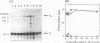Abstract
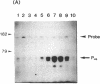
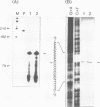
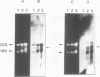
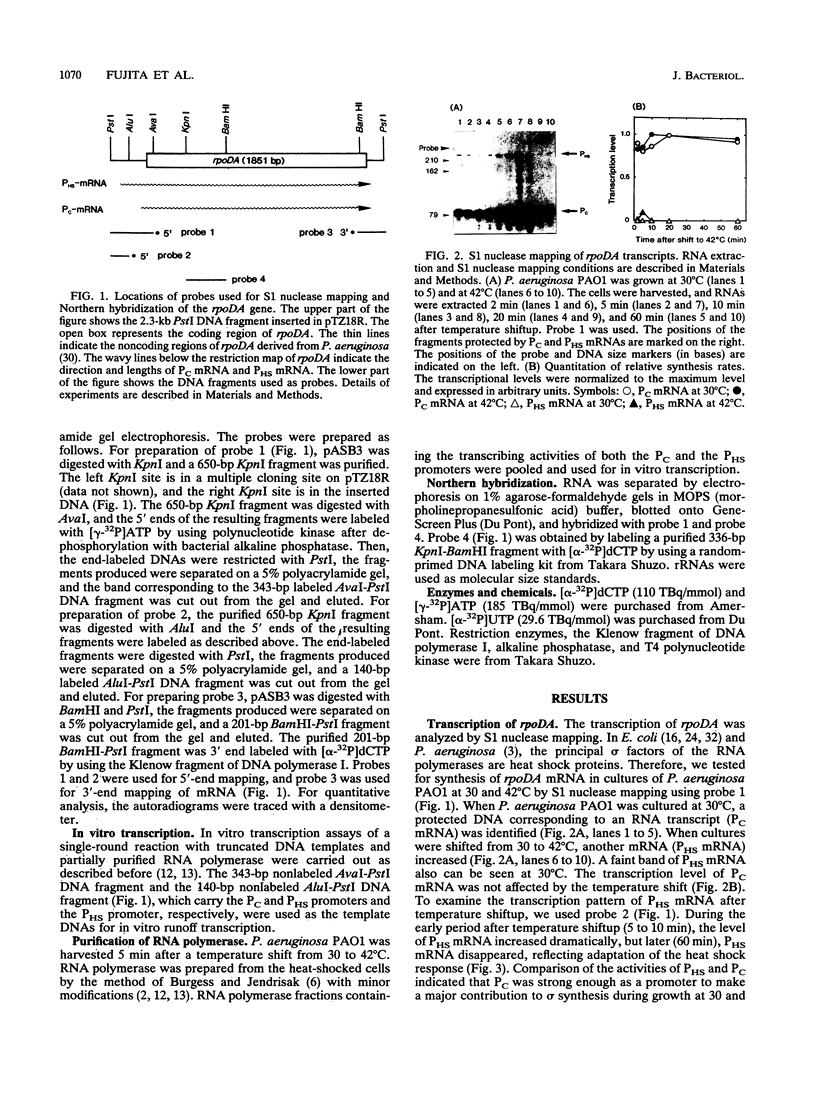
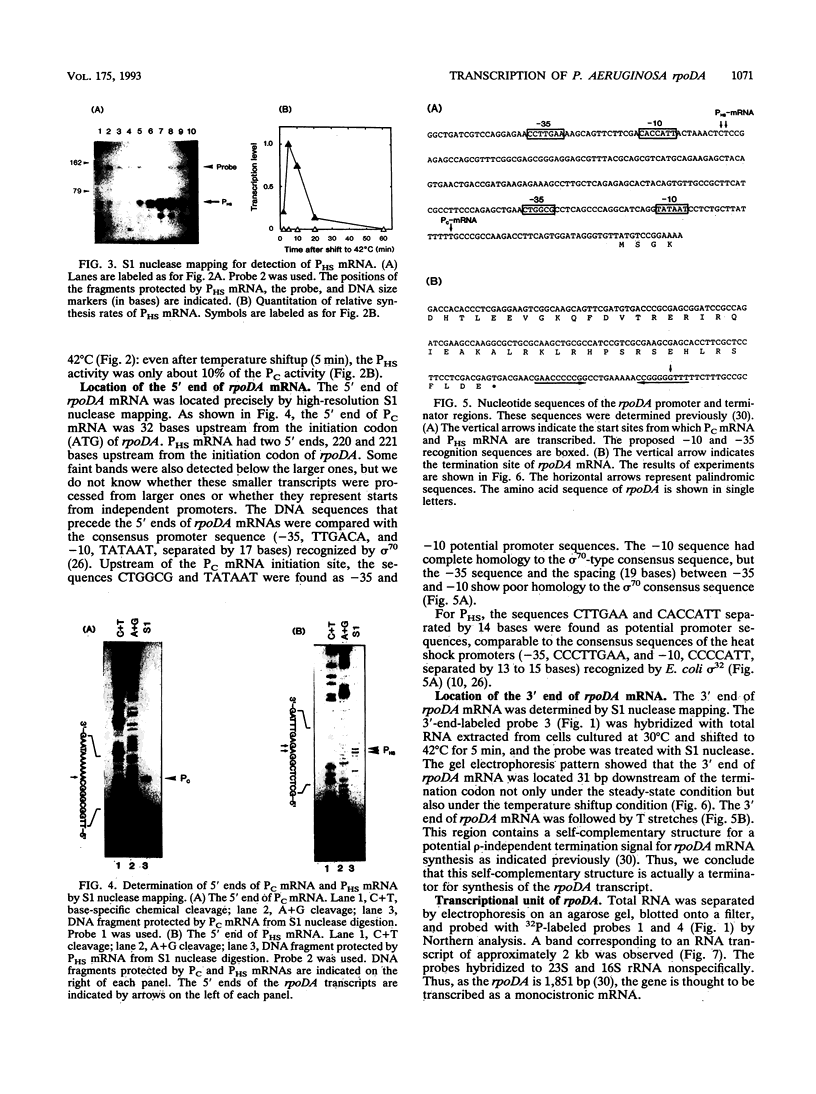
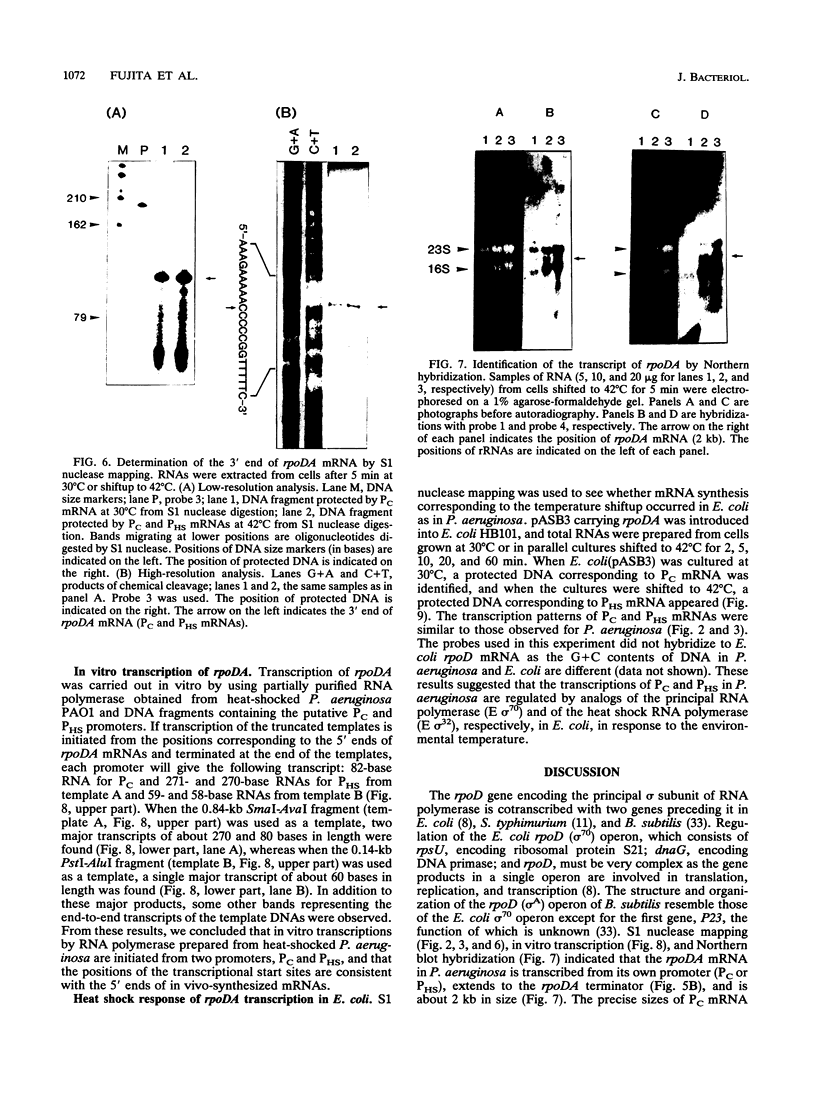
S1 nuclease mapping and Northern (RNA) hybridization revealed that the rpoDA gene encoding the principal sigma subunit of Pseudomonas aeruginosa PAO1 is transcribed as a monocistronic mRNA of 2 kb and that the transcription from the rpoDA promoter (PC) starts 32 bases upstream from the first nucleotide of the initiation codon during the steady-state growth condition at a low temperature (30 degrees C). The transcript terminates 31 bases downstream from the last nucleotide of the termination codon. When the growth temperature was shifted to 42 degrees C, the synthesis of rpoDA mRNA from a heat shock promoter was transiently induced, although transcription was still occurring from PC during the heat shock period. The transcription initiation site of the heat shock promoter (PHS) is located about 220 bases upstream of the initiation codon of rpoDA. In addition, both promoters were utilized in vitro by RNA polymerase partially purified from heat-shocked cells of P. aeruginosa PAO1. When the rpoDA was introduced into Escherichia coli, the transcription patterns of rpoDA at 30 and 42 degrees C were similar to those observed for P. aeruginosa. These results suggested that the transcription of rpoDA in P. aeruginosa is regulated by the principal RNA polymerase and the heat shock RNA polymerase in response to the environmental temperature.
Full text
PDF
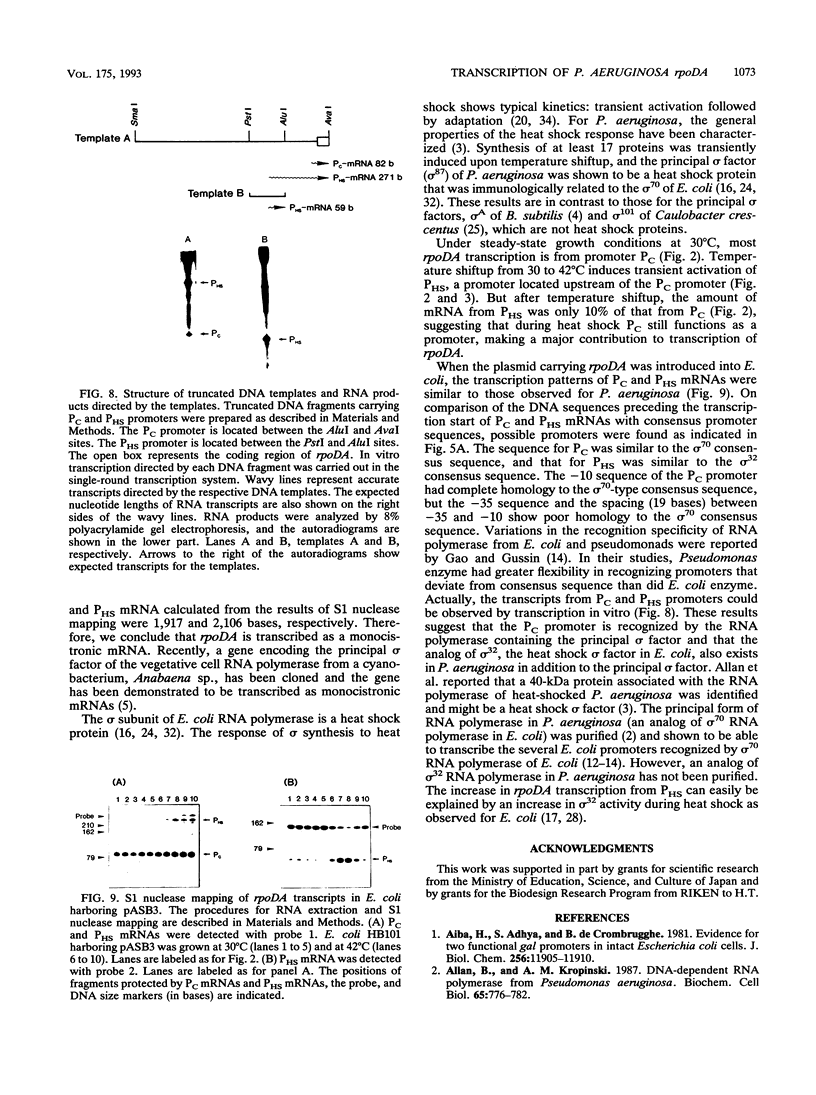

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Allan B., Kropinski A. M. DNA-dependent RNA polymerase from Pseudomonas aeruginosa. Biochem Cell Biol. 1987 Sep;65(9):776–782. doi: 10.1139/o87-101. [DOI] [PubMed] [Google Scholar]
- Allan B., Linseman M., MacDonald L. A., Lam J. S., Kropinski A. M. Heat shock response of Pseudomonas aeruginosa. J Bacteriol. 1988 Aug;170(8):3668–3674. doi: 10.1128/jb.170.8.3668-3674.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnosti D. N., Singer V. L., Chamberlin M. J. Characterization of heat shock in Bacillus subtilis. J Bacteriol. 1986 Dec;168(3):1243–1249. doi: 10.1128/jb.168.3.1243-1249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahamsha B., Haselkorn R. Isolation and characterization of the gene encoding the principal sigma factor of the vegetative cell RNA polymerase from the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991 Apr;173(8):2442–2450. doi: 10.1128/jb.173.8.2442-2450.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Burton Z. F., Gross C. A., Watanabe K. K., Burgess R. R. The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell. 1983 Feb;32(2):335–349. doi: 10.1016/0092-8674(83)90453-1. [DOI] [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner M. J., Chater K. F., Bibb M. J. Cloning, disruption, and transcriptional analysis of three RNA polymerase sigma factor genes of Streptomyces coelicolor A3(2). J Bacteriol. 1990 Jun;172(6):3367–3378. doi: 10.1128/jb.172.6.3367-3378.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing D. W., Bardwell J. C., Craig E. A., Woolford C., Hendrix R. W., Gross C. A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985 May;82(9):2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson B. D., Burton Z. F., Watanabe K. K., Burgess R. R. Nucleotide sequence of the rpsU-dnaG-rpoD operon from Salmonella typhimurium and a comparison of this sequence with the homologous operon of Escherichia coli. Gene. 1985;40(1):67–78. doi: 10.1016/0378-1119(85)90025-3. [DOI] [PubMed] [Google Scholar]
- Fujita M., Amemura A. In vitro interactions of Pseudomonas RNA polymerases with tac and RNA I promoters. Biosci Biotechnol Biochem. 1992 Oct;56(10):1644–1648. doi: 10.1271/bbb.56.1644. [DOI] [PubMed] [Google Scholar]
- Fujita M., Amemura A. Purification and characterization of a DNA-dependent RNA polymerase from Pseudomonas putida. Biosci Biotechnol Biochem. 1992 Nov;56(11):1797–1800. doi: 10.1271/bbb.56.1797. [DOI] [PubMed] [Google Scholar]
- Gao J. G., Gussin G. N. RNA polymerases from Pseudomonas aeruginosa and Pseudomonas syringae respond to Escherichia coli activator proteins. J Bacteriol. 1991 Jan;173(1):394–397. doi: 10.1128/jb.173.1.394-397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitt M. A., Wang L. F., Doi R. H. A strong sequence homology exists between the major RNA polymerase sigma factors of Bacillus subtilis and Escherichia coli. J Biol Chem. 1985 Jun 25;260(12):7178–7185. [PubMed] [Google Scholar]
- Grossman A. D., Straus D. B., Walter W. A., Gross C. A. Sigma 32 synthesis can regulate the synthesis of heat shock proteins in Escherichia coli. Genes Dev. 1987 Apr;1(2):179–184. doi: 10.1101/gad.1.2.179. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Inouye S. Cloning and DNA sequence of the gene coding for the major sigma factor from Myxococcus xanthus. J Bacteriol. 1990 Jan;172(1):80–85. doi: 10.1128/jb.172.1.80-85.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaux P. G., Herendeen S. L., Bloch P. L., Neidhardt F. C. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. doi: 10.1016/0092-8674(78)90317-3. [DOI] [PubMed] [Google Scholar]
- Losick R., Pero J. Cascades of Sigma factors. Cell. 1981 Sep;25(3):582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Mulvey M. R., Loewen P. C. Nucleotide sequence of katF of Escherichia coli suggests KatF protein is a novel sigma transcription factor. Nucleic Acids Res. 1989 Dec 11;17(23):9979–9991. doi: 10.1093/nar/17.23.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Reuter S. H., Shapiro L. Asymmetric segregation of heat-shock proteins upon cell division in Caulobacter crescentus. J Mol Biol. 1987 Apr 20;194(4):653–662. doi: 10.1016/0022-2836(87)90242-7. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Shiina T., Tanaka K., Takahashi H. Sequence of hrdB, an essential gene encoding sigma-like transcription factor of Streptomyces coelicolor A3(2): homology to principal sigma factors. Gene. 1991 Oct 30;107(1):145–148. doi: 10.1016/0378-1119(91)90308-x. [DOI] [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. The heat shock response of E. coli is regulated by changes in the concentration of sigma 32. Nature. 1987 Sep 24;329(6137):348–351. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Shiina T., Takahashi H. Multiple principal sigma factor homologs in eubacteria: identification of the "rpoD box". Science. 1988 Nov 18;242(4881):1040–1042. doi: 10.1126/science.3194753. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Takahashi H. Cloning and analysis of the gene (rpoDA) for the principal sigma factor of Pseudomonas aeruginosa. Biochim Biophys Acta. 1991 May 2;1089(1):113–119. doi: 10.1016/0167-4781(91)90092-z. [DOI] [PubMed] [Google Scholar]
- Taylor W. E., Straus D. B., Grossman A. D., Burton Z. F., Gross C. A., Burgess R. R. Transcription from a heat-inducible promoter causes heat shock regulation of the sigma subunit of E. coli RNA polymerase. Cell. 1984 Sep;38(2):371–381. doi: 10.1016/0092-8674(84)90492-6. [DOI] [PubMed] [Google Scholar]
- Wang L. F., Doi R. H. Nucleotide sequence and organization of Bacillus subtilis RNA polymerase major sigma (sigma 43) operon. Nucleic Acids Res. 1986 May 27;14(10):4293–4307. doi: 10.1093/nar/14.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Ito K., Nakamura Y., Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978 Jun;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]