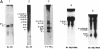Abstract
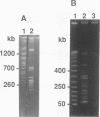
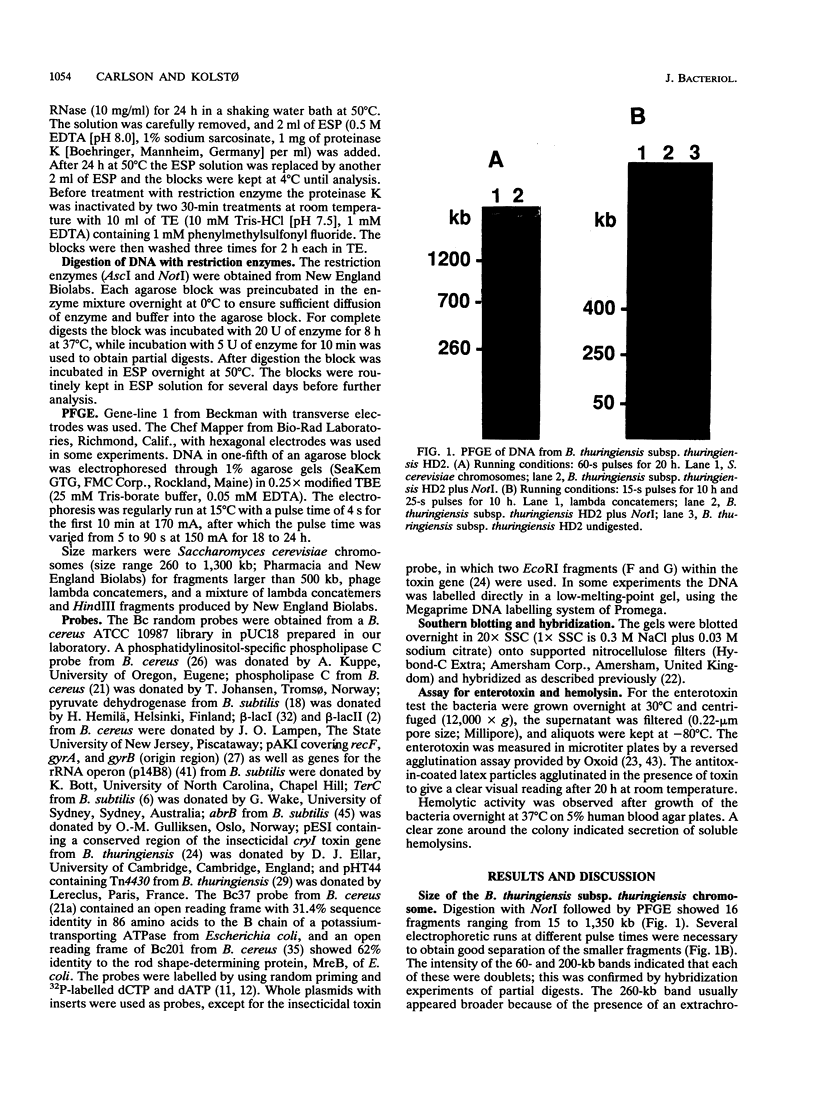
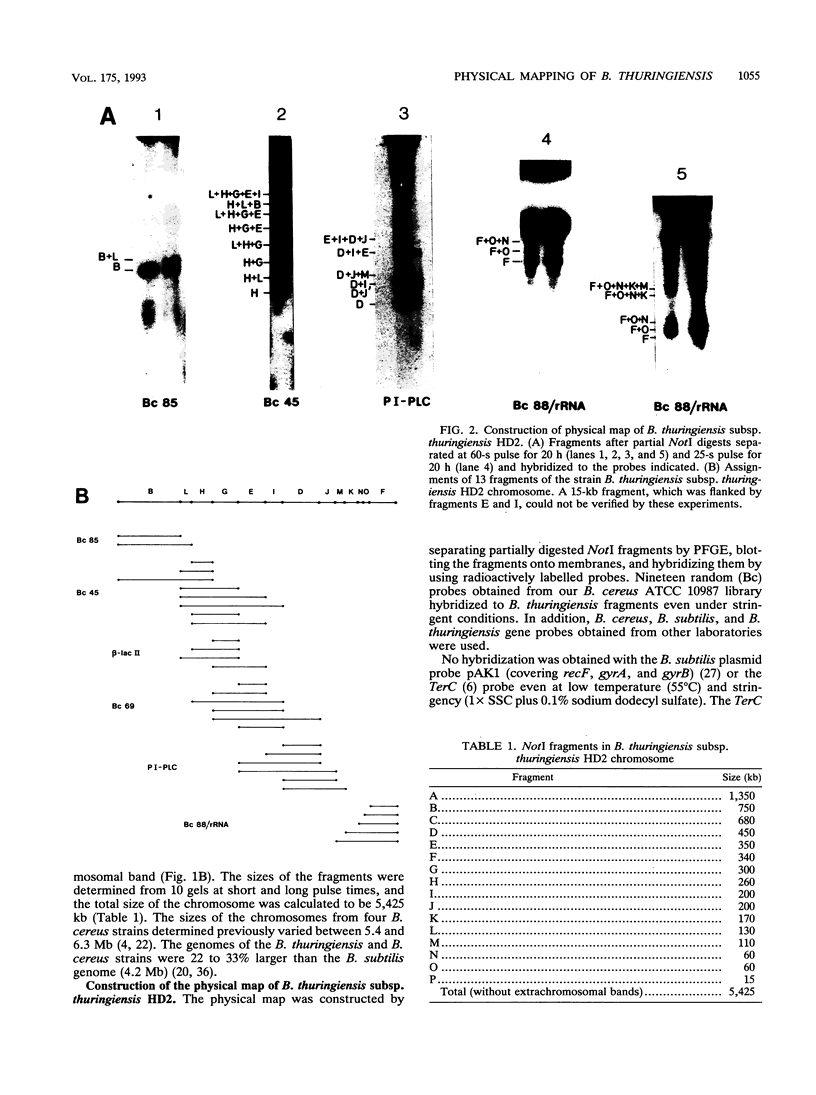
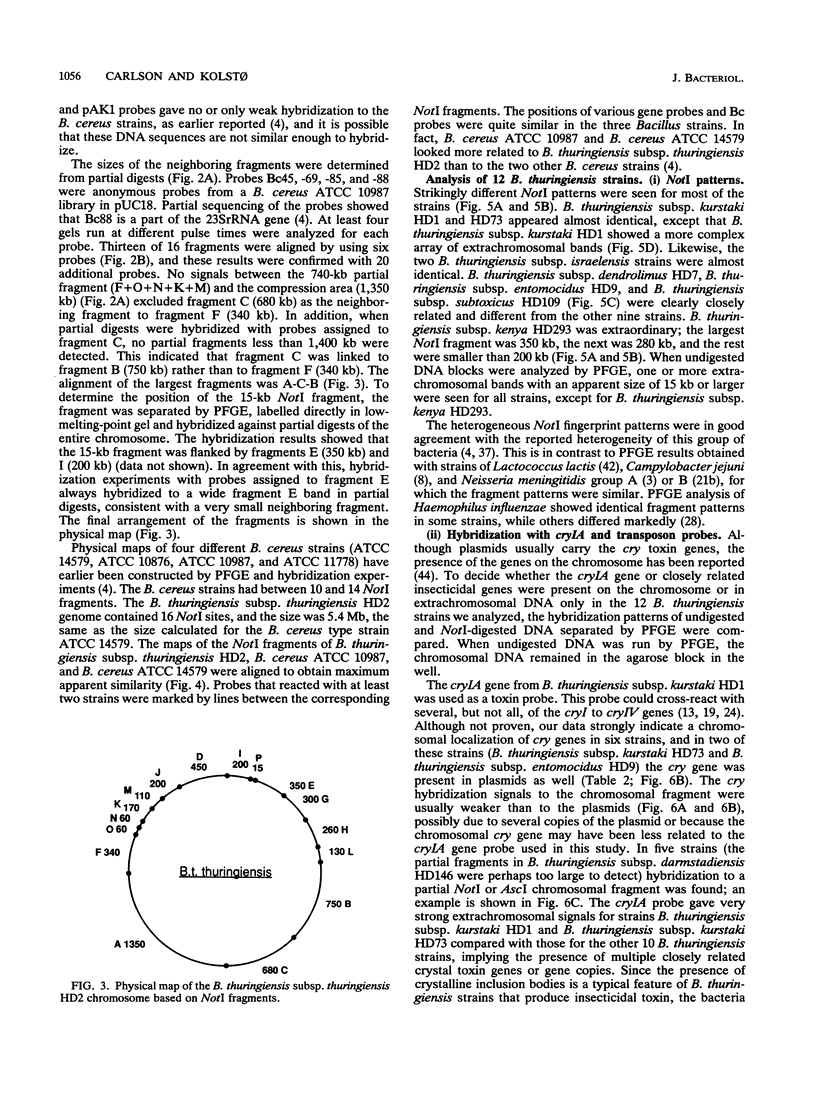
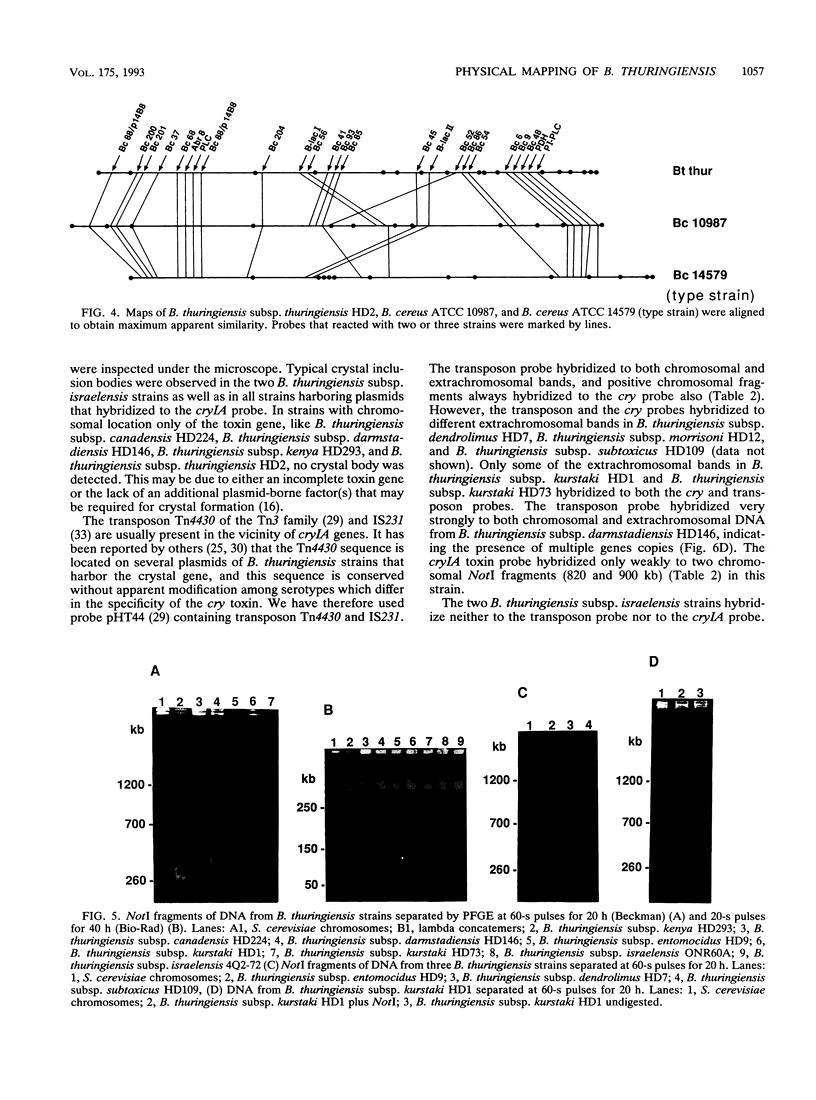
Bacillus thuringiensis is the source of the most widely used biological pesticide, through its production of insecticidal toxins. The toxin genes are often localized on plasmids. We have constructed a physical map of a Bacillus thuringiensis chromosome by aligning 16 fragments obtained by digestion with the restriction enzyme NotI. The fragments ranged from 15 to 1,350 kb. The size of the chromosome was 5.4 Mb. The NotI DNA fingerprint patterns of 12 different B. thuringiensis strains showed marked variation. The cryIA-type toxin gene was present on the chromosome in four strains, was extrachromosomal in four strains, and was both chromosomal and extrachromosomal in two strains. A Tn4430 transposon probe hybridized to 5 of the 10 cryIA-positive chromosomal fragments, while cryIA and the transposon often hybridized to different extrachromosomal bands. Ten of the strains were hemolytic when grown on agar plates containing human erythrocytes. Nine of the strains were positive when assayed for the presence of Bacillus cereus enterotoxin. We conclude that B. thuringiensis is very closely related to B. cereus and that the distinction between B. cereus and B. thuringiensis should be reconsidered.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Daniel M., Fleming J., Hermoso J. M., Pang C., Waley S. G. The amino acid sequence of the zinc-requiring beta-lactamase II from the bacterium Bacillus cereus 569. FEBS Lett. 1985 Sep 23;189(2):207–211. doi: 10.1016/0014-5793(85)81024-3. [DOI] [PubMed] [Google Scholar]
- Bygraves J. A., Maiden M. C. Analysis of the clonal relationships between strains of Neisseria meningitidis by pulsed field gel electrophoresis. J Gen Microbiol. 1992 Mar;138(3):523–531. doi: 10.1099/00221287-138-3-523. [DOI] [PubMed] [Google Scholar]
- Carlson C. R., Grønstad A., Kolstø A. B. Physical maps of the genomes of three Bacillus cereus strains. J Bacteriol. 1992 Jun;174(11):3750–3756. doi: 10.1128/jb.174.11.3750-3756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan C. M., Haarsma J. A., Smith M. T., Wake R. G. Sequence features of the replication terminus of the Bacillus subtilis chromosome. Nucleic Acids Res. 1987 Oct 26;15(20):8501–8509. doi: 10.1093/nar/15.20.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Dai S. M., Frutos R., Federici B. A., Gill S. S. Properties of a 72-kilodalton mosquitocidal protein from Bacillus thuringiensis subsp. morrisoni PG-14 expressed in B. thuringiensis subsp. kurstaki by using the shuttle vector pHT3101. Appl Environ Microbiol. 1992 Feb;58(2):507–512. doi: 10.1128/aem.58.2.507-512.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N., Taylor D. E. Use of pulsed-field agarose gel electrophoresis to size genomes of Campylobacter species and to construct a SalI map of Campylobacter jejuni UA580. J Bacteriol. 1990 Sep;172(9):5211–5217. doi: 10.1128/jb.172.9.5211-5217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansson A., Naidu A. S., Nilsson I., Wadström T., Pettersson H. E. Toxin production by Bacillus cereus dairy isolates in milk at low temperatures. Appl Environ Microbiol. 1989 Oct;55(10):2595–2600. doi: 10.1128/aem.55.10.2595-2600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- González J. M., Jr, Brown B. J., Carlton B. C. Transfer of Bacillus thuringiensis plasmids coding for delta-endotoxin among strains of B. thuringiensis and B. cereus. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6951–6955. doi: 10.1073/pnas.79.22.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J. M., Jr, Carlton B. C. A large transmissible plasmid is required for crystal toxin production in Bacillus thuringiensis variety israelensis. Plasmid. 1984 Jan;11(1):28–38. doi: 10.1016/0147-619x(84)90004-0. [DOI] [PubMed] [Google Scholar]
- González J. M., Jr, Carlton B. C. Patterns of plasmid DNA in crystalliferous and acrystalliferous strains of Bacillus thuringiensis. Plasmid. 1980 Jan;3(1):92–98. doi: 10.1016/s0147-619x(80)90038-4. [DOI] [PubMed] [Google Scholar]
- Hemilä H., Palva A., Paulin L., Arvidson S., Palva I. Secretory S complex of Bacillus subtilis: sequence analysis and identity to pyruvate dehydrogenase. J Bacteriol. 1990 Sep;172(9):5052–5063. doi: 10.1128/jb.172.9.5052-5063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya M., Tanaka T. Complete physical map of the Bacillus subtilis 168 chromosome constructed by a gene-directed mutagenesis method. J Mol Biol. 1991 Aug 5;220(3):631–648. doi: 10.1016/0022-2836(91)90106-g. [DOI] [PubMed] [Google Scholar]
- Johansen T., Holm T., Guddal P. H., Sletten K., Haugli F. B., Little C. Cloning and sequencing of the gene encoding the phosphatidylcholine-preferring phospholipase C of Bacillus cereus. Gene. 1988 May 30;65(2):293–304. doi: 10.1016/0378-1119(88)90466-0. [DOI] [PubMed] [Google Scholar]
- Kolstø A. B., Grønstad A., Oppegaard H. Physical map of the Bacillus cereus chromosome. J Bacteriol. 1990 Jul;172(7):3821–3825. doi: 10.1128/jb.172.7.3821-3825.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad J. W., Schnepf H. E., Whiteley H. R. Diversity of locations for Bacillus thuringiensis crystal protein genes. J Bacteriol. 1983 Apr;154(1):419–428. doi: 10.1128/jb.154.1.419-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad J. W., Whiteley H. R. Inverted repeat sequences flank a Bacillus thuringiensis crystal protein gene. J Bacteriol. 1984 Oct;160(1):95–102. doi: 10.1128/jb.160.1.95-102.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppe A., Evans L. M., McMillen D. A., Griffith O. H. Phosphatidylinositol-specific phospholipase C of Bacillus cereus: cloning, sequencing, and relationship to other phospholipases. J Bacteriol. 1989 Nov;171(11):6077–6083. doi: 10.1128/jb.171.11.6077-6083.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe M. F., Bott K. F. Genetic and physical organization of the cloned gyrA and gyrB genes of Bacillus subtilis. J Bacteriol. 1985 Apr;162(1):78–84. doi: 10.1128/jb.162.1.78-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O., Redfield R. J. Organization of the Haemophilus influenzae Rd genome. J Bacteriol. 1989 Jun;171(6):3016–3024. doi: 10.1128/jb.171.6.3016-3024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lereclus D., Mahillon J., Menou G., Lecadet M. M. Identification of Tn4430, a transposon of Bacillus thuringiensis functional in Escherichia coli. Mol Gen Genet. 1986 Jul;204(1):52–57. doi: 10.1007/BF00330186. [DOI] [PubMed] [Google Scholar]
- Lereclus D., Ribier J., Klier A., Menou G., Lecadet M. M. A transposon-like structure related to the delta-endotoxin gene of Bacillus thuringiensis. EMBO J. 1984 Nov;3(11):2561–2567. doi: 10.1002/j.1460-2075.1984.tb02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lereclus D., Vallade M., Chaufaux J., Arantes O., Rambaud S. Expansion of insecticidal host range of Bacillus thuringiensis by in vivo genetic recombination. Biotechnology (N Y) 1992 Apr;10(4):418–421. doi: 10.1038/nbt0492-418. [DOI] [PubMed] [Google Scholar]
- Madonna M. J., Zhu Y. F., Lampen J. O. Nucleotide sequence of the beta-lactamase I gene of Bacillus cereus strains 569/H and 5/B. Nucleic Acids Res. 1987 Feb 25;15(4):1877–1877. doi: 10.1093/nar/15.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahillon J., Seurinck J., van Rompuy L., Delcour J., Zabeau M. Nucleotide sequence and structural organization of an insertion sequence element (IS231) from Bacillus thuringiensis strain berliner 1715. EMBO J. 1985 Dec 30;4(13B):3895–3899. doi: 10.1002/j.1460-2075.1985.tb04163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson L., Moar W. J., van Frankenhuyzen K., Bossé M., Brousseau R. Insecticidal properties of a crystal protein gene product isolated from Bacillus thuringiensis subsp. kenyae. Appl Environ Microbiol. 1992 Feb;58(2):642–646. doi: 10.1128/aem.58.2.642-646.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahara A., Naterstad K., Kristensen T., Lopez R., Bork P., Kolstø A. B. Cloning of a gene from Bacillus cereus with homology to the mreB gene from Escherichia coli. Gene. 1992 Dec 1;122(1):181–185. doi: 10.1016/0378-1119(92)90047-s. [DOI] [PubMed] [Google Scholar]
- Priest F. G., Goodfellow M., Todd C. A numerical classification of the genus Bacillus. J Gen Microbiol. 1988 Jul;134(7):1847–1882. doi: 10.1099/00221287-134-7-1847. [DOI] [PubMed] [Google Scholar]
- Schnepf H. E., Whiteley H. R. Cloning and expression of the Bacillus thuringiensis crystal protein gene in Escherichia coli. Proc Natl Acad Sci U S A. 1981 May;78(5):2893–2897. doi: 10.1073/pnas.78.5.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa K., Sugiyama J., Terada T., Matsusaka N., Sugii S. Improved methods for purification of an enterotoxin produced by Bacillus cereus. FEMS Microbiol Lett. 1991 May 1;64(1):1–5. doi: 10.1016/0378-1097(91)90199-k. [DOI] [PubMed] [Google Scholar]
- Stewart G. C., Wilson F. E., Bott K. F. Detailed physical mapping of the ribosomal RNA genes of Bacillus subtilis. Gene. 1982 Sep;19(2):153–162. doi: 10.1016/0378-1119(82)90001-4. [DOI] [PubMed] [Google Scholar]
- Tanskanen E. I., Tulloch D. L., Hillier A. J., Davidson B. E. Pulsed-Field Gel Electrophoresis of SmaI Digests of Lactococcal Genomic DNA, a Novel Method of Strain Identification. Appl Environ Microbiol. 1990 Oct;56(10):3105–3111. doi: 10.1128/aem.56.10.3105-3111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley H. R., Schnepf H. E. The molecular biology of parasporal crystal body formation in Bacillus thuringiensis. Annu Rev Microbiol. 1986;40:549–576. doi: 10.1146/annurev.mi.40.100186.003001. [DOI] [PubMed] [Google Scholar]
- Zuber P., Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]