Abstract
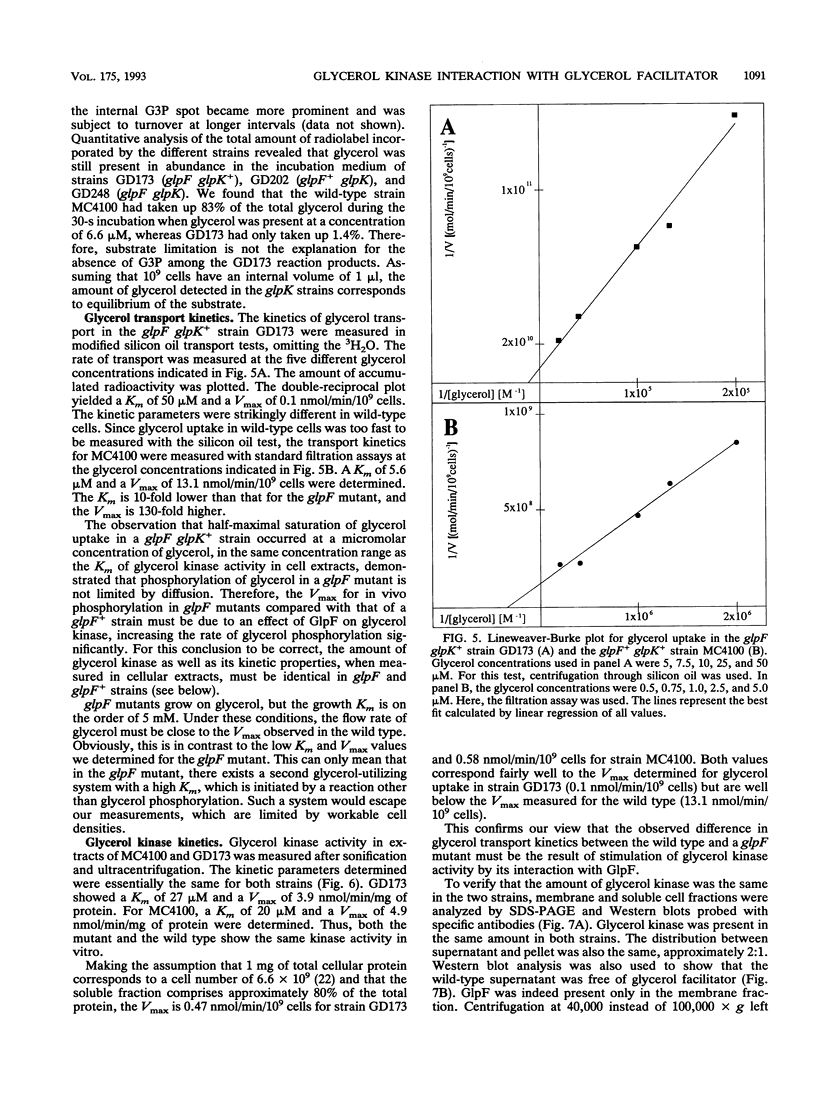
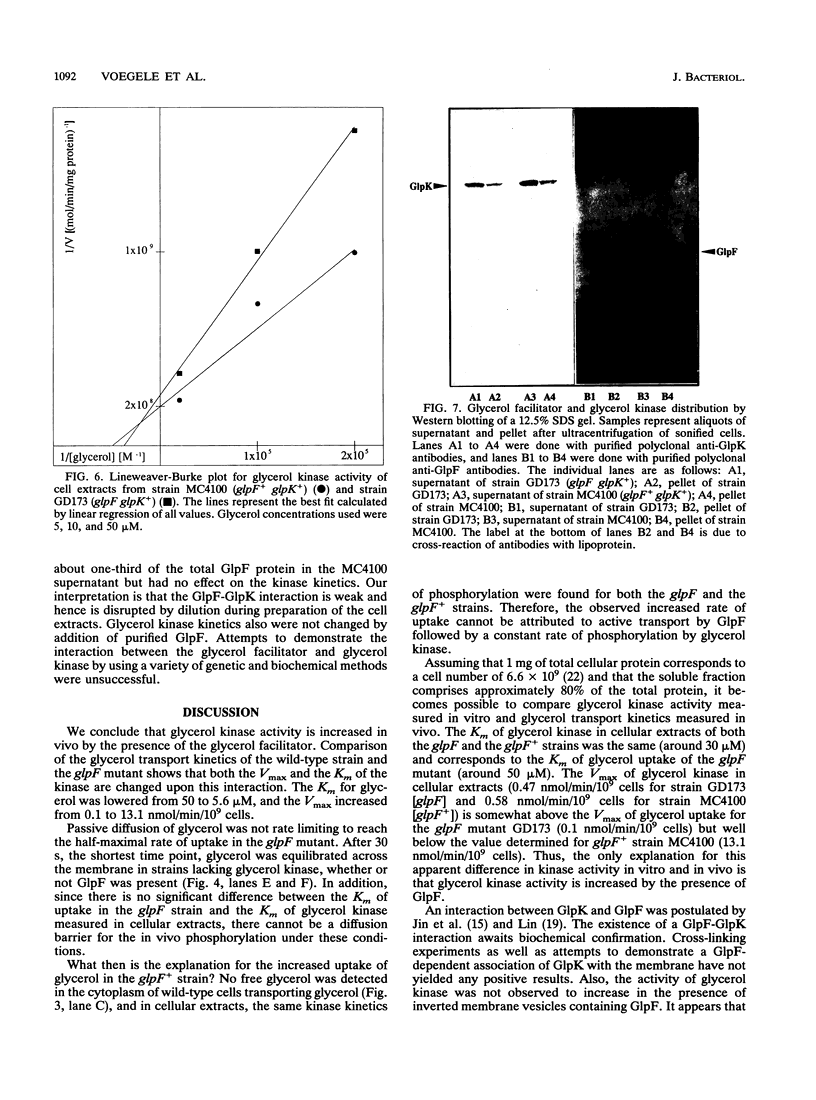
Glycerol transport is commonly cited as the only example of facilitated diffusion across the Escherichia coli cytoplasmic membrane. Two proteins, the glycerol facilitator and glycerol kinase, are involved in the entry of external glycerol into cellular metabolism. The glycerol facilitator is thought to act as a carrier or to form a selective pore in the cytoplasmic membrane, whereas the kinase traps the glycerol inside the cell as sn-glycerol-3-phosphate. We found that the kinetics of glycerol uptake in a facilitator-minus strain are significantly different from the kinetics of glycerol uptake in the wild type. Free glycerol was not observed inside wild-type cells transporting glycerol, and diffusion of glycerol across the cytoplasmic membrane was not the rate-limiting step for phosphorylation in facilitator-minus mutants. Therefore, the kinetics of glycerol phosphorylation are different, depending on the presence or absence of the facilitator protein. We conclude that there is an interaction between the glycerol facilitator protein and glycerol kinase that stimulates kinase activity, analogous to the hexokinase- and glycerol kinase-porin interactions in mitochondria.
Full text
PDF



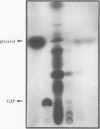
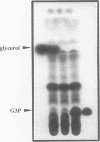
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASNIS R. E., BRODIE A. F. A glycerol dehydrogenase from Escherichia coli. J Biol Chem. 1953 Jul;203(1):153–159. [PubMed] [Google Scholar]
- Baker M. E., Saier M. H., Jr A common ancestor for bovine lens fiber major intrinsic protein, soybean nodulin-26 protein, and E. coli glycerol facilitator. Cell. 1990 Jan 26;60(2):185–186. doi: 10.1016/0092-8674(90)90731-s. [DOI] [PubMed] [Google Scholar]
- Berman-Kurtz M., Lin E. C., Richey D. P. Promoter-like mutant with increased expression of the glycerol kinase operon of Escherichia coli. J Bacteriol. 1971 Jun;106(3):724–731. doi: 10.1128/jb.106.3.724-731.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdiczka D. Interaction of mitochondrial porin with cytosolic proteins. Experientia. 1990 Feb 15;46(2):161–167. doi: 10.1007/BF02027312. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Lin E. C. Chromosomal location of the structural gene for glycerol kinase in Escherichia coli. J Bacteriol. 1966 May;91(5):1763–1766. doi: 10.1128/jb.91.5.1763-1766.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze M. O., McElhaney R. N. The effect of alterations in the fluidity and phase state of the membrane lipids on the passive permeation and facilitated diffusion of glycerol in Escherichia coli. J Gen Microbiol. 1981 Jun;124(2):299–307. doi: 10.1099/00221287-124-2-299. [DOI] [PubMed] [Google Scholar]
- Freedberg W. B., Lin E. C. Three kinds of controls affecting the expression of the glp regulon in Escherichia coli. J Bacteriol. 1973 Sep;115(3):816–823. doi: 10.1128/jb.115.3.816-823.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett S., Taylor R. K., Silhavy T. J., Berman M. L. Isolation and characterization of delta ompB strains of Escherichia coli by a general method based on gene fusions. J Bacteriol. 1985 May;162(2):840–844. doi: 10.1128/jb.162.2.840-844.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- Heller K. B., Lin E. C., Wilson T. H. Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol. 1980 Oct;144(1):274–278. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R. Z., Forage R. G., Lin E. C. Glycerol kinase as a substitute for dihydroxyacetone kinase in a mutant of Klebsiella pneumoniae. J Bacteriol. 1982 Dec;152(3):1303–1307. doi: 10.1128/jb.152.3.1303-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M., Kurokawa M., Ishibashi S. Binding and function of mitochondrial glycerol kinase in comparison with those of mitochondrial hexokinase. Arch Biochem Biophys. 1985 Feb 15;237(1):135–141. doi: 10.1016/0003-9861(85)90262-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCabe E. R. Human glycerol kinase deficiency: an inborn error of compartmental metabolism. Biochem Med. 1983 Oct;30(2):215–230. doi: 10.1016/0006-2944(83)90088-1. [DOI] [PubMed] [Google Scholar]
- Muramatsu S., Mizuno T. Nucleotide sequence of the region encompassing the glpKF operon and its upstream region containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res. 1989 Jun 12;17(11):4378–4378. [PMC free article] [PubMed] [Google Scholar]
- Ostlund A. K., Göhring U., Krause J., Brdiczka D. The binding of glycerol kinase to the outer membrane of rat liver mitochondria: its importance in metabolic regulation. Biochem Med. 1983 Oct;30(2):231–245. doi: 10.1016/0006-2944(83)90089-3. [DOI] [PubMed] [Google Scholar]
- Pao G. M., Wu L. F., Johnson K. D., Höfte H., Chrispeels M. J., Sweet G., Sandal N. N., Saier M. H., Jr Evolution of the MIP family of integral membrane transport proteins. Mol Microbiol. 1991 Jan;5(1):33–37. doi: 10.1111/j.1365-2958.1991.tb01823.x. [DOI] [PubMed] [Google Scholar]
- Pettigrew D. W., Ma D. P., Conrad C. A., Johnson J. R. Escherichia coli glycerol kinase. Cloning and sequencing of the glpK gene and the primary structure of the enzyme. J Biol Chem. 1988 Jan 5;263(1):135–139. [PubMed] [Google Scholar]
- Postma P. W., Epstein W., Schuitema A. R., Nelson S. O. Interaction between IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and glycerol kinase of Salmonella typhimurium. J Bacteriol. 1984 Apr;158(1):351–353. doi: 10.1128/jb.158.1.351-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey D. P., Lin E. C. Importance of facilitated diffusion for effective utilization of glycerol by Escherichia coli. J Bacteriol. 1972 Nov;112(2):784–790. doi: 10.1128/jb.112.2.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanno Y., Wilson T. H., Lin E. C. Control of permeation to glycerol in cells of Escherichia coli. Biochem Biophys Res Commun. 1968 Jul 26;32(2):344–349. doi: 10.1016/0006-291x(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Schweizer H., Boos W., Larson T. J. Repressor for the sn-glycerol-3-phosphate regulon of Escherichia coli K-12: cloning of the glpR gene and identification of its product. J Bacteriol. 1985 Feb;161(2):563–566. doi: 10.1128/jb.161.2.563-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer W. K., McCabe E. R. Subcellular distribution and kinetic properties of soluble and particulate-associated bovine adrenal glycerol kinase. Mol Cell Biochem. 1984 Sep;64(1):51–61. doi: 10.1007/BF00420928. [DOI] [PubMed] [Google Scholar]
- Sweet G., Gandor C., Voegele R., Wittekindt N., Beuerle J., Truniger V., Lin E. C., Boos W. Glycerol facilitator of Escherichia coli: cloning of glpF and identification of the glpF product. J Bacteriol. 1990 Jan;172(1):424–430. doi: 10.1128/jb.172.1.424-430.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. T., Ruch F. E., Jr, Lin C. C. Purification and properties of a nicotinamide adenine dinucleotide-linked dehydrogenase that serves an Escherichia coli mutant for glycerol catabolism. J Bacteriol. 1979 Oct;140(1):182–187. doi: 10.1128/jb.140.1.182-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner J. W., Paulus H. Catalytic and allosteric properties of glycerol kinase from Escherichia coli. J Biol Chem. 1973 Jun 10;248(11):3922–3932. [PubMed] [Google Scholar]
- Thorner J. W., Paulus H. Composition and subunit structure of glycerol kinase from Escherichia coli. J Biol Chem. 1971 Jun 25;246(12):3885–3894. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truniger V., Boos W., Sweet G. Molecular analysis of the glpFKX regions of Escherichia coli and Shigella flexneri. J Bacteriol. 1992 Nov;174(21):6981–6991. doi: 10.1128/jb.174.21.6981-6991.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Kistler W. S., Lin E. C. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J Bacteriol. 1970 Jun;102(3):753–759. doi: 10.1128/jb.102.3.753-759.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]