Abstract
Previous findings suggest that the rostral anterior cingulate cortex (rACC) is involved in memory for emotionally arousing training. There is also extensive evidence that the basolateral amygdala (BLA) modulates the consolidation of emotional arousing training experiences via interactions with other brain regions. The present experiments examined the effects of posttraining intra-rACC infusions of the cholinergic agonist oxotremorine (OXO) on inhibitory avoidance (IA) retention and investigated whether the BLA and rACC interact in enabling OXO effects on memory. In the first experiment, male Sprague–Dawley rats were implanted with bilateral cannulae above the rACC and given immediate posttraining OXO infusions. OXO (0.5 or 3 ng) induced significant enhancement of retention performance on a 48-h test. In the second experiment, unilateral posttraining OXO infusions (0.5, 3.0 or 10 ng) enhanced retention when infused into rACC, but not caudal ACC, consistent with previous evidence that ACC is composed of functionally distinct regions. A third experiment investigated the effects of posttraining intra-rACC OXO infusions (0.5 or 10 ng) in rats with bilateral sham or NMDA-induced lesions of the BLA. The BLA lesions did not impair IA retention, but blocked the enhancement induced by posttraining intra-rACC OXO infusions. Lastly, unilateral NMDA lesions of rACC blocked the enhancement of IA retention induced by posttraining ipsilateral OXO infusions into the BLA. These findings support the hypothesis that the rACC is involved in modulating the storage of emotional events and provide additional evidence that the BLA modulates memory consolidation through interactions with efferent brain regions, including the cortex.
Keywords: Oxotremorine, Basolateral amygdala, Memory, Inhibitory avoidance, Cholinergic stimulation, Cingulate cortex
1. Introduction
Substantial evidence from animal studies indicates that the anterior cingulate cortex (ACC) is involved in memory for training on aversive tasks. ACC lesions impair acquisition of active avoidance responses (Gabriel, Kubota, Sparen-borg, Straube, & Vogt, 1991; Kimble & Gostnell, 1968; Peretz, 1960) and posttraining infusions of various neuro-transmitter agonists and antagonists into the ACC affect retention of aversively motivated training (Farr, Uezu, Creonte, Flood, & Morley, 2000). Evidence from both human and animal studies suggests the ACC is composed of two functionally distinct regions. Findings of fMRI studies in human subjects indicate that emotional tasks activate the rostral ACC (rACC), whereas cognitive tasks activate caudal ACC (cACC) (Bush et al., 1998; Davis et al., 2005; Phan, Liberzon, Welsh, Britton, & Taylor, 2003; Whalen et al., 1998). Experiments investigating the effects of rACC and cACC lesions in rats suggest that only the rACC is involved in aversive learning. Lesions of the rACC impair conditioned place avoidance (CPA) induced by hindpaw-formalin injections, whereas cACC lesions do not prevent such learning (Johansen, Fields, & Manning, 2001). Pre-training intra-rACC drug infusions also affect acquisition of inhibitory avoidance (IA) and CPA (Johansen & Fields, 2004; Riekkinen, Kuitunen, & Riekkinen, 1995) and rACC lesions impair classical conditioning of a tone paired with a noxious CO2 laser pulse to the hindpaw (Kung, Su, Fan, Chai, & Shyu, 2003). Furthermore, posttraining rACC lesions and infusions of cholinergic antagonists impair retention (Hudspeth & Wilsoncroft, 1969; Riekkinen et al., 1995).
Extensive evidence indicates the basolateral amygdala (BLA) modulates memory consolidation via interactions with other brain areas (McGaugh, 2004; Packard, Cahill, & McGaugh, 1994). Lesions or pharmacological inactivation of BLA functioning block the memory-modulatory effects of drugs infused into other brain regions, including the hippocampus (Roozendaal & McGaugh, 1997; Roozendaal, Nguyen, Power, & McGaugh, 1999), entorhinal cortex (Roesler, Roozendaal, & McGaugh, 2002), medial prefrontal cortex (Roozendaal, McReynolds, & McGaugh, 2004; McGaugh, 2004), insular cortex (Miranda & McGaugh, 2004) and nucleus accumbens (Roozendaal, de Quervain, Ferry, Setlow, & McGaugh, 2001). Evidence of direct connections between the BLA and rACC (Krettek & Price, 1977; McDonald, 1991; Sarter & Markowitsch, 1983; Sripanidkulchai, Sripanidkulchai, & Wyss, 1984) suggests that the rACC and BLA may interact in consolidating aversive memories. To address this issue, the current experiments investigated the involvement of rostral and caudal ACC in modulating the storage of memory for IA training and, additionally, investigated whether the memory-modulating effects of treatments affecting either the rACC or BLA require normal functioning of the other region. We used the muscarinic cholinergic agonist oxotremorine (OXO) based on previous findings that muscarinic agonists and antagonists infusions into the ACC or BLA affect retention of aversive training (Farr et al., 2000; Introini-Collison & McGaugh, 1988; Malin & McGaugh, 2006; Riekkinen et al., 1995). In Experiment 1, rats received IA training followed by immediate bilateral OXO infusions into the rACC. Experiment 2 investigated the effects of unilateral posttraining OXO infusions administered selectively into either the rACC or cACC. Experiment 3 examined the effect of posttraining intra-rACC OXO infusions in rats with BLA lesions. Lastly, Experiment 4 investigated whether lesions of the rACC alter the memory-modulating effect of posttraining OXO infusions into the BLA.
2. Materials and methods
2.1. Subjects
Male Sprague–Dawley rats (approximately 300 g at the time of surgery) from Charles River Laboratories (Wilmington, MA, USA) were housed individually in a temperature-controlled (22 °C) vivarium and maintained on a 12-h light/dark cycle (7 A.M.–7 P.M.) with food and water ad libitum. Rats were given 1 week to acclimate to the vivarium prior to surgery. Behavioral procedures began 6–10 days after surgery. All training and testing occurred between 10:00 A.M. and 5:00 P.M. All procedures were in accordance with NIH guidelines and were approved by the UC Irvine Institutional Animal Care and Use Committee.
2.2. Surgery
The rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and given atropine sulfate (0.1 mg/2 ml, i.p.) to maintain respiration and 0.9% sterile saline to prevent dehydration (3.0 ml). The animals were then placed in a small animal stereotaxic frame (Kopf Instruments, Tujunga, CA, USA). In Experiment 1, animals were implanted bilaterally with stainless steel guide cannulae (10 mm; 23 gauge) aimed above the rACC. The following stereotaxic coordinates were used: anteroposterior (AP), +2.7 mm from bregma; mediolateral (ML), ±0.5 mm from midline; dorsoventral (DV), −1.5 mm from skull surface. In Experiment 2, animals were implanted with unilateral cannulae, counterbalanced for side, aimed above either the rACC or cACC. The stereotaxic coordinates for cACC were: AP, +0.2 mm; ML, ±0.5 mm; DV, −1.5. In Experiment 3, bilateral neurotoxic lesions of the BLA were made by infusing N-methyl-D-aspartate (NMDA; Sigma, St. Louis, MO, USA; 0.2 μL, 0.55 μL/min) through an infusion needle that was lowered into the BLA (AP, −2.8 mm; ML ±5.0 mm; DV, −8.5 mm). The NMDA was dissolved into 0.9% saline (12.5 mg/mL) and infused through a 30 gauge needle that was attached via polyethylene tubing to a 10 μL Hamilton syringe and driven by an automated syringe pump (Sage Instruments, Boston, MA, USA). Sham operations used the same procedure, with the exception that the infusion needle was lowered to a level just dorsal to the BLA (DV, −6.5 mm) and no solution was infused. After lesioning of the BLA, animals were implanted with unilateral cannulae, counterbalanced for side, aimed above the rACC. The cannulae were affixed to the skull with dental cement and two anchoring surgical screws (Small Parts, Inc., Miami Lakes, FL, USA) and insect pins (10 mm long 00 insect dissection pins) were inserted into the cannulae to maintain patency. The same procedures were used in Experiment 4 to lesion the rACC unilaterally on the right side (0.5 μL, 0.55 μL/min; AP, +2.1, ML −0.5, DV, −2.6) and implant ipsilateral cannulae (15mm) into the BLA (AP, −2.8, ML, −5.0, DV, −6.5). The lesions and cannulae were limited to the right side based on recent evidence from our laboratory that infusions into the right BLA are more effective than those into the left side (Lalumiere & McGaugh, 2005). After the surgery, the rats were kept in an incubator until they awoke from the anesthesia.
2.3. Behavioral procedures
Each rat was handled for at least 1 min per day for 3 days before the start of training, to allow habituation to the infusion procedure. The rats were trained and tested in a step-through inhibitory avoidance task. The IA apparatus consisted of a trough-shaped alley (91 cm long, 15 cm deep) divided into two compartments by a retractable door: an illuminated safe compartment (31 cm long) and a dark shock compartment (60 cm long). On the training trial each rat was placed in the lit start compartment facing away from the shock compartment. After the rat stepped with all four paws into the dark compartment, the retractable door was closed and an inescapable mild footshock (0.38 mA, 1.0 s for Experiments 1–3; 0.45 mA, 1.0 s for Experiment 4) was administered. The rat was removed from the dark compartment 15 s after termination of the footshock and immediately given its appropriate intra-rACC (Experiments 1–3), cACC (Experiment 2) or BLA (Experiment 4) infusion. On the retention tested 48 h later each rat was placed into the light compartment with the retractable door open and permitted to explore the box freely. The latency to enter the dark (shock) compartment with all four paws was recorded with a timer by a nearby experimenter as a measure of retention. A maximum of 600 s was recorded on the retention test.
2.4. Drugs and infusion procedures
For each experiment the drug solutions were infused directly into the rACC, cACC or BLA immediately following IA training to selectively affect the consolidation phase of memory. Control animals received infusions of the vehicle solution (0.9% sterile saline). All other animals received infusions of the non-selective muscarinic cholinergic agonist oxotremorine (OXO) (0.5, 3, or 10 ng in 0.5 μl for rACC and cACC; 10 or 100 ng in 0.2 μl for BLA). OXO was obtained from Sigma (St. Louis, MO, USA). The doses of OXO for ACC and BLA infusions were chosen based on those used in previous studies (Farr et al., 2000; Introini-Collison & McGaugh, 1988; Malin & McGaugh, 2006).
The drug solutions were infused at a constant rate, over 57.5 s for rACC and cACC infusions and 32 s for BLA infusions, by an automated syringe pump (Sage Instruments, Boston, MA, USA) through a 30 gauge needle (extending 1 mm past the cannulae for rACC and cACC, 2 mm for BLA) that was attached by polyethylene tubing to a 10 μl Hamilton syringe.
2.5. Histology
After the behavioral tests were completed, the rats were anesthetized with an overdose of sodium pentobarbital (100 mg/kg i.p.) and perfused intracardially with 0.9% saline and then 10% formaldehyde. After decapitation, the brains were removed and placed in 10% formaldehyde for a minimum of 24 h, and were then cryoprotected in a 30% sucrose solution. Coronal slices of 50 μm were taken with a freezing microtome, mounted on gelatin-coated slides, and stained with thionin. The sections were examined under a light microscope to determine injection needle placement and size and extent of lesions according to the standardized atlas plates of Paxinos and Watson (2005).
2.6. Statistics
The behavioral data from Experiments 1 and 2 were analyzed with a one-way ANOVA, with intra-rACC drug treatment as the between-subjects variable. The behavioral data of Experiment 3 were analyzed with a two-way ANOVA, with intra-rACC drug treatment as the repeated measure and BLA lesion as the between-subjects variable. The behavioral data of Experiment 4 were also analyzed with a two-way ANOVA, with intra-BLA drug treatment as the repeated measure and rACC lesion as the between-subjects variable. For each experiment, Fisher's post-hoc tests were performed to determine the source of detected significance. p values of <.05 were considered significant. All measures are expressed as means ± SEM.
3. Results
3.1. Histology
Figs. 1 and 3 show representative photomicrographs of needle tracks terminating in the rACC and cACC. Animals with improper injection needle placements were excluded from the analysis . Fig. 5A shows a representative BLA lesion, and Fig. 5B shows a sham lesion control . Fig. 7A and B show representative rACC NMDA lesions and sham lesions, respectively. Only animals with accurate lesions of the respective brain region were included in the analysis. Animals with lesions that were either too small or extended significantly into other brain regions were excluded from the analysis. In some cases, minor damage was seen in the cortex adjacent to the BLA, but the central nucleus of the amygdala was intact in all animals. The smallest and largest extents of BLA and rACC lesions are shown in Figs. 5C and 7C. Fig. 8 shows a representative photomicrograph of a needle track terminating in the BLA.
Fig. 1.
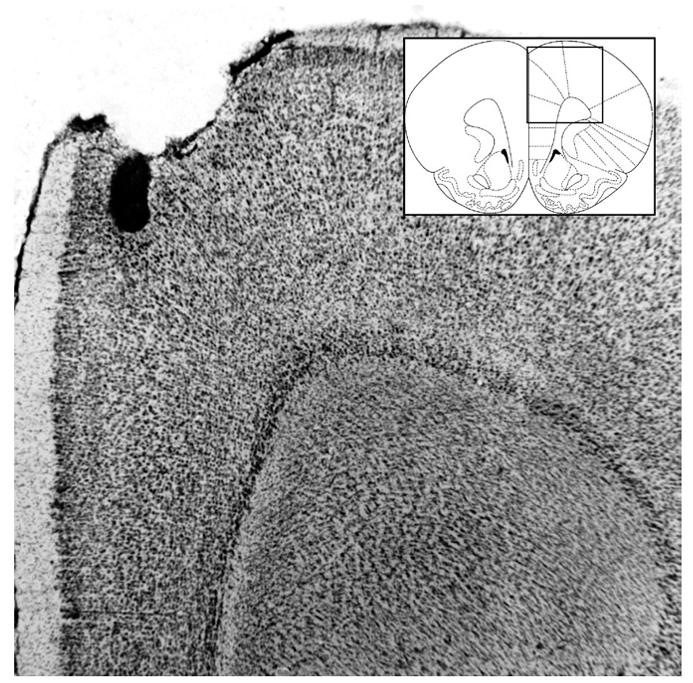
Representative photomicrograph of needle track terminating in the rACC with a diagram of rACC and surrounding structures (Paxinos & Watson, 2005).
Fig. 3.
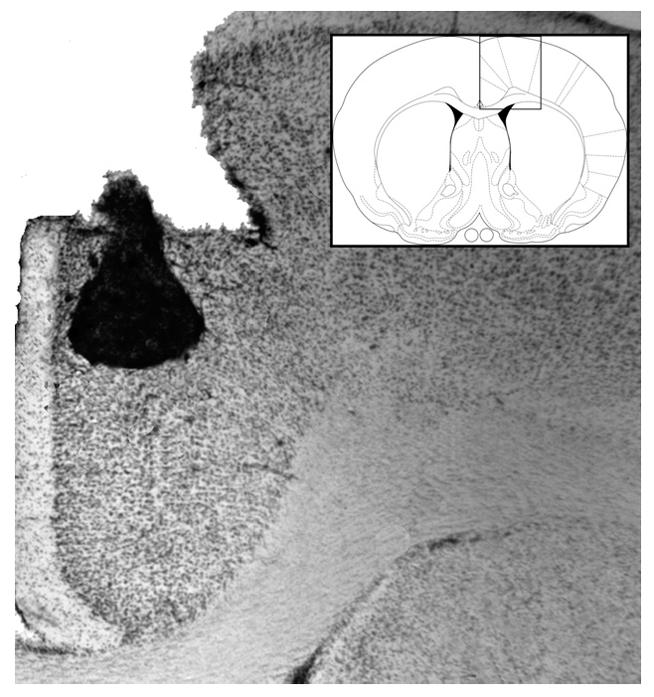
Representative photomicrograph of needle track terminating in the cACC with a diagram of cACC and surrounding structures (Paxinos & Watson, 2005).
Fig. 5.
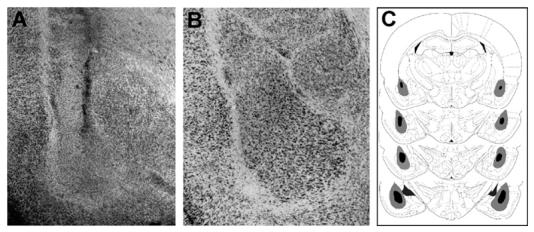
(A) Representative photomicrograph of BLA lesion. (B) Representative photomicrograph of sham BLA lesion. (C) Smallest (black area) and largest (gray area) BLA lesions from rats used in the experiment (Paxinos & Watson, 2005).
Fig. 7.
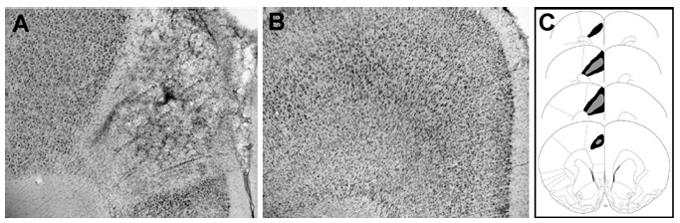
(A) Representative photomicrograph of rACC lesion. (B) Representative photomicrograph of sham rACC lesion. (C) Smallest (black area) and largest (gray area) rACC lesions from rats used in the experiment (Paxinos & Watson, 2005).
Fig. 8.
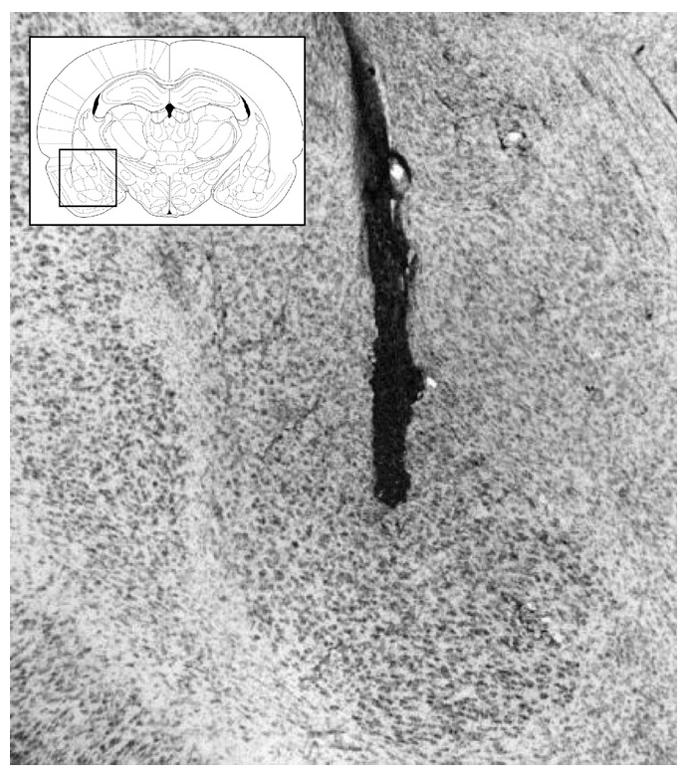
Representative photomicrograph of needle track terminating in the BLA with a diagram of BLA and surrounding structures (Paxinos & Watson, 2005).
3.2. Experiment 1
The results of animals given immediate posttraining bilateral intra-rACC infusions are shown in Fig. 2. A one-way ANOVA revealed a significant effect (F(2,29) = 3.910; p<.05). The retention latencies of the rats given 0.5 and 3 ng doses of OXO were significantly longer than those of the saline control group (p<.05 for both comparisons).
Fig. 2.
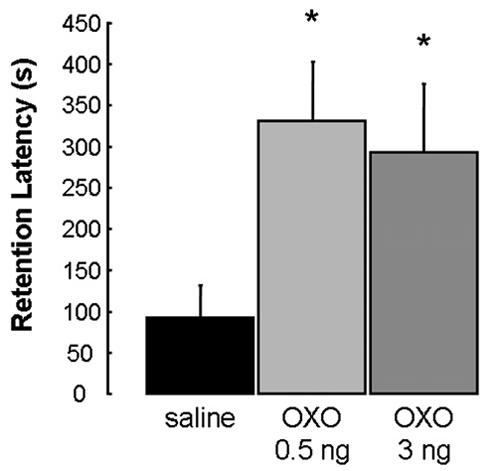
Enhanced retention of rats that received bilateral infusions of OXO into the rACC immediately following IA training. Results represent mean + SEM retention latencies in seconds. *p < .05 compared with vehicle group (n = 11, 12 and 9, respectively).
3.3. Experiment 2
Fig. 4 shows the retention latencies of animals given immediate posttraining unilateral infusions into the rACC and cACC. A one-way ANOVA of the intra-rACC infusions revealed a significant effect (F(3,64) = 5.073; p<.005). Animals given infusions of all three doses of OXO into the rACC had significantly longer retention latencies than those of saline controls (p<.005 for 0.5 and 3 ng OXO, p<.05 for 10 ng OXO). A one-way ANOVA of the intra-cACC infusions showed no significant drug effect (F(3,34)=0.032; p>.05). The retention latencies of animals given IA training with no footshock and immediate post-training OXO infusions into the rACC were similar to those of saline controls. A one-way ANOVA revealed no significant drug effect (F(1,12)=0.541; p>.05; data not shown).
Fig. 4.
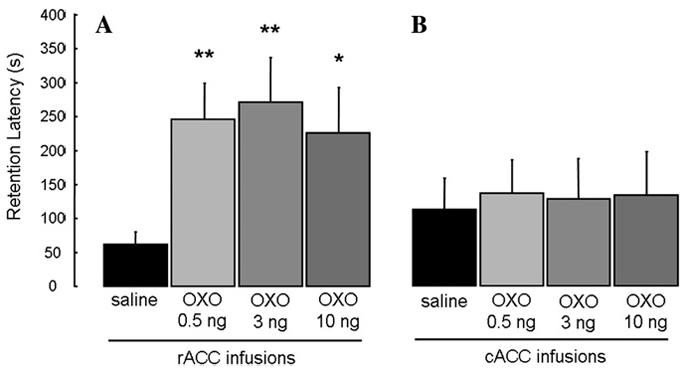
Enhanced retention of rats that received unilateral infusions of OXO into the rACC (A), but not cACC (B), immediately following IA training. Results represent mean + SEM retention latencies in seconds. *p < .05; **p < .005 compared with vehicle group. (A) (n = 25, 16, 15, and 12, respectively); (B) (n = 9, 8, 10, and 11, respectively).
3.4. Experiment 3
The retention latencies of animals given excitotoxic or sham lesions of the BLA and immediate posttraining intra-rACC infusions are shown in Fig. 6. A two-way ANOVA revealed a significant main effect of the BLA lesions (F(1,66)=23.602, p<.0001), a significant main effect of the intra-rACC infusions (F(2,66)=4.413, p<.05), and a significant interaction between the BLA lesions and the intra-rACC infusions (F(2,66)=4.081, p<.05). The retention latencies of sham-lesioned rats given intra-rACC infusions of 0.5 and 10 ng OXO were significantly longer than those of saline controls (p<.001 for 0.5 ng and p<.05 for 10 ng) and their respective lesion counterparts (p<.0001 for 0.5 ng and p<.05 for 10 ng). Furthermore, the retention latencies of BLA-lesioned rats given intra-rACC infusions of OXO did not differ from those of saline controls (p>.05). In addition, the latencies of the BLA-lesioned rats given saline into the rACC did not differ from those of the sham-lesioned saline controls (p>.05).
Fig. 6.
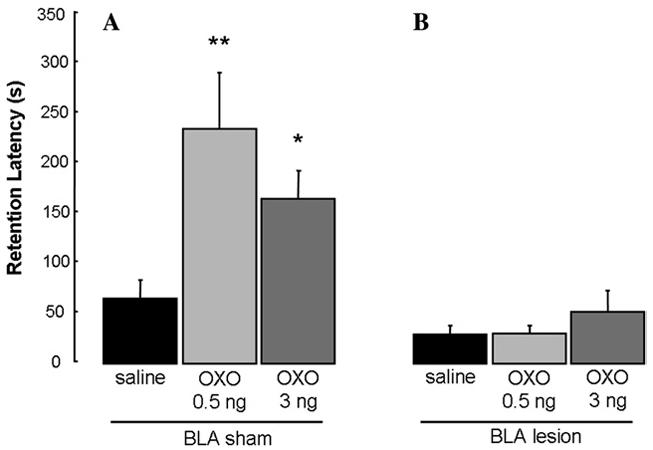
Enhanced retention of rats that received unilateral infusions of OXO into the rACC (A) was blocked by bilateral BLA lesions (B). *p < .05; **p < .01 compared with vehicle group. (A) (n = 11, 13, and 12, respectively) (B) (12, 13, and 11, respectively).
3.5. Experiment 4
Fig. 9 shows the retention latencies of animals given unilateral excitotoxic or sham lesions of the rACC and immediate posttraining intra-BLA infusions. A two-way ANOVA revealed a significant main effect of the rACC lesions (F(1,70)=17.540, p<.0001), a significant main effect of the intra-BLA infusions (F(2,70)=3.615, p<.05), and a significant interaction between the rACC lesions and the intra-BLA infusions (F(2, 70)=5.393, p<.01). The retention latencies of sham-lesioned rats given intra-BLA infusions of 10 and 100 ng OXO were significantly longer than those of saline controls (p<.01 for 10 ng and p<.0001 for 100 ng) and their respective lesion counterparts (p<.001 for 10 ng and p<.0001 for 100 ng). The retention latencies of rACC-lesioned rats given infusions of OXO into the BLA did not differ from those of saline controls (p>.05). Finally, rACC-lesioned rats that received intra-BLA infusions of OXO did not differ from the sham-lesioned saline controls (p>.05).
Fig. 9.
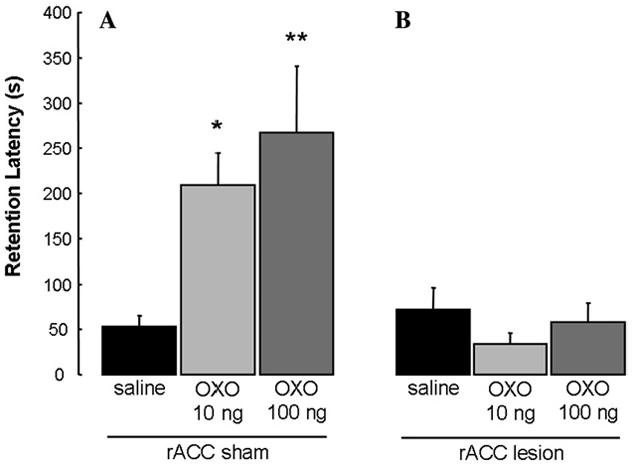
Enhanced retention of rats that received unilateral infusions of OXO into the BLA (A) was blocked by unilateral rACC lesions (B). *p < .01; **p < .0001 compared with vehicle group. (A) (n = 14, 16, and 11, respectively) (B) (8, 14, and 13, respectively).
4. Discussion
These findings provide evidence that (1) the ACC is involved in memory consolidation for IA training, (2) the involvement is restricted selectively to rACC and, (3) the memory-modulatory effects of OXO infusions into the rACC or BLA are blocked by a lesion of the other region. Bilateral infusions of the muscarinic cholinergic agonist OXO administered into the rACC posttraining enhanced IA retention. Posttraining unilateral OXO infusions enhanced retention when administered into rACC but not when administered into the immediately adjacent cACC. Unilateral posttraining rACC infusions did not enhance memory in rats with BLA lesions. Similarly, unilateral intra-BLA OXO infusions did not enhance IA retention in rats with lesions of the rACC. These results are consistent with previous findings that the rACC is involved in memory consolidation of aversive experiences as well as extensive evidence that the BLA interacts with other brain regions during the consolidation of emotionally arousing experiences.
The findings of both anatomical and behavioral studies suggest that ACC is best characterized as a network of distinct regions. A recent study of intrinsic connectivity within the ACC of rats showed that rostral areas of ACC are predominantly interconnected with each other and have little communication with other cingulate regions, including cACC (Jones, Groenewegen, & Witter, 2005). Our finding that OXO infusions enhanced memory for IA when administered posttraining into rACC, but not when infused into the adjacent cACC, fits well with evidence that lesions of the rostral, but not the cACC, impaired hindpaw-formalin induced CPA (Johansen et al., 2001). Thus, only rACC appears to be implicated in modulating memory for IA and CPA, both of which are aversive, emotionally arousing tasks. These findings provide strong evidence that ACC is composed of at least two functionally distinct regions that differ in their involvement in emotionally arousing tasks.
One of the major aims of the present experiments was to determine whether BLA lesions block the effect of post-training intra-rACC infusions. These two regions are directly connected, and thus there is an anatomical basis for a potential interaction. As discussed above, it is well established that the BLA modulates memory by influencing efferent brain structures (McGaugh, 2002, 2004). BLA stimulation induces changes in activity of the neocortex and hippocampus, Arc protein and c-fos expression in the hippocampus, c-fos expression in the caudate nucleus, and influences the stabilization of thalamo-cortical long-term potentiation (Dringenberg & Vanderwolf, 1996, 1997; Dringenberg, Kuo, & Tomaszek, 2004; McIntyre et al., 2005; Packard, Williams, Cahill, & McGaugh, 1995). Moreover, previous findings from our laboratory indicate that lesions or pharmacological inactivation of the BLA prevent the memory-modulatory effects of drugs infused into other brain regions, including the hippocampus (Roozendaal & McGaugh, 1997; Roozendaal et al., 1999), entorhinal cortex (Roesler et al., 2002), medial prefrontal cortex (Roozendaal et al., 2004), insular cortex (Miranda & McGaugh, 2004) and nucleus accumbens (Roozendaal et al., 2001). The findings of Experiment 3 clearly indicate that BLA lesions also prevent the memory enhancement induced by posttraining intra-rACC OXO infusions.
Another objective of the current experiments was to determine whether lesions of the rACC affect the memory-modulatory influence of intra-BLA OXO infusions. As discussed above, BLA lesions block the efficacy of drug infusions into a variety of efferent brain regions. However, previous experiments had not examined whether a lesion of one of these efferent brain regions would have a comparable effect on intra-BLA drug infusions. Our findings indicate that rACC lesions block the memory enhancement of IA retention induced by intra-BLA OXO infusions. Importantly, rACC lesions alone had no effect on retention, as the lesions were unilateral and the ipsilateral rACC was intact and functioning. The finding that a lesion of either region, BLA or rACC, blocks the efficacy of infusions into the other region might be interpreted as evidence that the BLA and the rACC play interchangeable roles during memory consolidation. However, other evidence very strongly suggests that the rACC and BLA are involved in different aspects of memory consolidation. As noted above, there is considerable evidence that the BLA modulates memory consolidation of many tasks other than IA, such as spatial-water maze, appetitive T-maze tasks, conditioned taste aversion, contextual and cued fear conditioning, and conditioned place preference (e.g., LaLumiere, Buen, & McGaugh, 2003; Miranda & McGaugh, 2004; Roozendaal & McGaugh, 1997, 2004; Roozendaal, Hui, Hui, Berlau, Mc Gaugh, & Weinberger, 2006; Schroeder & Packard, 2002, 2003). In contrast, recent evidence suggests that the rACC may only modulate memory for aversive tasks that involve a nociceptive stimulus. As noted above, rACC lesions impaired CPA for a nociceptive hindpaw-formalin injection. However, in a subsequent experiment, rACC lesions did not disrupt the acquisition of CPA when the aversive stimulus was a nonnociceptive i.p. κ-opiod injection (Johansen et al., 2001). Moreover, we recently found a functional dissociation between the BLA and rACC in modulating memory for different aspects of training experiences. In that experiment (Malin & McGaugh, 2006) we first exposed rats to the inhibitory avoidance training context and then, on a subsequent day gave them a brief footshock in that context. Memory was assessed 24 h later by latencies to enter the shock compartment. Intra-rACC OXO infusions enhanced retention latencies when administered after the footshock, but not context, training. In contrast, infusions of OXO into the BLA induced an enhancement of retention when administered after either the context training or the footshock training. An additional finding was that intra-hippocampal infusions of OXO enhanced retention only when administered after the context exposure. If the BLA and rACC play interchangeable roles in IA memory consolidation, then intra-BLA OXO infusions would have been expected to induce effects comparable to those given into the rACC. These findings clearly suggest that the rACC is selectively involved in influencing memory of the footshock experience of training, whereas the BLA is more liberally involved in influencing memory for both the context and footshock components of training (Malin & McGaugh, 2006). These findings are consistent with extensive evidence cited above indicating that the BLA modulates the consolidation of memory for a wide variety of training experiences (McGaugh, 2004). Our experiments did not investigate whether lesions of the rACC would block the memory-modulatory influences induced by stimulation of other brain regions. Whether such effects, if obtained, would be restricted to modulation of memory for nociceptive stimulation remains to be investigated.
Importantly, our findings do not address the issue of the locus of the permanent memory of IA training; rather, the present findings examined the role of the rACC and BLA in modulating memory storage. As the current experiments utilized post-training infusions into the rACC to influence the consolidation phase of memory, the role of the rACC in the storage of memory for IA training cannot be inferred. Instead, the present findings indicate that the rACC plays a role in modulating the consolidation of this memory. Recent findings that lidocaine infusions into ACC disrupt retention performance when administered 30 days or longer after training but not 1 day after training (Frankland, Bontempi, Talton, Kaczmarek, & Silva, 2004; Maviel, Durkin, Menzaghi, & Bontempi, 2004) have suggested that the ACC may be a locus of storage of long-term memory or a site of integration of cortical representations. However, as the ACC infusions used in these studies did not selectively target the rACC or cACC, it remains possible that these two regions of ACC may be differentially involved in the storage of memory. Moreover, previous studies reporting that rACC lesions given 1 day after training, or that scopolamine infusions into the rACC before retention testing, do not affect CPA or IA retention performance (Johansen & Fields, 2004; Riekkinen et al., 1995) very strongly suggest that rACC is not the locus of permanent memory of the training. These findings, together with the present experiments, instead suggest that the rACC is selectively involved in the early stages of memory consolidation occurring shortly after training. It is not yet clear how these findings are to be resolved with those of Maviel et al. (2004) and Frankland and Bontempi (2005). But such evidence suggests that the memory-modulating effects of posttraining intra-rACC OXO infusions are likely to be due to interactions of the rACC with other brain regions rather than to strengthening of memory processes within rACC.
Recently, Johansen and Fields (2004) reported that activation of the rACC is sufficient to produce avoidance learning on a CPA task. In their experiment, rats given intra-rACC infusions of a glutamatergic agonist just prior to conditioning, in the absence of a peripheral stimulus (such as a hindpaw-formalin injection), displayed CPA for that context during testing. Based on this finding, the authors suggest that rACC neuronal activation can replace a peripheral stimulus to produce conditioned avoidance. Thus, to rule out the possibility that posttraining intra-rACC infusions alone induced enhanced IA retention performance in the current experiments, we included a group of animals that was trained on IA, but not given footshock, and then immediately given intra-rACC OXO infusions. With such training conditions, intra-rACC OXO infusions did not affect retention performance (results not shown). This finding clearly indicates that in our present experiments the posttraining OXO infusions enhanced IA retention by influencing memory consolidation rather than serving as an aversive stimulus.
In summary, the present findings show that the rACC (but not cACC) is involved in memory consolidation of IA training, and that the BLA and rACC interact in enabling posttraining infusions to enhance memory. These findings provide further evidence for the involvement of the rACC in memory for emotional events and for a critical role of the BLA in modulating memory through its interactions with efferent brain structures.
Acknowledgments
This work was supported by National Institutes of Health and United States Public Health Service Grant MH15256 to J.L.M. We thank Kelly Lei, Bita Binesh, Broc Mushet and Alexander Leigh for their excellent technical assistance.
References
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: an interference task specialized for functional neuroimaging-validation study with functional MRI. Human Brain Mapping. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Taylor KS, Hutchison WD, Dostrovsky JO, McAndrews MP, Richter EO, et al. Human anterior cingulate cortex neurons encode cognitive and emotional demands. The Journal of Neuroscience. 2005;2:8402–8406. doi: 10.1523/JNEUROSCI.2315-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Cholinergic activation of the electrocorticogram: an amygdaloid activating system. Experimental Brain Research. 1996;108:285–296. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Neocortical activation: modulation by multiple pathways acting on central cholinergic and serotonergic systems. Experimental Brain Research. 1997;116:160–174. doi: 10.1007/pl00005736. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Kuo MC, Tomaszek S. Stabilization of thalamo-cortical long-term potentiation by the amygdala: cholinergic and transcription-dependent mechanisms. The European Journal of Neuroscience. 2004;20:557–565. doi: 10.1111/j.1460-9568.2004.03515.x. [DOI] [PubMed] [Google Scholar]
- Farr SA, Uezu K, Creonte TA, Flood JF, Morley JE. Modulation of memory processing in the cingulate cortex of mice. Pharmacology, Biochemistry, and Behavior. 2000;65:363–368. doi: 10.1016/s0091-3057(99)00226-9. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nature Reviews Neuroscience. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Kubota Y, Sparenborg S, Straube K, Vogt BA. Effects of cingulate cortical lesions on avoidance learning and training-induced unit activity in rabbits. Experimental Brain Research. 1991;86:585–600. doi: 10.1007/BF00230532. [DOI] [PubMed] [Google Scholar]
- Hudspeth WJ, Wilsoncroft WE. Retrograde amnesia: time dependent effects of rhinencephalic lesions. Journal of Neurobiology. 1969;1:221–232. doi: 10.1002/neu.480010209. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, McGaugh JL. Modulation of memory by post-training epinephrine: involvement of cholinergic mechanisms. Psychopharmacology (Berl) 1988;94:379–385. doi: 10.1007/BF00174693. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nature Reviews Neuroscience. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- Jones BF, Groenewegen HJ, Witter MP. Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neuroscience. 2005;133:193–207. doi: 10.1016/j.neuroscience.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Kimble DP, Gostnell D. Role of cingulate cortex in shock avoidance behavior of rats. Journal of Comparative and Physiological Psychology. 1968;65:290–294. doi: 10.1037/h0025532. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. The Journal of Comparative Neurology. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- Kung JC, Su NM, Fan RJ, Chai SC, Shyu BC. Contribution of the anterior cingulate cortex to laser-pain conditioning in rats. Brain Research. 2003;970:58–72. doi: 10.1016/s0006-8993(02)04276-2. [DOI] [PubMed] [Google Scholar]
- Lalumiere RT, McGaugh JL. Memory enhancement induced by post- training intrabasolateral amygdala infusions of beta-adrenergic or muscarinic agonists requires activation of dopamine receptors: Involvement of right, but not left, basolateral amygdala. Learning and Memory. 2005;12:527–532. doi: 10.1101/lm.97405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, McGaugh JL. Posttraining intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. The Journal of Neuroscience. 2003;23:6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends in Neurosciences. 2002;25:456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, et al. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MI, McGaugh JL. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: involvement of the basolateral amygdala. Learning and Memory. 2004;11:312–317. doi: 10.1101/lm.72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M, Williams CL, Cahill L, McGaugh JL. The anatomy of a memory modulatory system: from periphery to brain. In: Spear N, Spear L, Woodruff M, editors. Neurobehavioral plasticity, learning, development and response to brain insults. Lawrence Erlbaum Associates; Hillsdale, NJ: 1995. pp. 149–184. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5 ed. Elsevier Academic Press; Amsterdam: 2005. [Google Scholar]
- Peretz E. The effects of lesions of the anterior cingulate cortex on the behavior of the rat. Journal of Comparive and Physiological Psychology. 1960;53:540–548. doi: 10.1037/h0040297. [DOI] [PubMed] [Google Scholar]
- Phan KL, Liberzon I, Welsh RC, Britton JC, Taylor SF. Habituation of rostral anterior cingulate cortex to repeated emotionally salient pictures. Neuropsychopharmacology. 2003;28:1344–1350. doi: 10.1038/sj.npp.1300186. [DOI] [PubMed] [Google Scholar]
- Riekkinen P, Kuitunen J, Riekkinen M. Effects of scopolamine infusions into the anterior and posterior cingulate on passive avoidance and water maze navigation. Brain Research. 1995;685:46–54. doi: 10.1016/0006-8993(95)00422-m. [DOI] [PubMed] [Google Scholar]
- Roesler R, Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of 8-Br-cAMP infused into the entorhinal cortex of rats after training. The European Journal of Neuroscience. 2002;15:905–910. doi: 10.1046/j.1460-9568.2002.01924.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. The European Journal of Neuroscience. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. The Journal of Neuroscience. 2004;24:1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, de Quervain DJ, Ferry B, Setlow B, McGaugh JL. Basolateral amygdala-nucleus accumbens interactions in mediating glucocorticoid enhancement of memory consolidation. The Journal of Neuroscience. 2001;21:2518–2525. doi: 10.1523/JNEUROSCI.21-07-02518.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiology of Learning and Memory. 2006;86:249–255. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Sarter M, Markowitsch HJ. Convergence of basolateral amygdaloid and mediodorsal thalamic projections in different areas of the frontal cortex in the rat. Brain Research Bulletin. 1983;10:607–622. doi: 10.1016/0361-9230(83)90029-1. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Posttraining intra-basolateral amygdala scopolamine impairs food- and amphetamine-induced conditioned place preferences. Behavioral Neuroscience. 2002;116:922–927. doi: 10.1037//0735-7044.116.5.922. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Systemic or intra-amygdala injections of glucose facilitate memory consolidation for extinction of drug-induced conditioned reward. The European Journal of Neuroscience. 2003;17:1482–1488. doi: 10.1046/j.1460-9568.2003.02578.x. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K, Sripanidkulchai B, Wyss JM. The cortical projection of the basolateral amygdaloid nucleus in the rat: a retrograde fluorescent dye study. The Journal of Comparative Neurology. 1984;229:419–431. doi: 10.1002/cne.902290310. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]


