Abstract
Iron acquisition by the gram-negative pathogens Bordetella bronchiseptica and Bordetella pertussis is thought to occur by hydroxamate siderophore-mediated transport as well as an apparently siderophore-independent process by which host transferrins bind to bacterial surface receptors. We constructed B. bronchiseptica mutants deficient in siderophore activity by insertional mutagenesis with miniTn5/lacZ1. The mutants could be placed into four distinct complementation groups, as determined from cross-feeding assays which demonstrated restored siderophore synthesis. Mutants deficient in siderophore activity were BRM1, BRM6, and BRM9, exhibiting approximately 36 to 41% of wild-type siderophore levels, and BRM3 and BRM8, which appeared to produce very little or no detectable siderophore. Mutant BRM4 was found to be a leucine auxotroph, while mutants BRM2 and BRM7 could synthesize siderophore only in low-iron medium which was supplemented with various amino acids. Evaluation of all transcriptional fusions revealed an apparent lack of iron-regulated lacZ expression. Genomic regions flanking the transposable element in the siderophore mutants were homologous with B. pertussis chromosomal DNA, while bioassays suggested siderophore cross-feeding between B. pertussis and B. bronchiseptica. These results indicate probable similarity between the siderophore biosynthetic and transport systems of the two species.
Full text
PDF


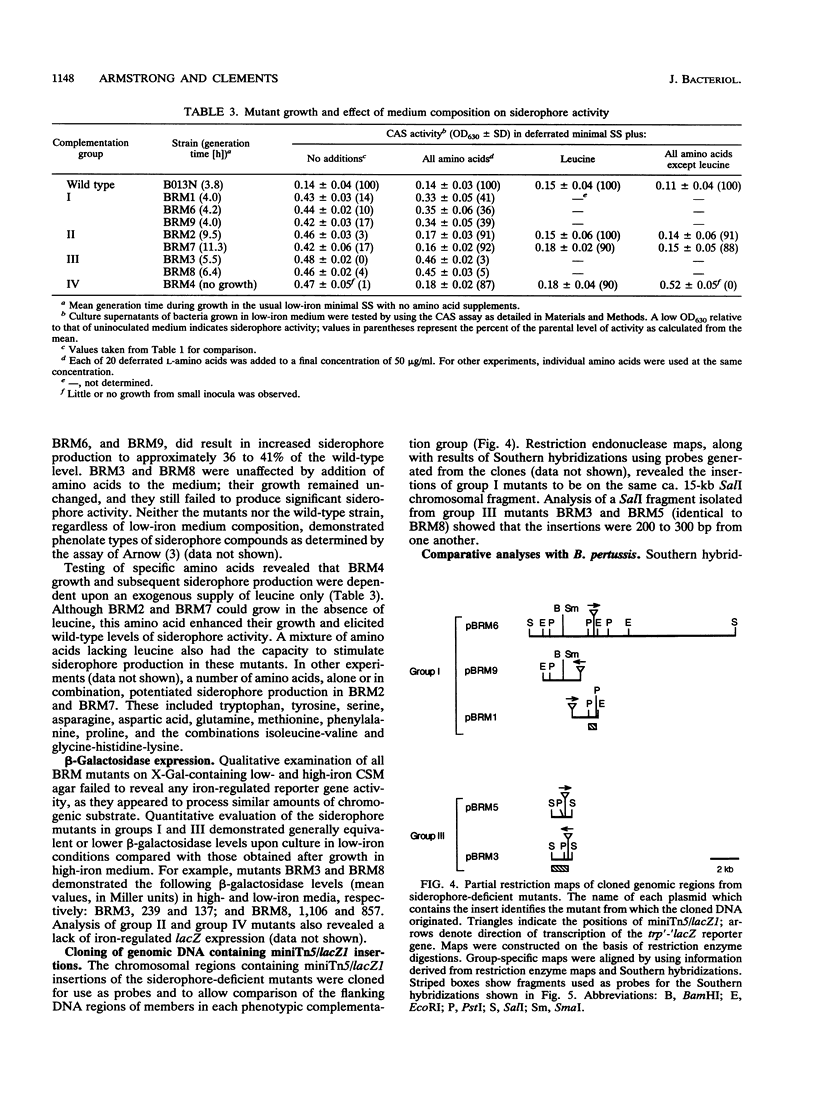
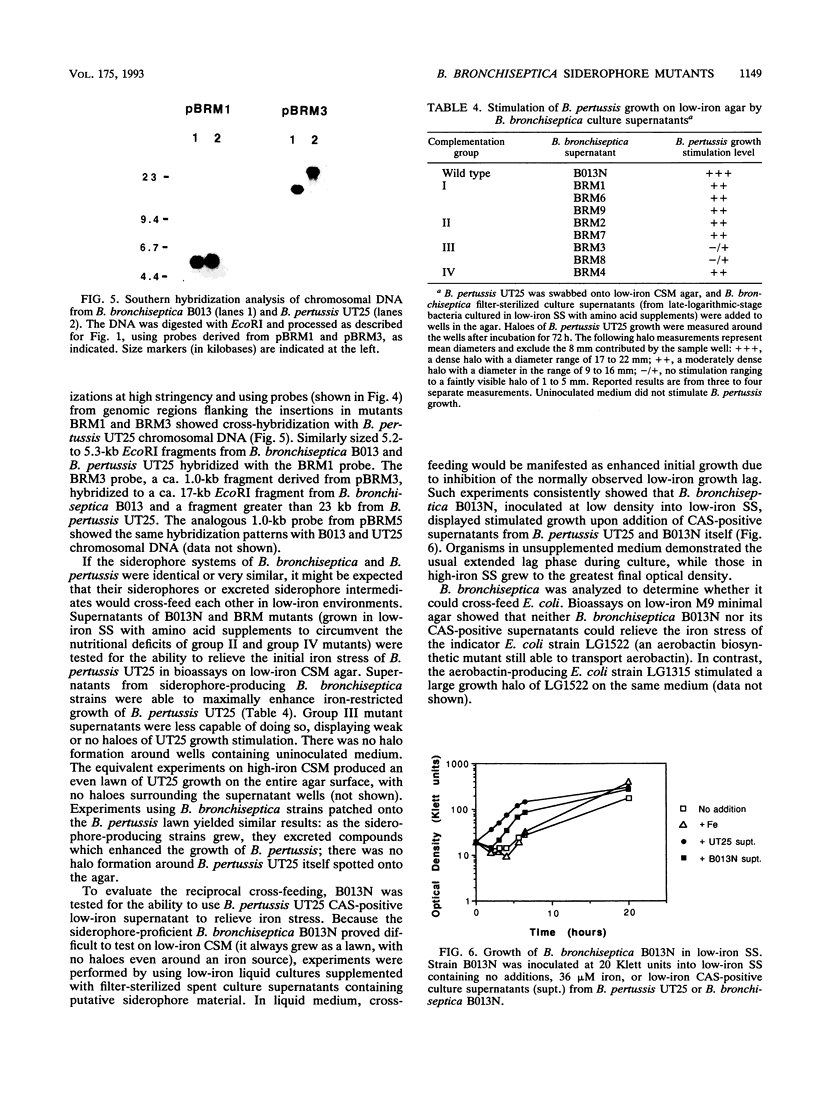



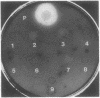
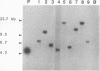
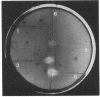
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agiato L. A., Dyer D. W. Siderophore production and membrane alterations by Bordetella pertussis in response to iron starvation. Infect Immun. 1992 Jan;60(1):117–123. doi: 10.1128/iai.60.1.117-123.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricó B., Miller J. F., Roy C., Stibitz S., Monack D., Falkow S., Gross R., Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987 Aug 25;26(17):5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Bauwens J. E., Spach D. H., Schacker T. W., Mustafa M. M., Bowden R. A. Bordetella bronchiseptica pneumonia and bacteremia following bone marrow transplantation. J Clin Microbiol. 1992 Sep;30(9):2474–2475. doi: 10.1128/jcm.30.9.2474-2475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman T. J., Ozenberger B. A., McIntosh M. A. Regulation of divergent transcription from the iron-responsive fepB-entC promoter-operator regions in Escherichia coli. J Mol Biol. 1990 Apr 20;212(4):669–682. doi: 10.1016/0022-2836(90)90229-F. [DOI] [PubMed] [Google Scholar]
- Brown D. R., Parker C. D. Cloning of the filamentous hemagglutinin of Bordetella pertussis and its expression in Escherichia coli. Infect Immun. 1987 Jan;55(1):154–161. doi: 10.1128/iai.55.1.154-161.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock J. O., Armstrong S. K., Shear J. L., Lies D. P., McIntosh M. A. Formation of ion channels by colicin B in planar lipid bilayers. J Membr Biol. 1990 Mar;114(1):79–95. doi: 10.1007/BF01869387. [DOI] [PubMed] [Google Scholar]
- Carbonetti N. H., Williams P. H. A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30. Infect Immun. 1984 Oct;46(1):7–12. doi: 10.1128/iai.46.1.7-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington D. O., Switzer W. P. Parenteral vaccination of young swine against Bordetella bronchiseptica. Am J Vet Res. 1979 Oct;40(10):1347–1351. [PubMed] [Google Scholar]
- Fleming T. P., Nahlik M. S., McIntosh M. A. Regulation of enterobactin iron transport in Escherichia coli: characterization of ent::Mu d(Apr lac) operon fusions. J Bacteriol. 1983 Dec;156(3):1171–1177. doi: 10.1128/jb.156.3.1171-1177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow R. A. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980 Dec;44(4):722–738. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorringe A. R., Woods G., Robinson A. Growth and siderophore production by Bordetella pertussis under iron-restricted conditions. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):101–105. doi: 10.1016/0378-1097(90)90265-r. [DOI] [PubMed] [Google Scholar]
- Herrero M., de Lorenzo V., Timmis K. N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990 Nov;172(11):6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi A., Suzuki Y., Ono S., Sato H., Sato Y. Heptakis(2,6-O-dimethyl)beta-cyclodextrin: a novel growth stimulant for Bordetella pertussis phase I. J Clin Microbiol. 1983 May;17(5):781–786. doi: 10.1128/jcm.17.5.781-786.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenstein D. A., Ciofalo L., Jordan M. C. Bordetella bronchiseptica bacteremia. West J Med. 1984 Jan;140(1):96–98. [PMC free article] [PubMed] [Google Scholar]
- Lee B. C., Schryvers A. B. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol Microbiol. 1988 Nov;2(6):827–829. doi: 10.1111/j.1365-2958.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Menozzi F. D., Gantiez C., Locht C. Identification and purification of transferrin- and lactoferrin-binding proteins of Bordetella pertussis and Bordetella bronchiseptica. Infect Immun. 1991 Nov;59(11):3982–3988. doi: 10.1128/iai.59.11.3982-3988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D. J., Williams P. Utilization of transferrin-bound iron by Haemophilus species of human and porcine origins. FEMS Microbiol Lett. 1989 Nov;53(1-2):123–127. doi: 10.1016/0378-1097(89)90378-9. [DOI] [PubMed] [Google Scholar]
- Musser J. M., Hewlett E. L., Peppler M. S., Selander R. K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986 Apr;166(1):230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Porter J. F., Parton R., Wardlaw A. C. Growth and survival of Bordetella bronchiseptica in natural waters and in buffered saline without added nutrients. Appl Environ Microbiol. 1991 Apr;57(4):1202–1206. doi: 10.1128/aem.57.4.1202-1206.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redhead K., Hill T. Acquisition of iron from transferrin by Bordetella pertussis. FEMS Microbiol Lett. 1991 Jan 15;61(2-3):303–307. doi: 10.1016/0378-1097(91)90570-z. [DOI] [PubMed] [Google Scholar]
- Redhead K., Hill T., Chart H. Interaction of lactoferrin and transferrins with the outer membrane of Bordetella pertussis. J Gen Microbiol. 1987 Apr;133(4):891–898. doi: 10.1099/00221287-133-4-891. [DOI] [PubMed] [Google Scholar]
- Schneider D. R., Parker C. D. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect Immun. 1982 Nov;38(2):548–553. doi: 10.1128/iai.38.2.548-553.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B. Characterization of the human transferrin and lactoferrin receptors in Haemophilus influenzae. Mol Microbiol. 1988 Jul;2(4):467–472. doi: 10.1111/j.1365-2958.1988.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect Immun. 1988 May;56(5):1144–1149. doi: 10.1128/iai.56.5.1144-1149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Aaronson W., Monack D., Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 1989 Mar 16;338(6212):266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- Tsai J., Dyer D. W., Sparling P. F. Loss of transferrin receptor activity in Neisseria meningitidis correlates with inability to use transferrin as an iron source. Infect Immun. 1988 Dec;56(12):3132–3138. doi: 10.1128/iai.56.12.3132-3138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Melton A. R., Walker K. E., Andraos-Selim C., Meidl J. J. Use of the promoter fusion transposon Tn5 lac to identify mutations in Bordetella pertussis vir-regulated genes. Infect Immun. 1989 Sep;57(9):2674–2682. doi: 10.1128/iai.57.9.2674-2682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Warner P. J. ColV plasmid-mediated, colicin V-independent iron uptake system of invasive strains of Escherichia coli. Infect Immun. 1980 Aug;29(2):411–416. doi: 10.1128/iai.29.2.411-416.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow G. C., Young I. G., Gibson F. Mu-induced polarity in the Escherichia coli K-12 ent gene cluster: evidence for a gene (entG) involved in the biosynthesis of enterochelin. J Bacteriol. 1975 Oct;124(1):1–6. doi: 10.1128/jb.124.1.1-6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Herrero M., Jakubzik U., Timmis K. N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990 Nov;172(11):6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Neilands J. B. Characterization of iucA and iucC genes of the aerobactin system of plasmid ColV-K30 in Escherichia coli. J Bacteriol. 1986 Jul;167(1):350–355. doi: 10.1128/jb.167.1.350-355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]