TNF receptor knockout, or a combination of the two [44].
The differences between the in vivo and the cell culture results make it difficult to conclude whether the pro-inflammatory cytokines contribute to aseptic loosening. One alternative is that in vivo experiments are more physiological and that therefore the current results do not support a role for the pro-inflammatory cytokines in aseptic loosening. We however favor the alternative that, in this case, the cell culture experiments can be more informative. We favor this alternative because the role of the pro-inflammatory cytokines may be obscured in vivo by compensation or by the low signal to noise ratio (see following paragraphs). Moreover, there is abundant clinical, cell culture, and animal evidence suggesting an association between aseptic loosening and the pro-inflammatory cytokines. Thus, all three cytokines are up-regulated during aseptic loosening [4–7, 15, 16] and by wear particles in vitro [1–3] and in vivo [8, 9]. The associations between an increased frequency of aseptic loosening and polymorphisms in the promoters for TNFα and IL-1 receptor antagonist [14, 19] are also consistent with this alternative and establish a clinical correlation for our cell culture results.
Multiple pro-inflammatory cytokines can compensate for each other [22, 45] and this compensation is more likely to occur in long-term genetic ablation experiments than in short-term antibody neutralization experiments. Moreover, since the two-step cell culture system used in this study isolates the antibodies from the cells that are stimulated by the wear particles to produce cytokines, the liklihood of compensation is further reduced. Thus, knock out of one or two cytokines may not be sufficient in vivo to observe the important physiological role of the cytokines. In contrast, RANKL is required in our cell culture system [34] and in the murine calvarial model [41–43]. This dependence on RANKL is due to it’s requirement for osteoclast differentiation, survival, and activity [46] in contrast to the ability of the pro-inflammatory cytokines to compensate for each other [22, 45]. The clinical implication of these results is that inhibition of RANKL signaling may be a more effective therapeutic strategy than inhibition of any one of the cytokines. This may be especially important in light of the growing realization that therapies directed against pro-inflammatory cytokines can induce serious infections and malignancy [47, 48].
The low signal to noise ratio in the in vivo and organ culture experiments (see next paragraph for details) compared with the cell culture experiments may be another factor that makes it difficult to detect the in vivo effects of gene knock outs. An additional caveat of this low signal to noise ratio is the need to study relatively large numbers of mice to obtain reliable results. For example, both we [49] and others [50] initially reported that IL-6 knock out significantly protects mice from particle-induced osteolysis. However, both groups found that further examination of larger numbers of mice did not replicate the initial findings (this study and O’Keefe, personal communication). Thus, all studies of particle-induced osteolysis with small numbers of mice, including the previous reports on the role of TNF [10–13], should be considered preliminary. To substantiate this point, we performed a power analysis using the means and standard deviations from the wild type mice implanted with titanium particles in Figures 3–6. To detect a decrease in osteolysis of 50% (α = 0.05, power = 0.8), n equals 22 parietal bones per group for the entire bone method and n equals 10 mice per group for the suture region method. The use of smaller numbers of mice is analogous to the clinical pilot study of TNFα blockade that failed to detect an effect on osteolysis due to therelatively small number of patients that were studied [51].
One of the major limitations of the murine model of wear particle-induced osteolysis is the low signal to noise ratio described in the previous paragraph. A major cause of this is the variability observed between individual mice. To illustrate this variability, we recommend publication of box plots as shown in Figures 3–6 rather than bar graphs. One source of the variability is likely to be due to use of two dimensional measurements of three dimensional structures. Measurement by uCT would alleviate this source of variability. Another source of the variability is the differences in extent of pre-existing marrow between individual mice, which may explain the differences observed in the level of apparent osteolysis between the wild type and TNF receptor double knock out mice following sham surgeries. Unfortunately, the pre-existing marrow is sometimes difficult to distinguish from the particle-induced osteolysis. A non-invasive method of analysis, such as uCT, would allow measurement of the pre-existing marrow volumes prior to particle implantation and subtraction, for each mouse, of that volume from the total non-bone area observed following particle implantation.
Our in vitro results indicate that TNFα, IL-1, and IL-6 synergistically contribute to aseptic loosening. Our results also highlight the need for better methods, such as uCT, of measuring particle-induced osteolysis in vivo. Such methods, together with the use of continuous particle infusion to more closely mimic the situation in patients [9], the study of the effects of particles in conjunction with other factors that may also contribute to aseptic loosening [2, 3], and the expanded use of transgenic, knock out, and gene therapy approaches [8, 10–13, 17, 18, 29, 42, 43, 52], promise continued advances in our understanding of particle-induced osteolysis.
Acknowledgments
The authors thank Jenifer Mikulan for assistance with animal surgeries, Teresa Pizzuto for preparing the microradiographs, Kari Smith for preparing the images shown in Figure 7, and all the members of the Greenfield lab for helpful discussions. Supported by NIH RO1 AR43769 to EMG. None of the authors have professional or financial affiliations that may be perceived to have biased the presentation.
Footnotes
This paper is dedicated to the memory of Miles A. Crenshaw, Ph.D.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright TM, Goodman SB, editors. Implant Wear in Total Joint Replacement. Rosemount, Ill: American Academy of Orthopaedic Surgeons; 2001. [Google Scholar]
- 2.Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Nalepka JL, Seabold JM. Does endotoxin contribute to aseptic loosening of orthopaedic implants? J Biomed Mater Res B Appl Biomater. 2005;72B:179–85. doi: 10.1002/jbm.b.30150. [DOI] [PubMed] [Google Scholar]
- 3.Greenfield E. Particulate Matter and Host Reactions. In: Wnek G, Bowlin G, editors. Encyclopedia of Biomaterials and Biomedical Engineering. New York: Taylor Francis; 2006. [DOI] [Google Scholar]
- 4.Hicks DG, Judkins AR, Sickel JZ, Rosier RN, Puzas JE, O’Keefe RJ. Granular histiocytosis of pelvic lymph nodes following total hip arthroplasty. The presence of wear debris, cytokine production, and immunologically activated macrophages. J Bone Joint Surg. 1996;78A:482–96. doi: 10.2106/00004623-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Konttinen Y, Lassus J, Natah S, Ceponis A, Solovieva S, Aspenberg P, Santavirta S. Tumor necrosis factor-alpha (TNF-alpha) in loosening of total hip replacement (THR) Clin Exp Rheumatol. 1996;14:643–8. [PubMed] [Google Scholar]
- 6.Stea S, Visentin M, Granchi D, Ciapetti G, Donati M, Sudanese A, Zanotti C, Toni A. Cytokines and osteolysis around total hip prostheses. Cytokine. 2000;12:1575–9. doi: 10.1006/cyto.2000.0753. [DOI] [PubMed] [Google Scholar]
- 7.Goodman S, Huie P, Song Y, Schurman D, Maloney W, Woolson S, Sibley R. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg. 1998;80B:531–9. doi: 10.1302/0301-620x.80b3.8158. [DOI] [PubMed] [Google Scholar]
- 8.Taki N, Tatro JM, Nalepka JL, Togawa D, Goldberg VM, Rimnac CM, Greenfield EM. Polyethylene and titanium particles induce osteolysis by similar, lymphocyte-independent, mechanisms. J Orthop Res. 2005;23:376–83. doi: 10.1016/j.orthres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi Y, Kim K, Itoh T. Gene expression of bone-resorbing cytokines in rat osteolysis model. J Orthop Sci. 2005;10:62–9. doi: 10.1007/s00776-004-0846-8. [DOI] [PubMed] [Google Scholar]
- 10.Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Amer J Pathol. 1999;154:203–10. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz EM, Lu AP, Goater JJ, Benz EB, Kollias G, Rosier RN, Puzas JE, O’Keefe RJ. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res. 2000;18:472–80. doi: 10.1002/jor.1100180321. [DOI] [PubMed] [Google Scholar]
- 12.Childs L, Goater J, O’Keefe R, Schwarz E. Effect of anti-tumor necrosis factor-alpha gene therapy on wear debris-induced osteolysis. J Bone Joint Surg. 2001;83A:1789–97. doi: 10.2106/00004623-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Childs L, Goater J, O’Keefe R, Schwarz E. Efficacy of etanercept for wear debris-induced osteolysis. J Bone Miner Res. 2001;16:338–47. doi: 10.1359/jbmr.2001.16.2.338. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson JM, Wilson A, Stockley I, Scott I, Macdonald D, Hamer A, Duff G, Eastell R. Variation in the TNF gene promoter and risk of osteolysis after total hip arthroplasty. J Bone Miner Res. 2003;18:1995–2001. doi: 10.1359/jbmr.2003.18.11.1995. [DOI] [PubMed] [Google Scholar]
- 15.Jiranek WA, Machado M, Jasty M, Jevsevar D, Wofe HJ, Goldring SR, Goldberg MJ, Harris WH. Production of cytokines around loosened cemented acetabular components. J Bone Joint Surg. 1994;75A:863–79. doi: 10.2106/00004623-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Konttinen Y, Kurvinen H, Takagi M, Michelsson J, Eklund K, Nordsletten L, Buo L, Aasen A, Santavirta S. Interleukin-1 and collagenases around loosening total hip prostheses. Clin Exp Rheumatol. 1996;14:255–62. [PubMed] [Google Scholar]
- 17.Epstein N, Warme B, Spanogle J, Ma T, Bragg B, Smith R, Goodman S. Interleukin-1 modulates periprosthetic tissue formation in an intramedullary model of particle-induced inflammation. J Orthop Res. 2005;23:501–10. doi: 10.1016/j.orthres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Yang SY, Wu B, Mayton L, Mukherjee P, Robbins PD, Evans CH, Wooley PH. Protective effects of IL-1Ra or vIL-10 gene transfer on a murine model of wear debris-induced osteolysis. Gene Ther. 2004;11:483–91. doi: 10.1038/sj.gt.3302192. [DOI] [PubMed] [Google Scholar]
- 19.Gordon A, Wilkinson JM, Wilson AG, Stockley I, MacDonald D, Eastell R. Polymorphisms in the interleukin-one gene cluster and the risk of aseptic loosening after total hip arthroplasty. J Bone Miner Res. 2003;18(S2):326. [Google Scholar]
- 20.Konttinen Y, Xu J, Waris E, Li T, Gomez-Barrena E, Nordsletten L, Santavirta S. Interleukin-6 in aseptic loosening of total hip replacement prostheses. Clin Exp Rheumatol. 2002;20:485–90. [PubMed] [Google Scholar]
- 21.Udagawa N, Takahashi N, Katagiri T, Tamura T, Wada S, Findlay DM, Martin TJ, Hirota H, Taga T, Kishimoto T, Suda T. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast precursors. J Exp Med. 1995;182:1461–8. doi: 10.1084/jem.182.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ragab AA, Nalepka JL, Bi Y, Greenfield EM. Cytokines synergistically induce osteoclast differentiation: support by immortalized or normal calvarial cells. Am J Physiol Cell Physiol. 2002;283:C679–C87. doi: 10.1152/ajpcell.00421.2001. [DOI] [PubMed] [Google Scholar]
- 23.Wong P, Quinn J, Sims N, van Nieuwenhuijze A, Campbell I, Wicks I. Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum. 2006;54:158–68. doi: 10.1002/art.21537. [DOI] [PubMed] [Google Scholar]
- 24.Ohishi M, Matsumura Y, Aki D, Mashima R, Taniguchi K, Kobayashi T, Kukita T, Iwamoto Y, Yoshimura A. Suppressors of cytokine signaling-1 and -3 regulate osteoclastogenesis in the presence of inflammatory cytokines. J Immunol. 2005;174:3024–31. doi: 10.4049/jimmunol.174.5.3024. [DOI] [PubMed] [Google Scholar]
- 25.Perry M, Ponsford F, Mortuza F, Learmonth I, Atkins R, Elson C. Osteolytic properties of the synovial-like tissue from aseptically failed joint prostheses. Br J Rheumatol. 1996;35:943–50. doi: 10.1093/rheumatology/35.10.943. [DOI] [PubMed] [Google Scholar]
- 26.Ragab AA, Van De Motter R, Lavish SA, Goldberg VM, Ninomiya JT, Carlin CR, Greenfield EM. Measurement and removal of adherent endotoxin from titanium particles and implant surfaces. J Orthop Res. 1999;17:803–9. doi: 10.1002/jor.1100170603. [DOI] [PubMed] [Google Scholar]
- 27.Bi Y, Van De Motter RR, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Titanium particles stimulate bone resorption by inducing differentiation of murine osteoclasts. J Bone Joint Surg. 2001;83A:501–8. doi: 10.2106/00004623-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Kaar SG, Ragab AA, Kaye SJ, Kilic BA, Jinno T, Goldberg VM, Bi Y, Stewart MC, Carter JR, Greenfield EM. Rapid repair of titanium particle-induced osteolysis is dramatically reduced in aged mice. J Orthop Res. 2001;19:171–8. doi: 10.1016/S0736-0266(00)00033-4. [DOI] [PubMed] [Google Scholar]
- 29.Bi Y, Seabold JM, Kaar SC, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopaedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16:2082–91. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- 30.Arend WP. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu S, Asou Y, Okuda N, Kato N, Rittling S, Shinomiya K, Muneta T, Denhardt D, Noda M. Osteopontin deficiency impairs wear debris-induced osteolysis. J Bone Miner Res. 2005;20:S258. doi: 10.1002/art.27400. [DOI] [PubMed] [Google Scholar]
- 32.Kassiotis G, Kollias G. TNF and receptors in organ-specific autoimmune disease: multi-layered functioning mirrored in animal models. J Clin Invest. 2001;107:1507–8. doi: 10.1172/JCI13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moro T, Takatori Y, Ishihara K, Konno T, Takigawa Y, Matsushita T, Chung U, Nakamura K, Kawaguchi H. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat Mater. 2004;3:829–36. doi: 10.1038/nmat1233. [DOI] [PubMed] [Google Scholar]
- 34.Nalepka JL, Lowe RW, Greenfield EM. The role of ODF/OPGL in stimulation of osteoclast differentiation by TNF-alpha. Trans Orthop Res Soc. 2001;26:40. [Google Scholar]
- 35.The American Society for Bone and Mineral Research President’s Committee on Nomenclature. Proposed standard nomenclature for new tumor necrosis factor members involved in the regulation of bone resorption. Bone. 2000;27:761–4. doi: 10.1016/s8756-3282(00)00420-8. [DOI] [PubMed] [Google Scholar]
- 36.Akisue T, Bauer TW, Farver CF, Mochida Y. The effect of particle wear debris on NFkappaB activation and pro-inflammatory cytokine release in differentiated THP-1 cells. J Biomed Mater Res. 2002;259:507–15. doi: 10.1002/jbm.1264. [DOI] [PubMed] [Google Scholar]
- 37.Maloney WJ, James RE, Smith RL. Human macrophage response to retrieved titanium alloy particles in vitro. Clin Orthop Rel Res. 1996;322:268–78. [PubMed] [Google Scholar]
- 38.Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Human monocyte response to particulate biomaterials generated in vivo and in vitro. J Orthop Res. 1995;13:792–801. doi: 10.1002/jor.1100130520. [DOI] [PubMed] [Google Scholar]
- 39.Nelson CL, McLaren AC, McLaren SG, Johnson JJ, Smeltzer MS. Is aseptic loosening truly aseptic? Clin Orthop Rel Res. 2005;437:25–30. doi: 10.1097/01.blo.0000175715.68624.3d. [DOI] [PubMed] [Google Scholar]
- 40.Sundfeldt M, Carlsson L, Johansson C, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77:177–97. doi: 10.1080/17453670610045902. [DOI] [PubMed] [Google Scholar]
- 41.Childs L, Paschalis E, Xing L, Dougall W, Anderson D, Boskey A, Puzas J, Rosier R, O’Keefe R, Boyce B, Schwarz E. In vivo RANK signaling blockade using the receptor activator of NF-kappaB:Fc effectively prevents and ameliorates wear debris-induced osteolysis via osteoclast depletion without inhibiting osteogenesis. J Bone Miner Res. 2002;17:192–9. doi: 10.1359/jbmr.2002.17.2.192. [DOI] [PubMed] [Google Scholar]
- 42.Goater J, O’Keefe R, Rosier R, Puzas J, Schwarz E. Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthop Res. 2002;20:169–73. doi: 10.1016/S0736-0266(01)00083-3. [DOI] [PubMed] [Google Scholar]
- 43.Ulrich-Vinther M, Carmody E, Goater J, Soballe K, O’Keefe R, Schwarz E. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J Bone Joint Surg. 2002;84A:1405–12. doi: 10.2106/00004623-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 44.Chiang CY, Kyritsis G, Graves DT, Amar S. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect Immun. 1999;67:4231–6. doi: 10.1128/iai.67.8.4231-4236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haddad J. Cytokines and related receptor-mediated signaling pathways. Biochem Biophys Res Commun. 2002;297:700–13. doi: 10.1016/s0006-291x(02)02287-8. [DOI] [PubMed] [Google Scholar]
- 46.Kong Y, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger J. OPGL is a key regulator of osteoclastogenesis, lympocyte development and lymph node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 47.Rychly D, DiPiro J. Infections associated with tumor necrosis factor-alpha antagonists. Pharmacotherapy. 2005;25:1181–92. doi: 10.1592/phco.2005.25.9.1181. [DOI] [PubMed] [Google Scholar]
- 48.Keystone E. Safety of biologic therapies--an update. J Rheumatol. 2005;74 (Suppl):8–12. [PubMed] [Google Scholar]
- 49.Taki N, Seabold J, Ragab A, Goldberg V, Greenfield E. Titanium induced osteolysis is decreased in interluekin-6 knock out mice. Trans Orthop Res Soc. 2002;27:1045. [Google Scholar]
- 50.Carmody E, O’Keefe R, Schwarz E. The role of interleukin-6 in periprosthetic osteolysis and aseptic loosening of prosthetic joint implants. Trans Orthop Res Soc. 2002;27:1052. [Google Scholar]
- 51.Schwarz E, Campbell D, Totterman S, Boyd A, O’Keefe R, Looney R. Use of volumetric computerized tomography as a primary outcome measure to evaluate drug efficacy in the prevention of peri-prosthetic osteolysis: a 1-year clinical pilot of etanercept vs. placebo J Orthop Res. 2003;21:1049–55. doi: 10.1016/S0736-0266(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 52.Yang S, Nasser S, Markel D, Robbins P, Wooley P. Human periprosthetic tissues implanted in severe combined immunodeficient mice respond to gene transfer of a cytokine inhibitor. J Bone Joint Surg. 2005;87A:1088–97. doi: 10.2106/JBJS.D.02052. [DOI] [PubMed] [Google Scholar]


