Abstract
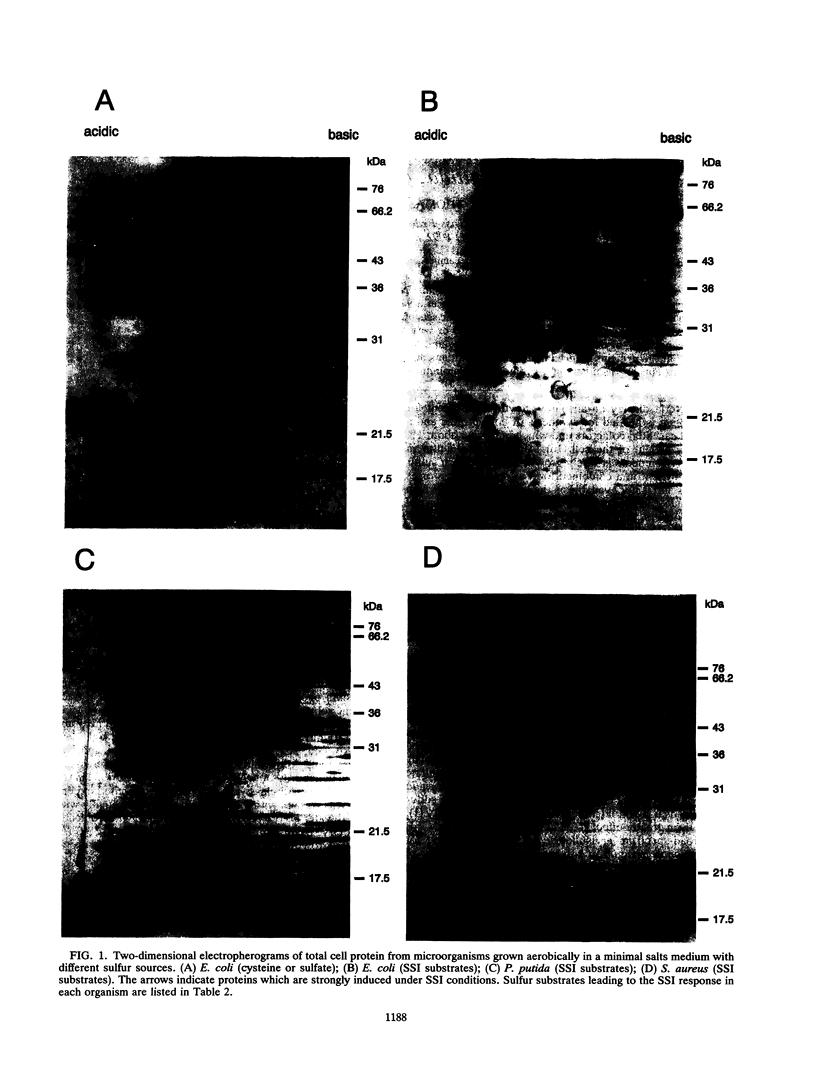
Two-dimensional gel electrophoresis of proteins from Escherichia coli, Pseudomonas putida, and Staphylococcus aureus, grown with methionine or one of a variety of organosulfates and organosulfonates as the sole source of sulfur, showed expression of specific sets of 7 to 14 proteins which were not observed during growth with sulfate or cysteine for all three species or with thiocyanate for P. putida and S. aureus. Under the same conditions, arylsulfatase activity in P. putida and S. aureus was seen to increase by up to 140-fold, suggesting that the proteins induced under these conditions may be involved in sulfur metabolism. We propose that these proteins are members of a sulfate starvation-induced stimulon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Murooka Y., Harada T. Regulation of arylsulfatase synthesis by sulfur compounds in Klebsiella aerogenes. J Bacteriol. 1975 Jan;121(1):29–35. doi: 10.1128/jb.121.1.29-35.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptist E. W., Hallquist S. G., Kredich N. M. Identification of the Salmonella typhimurium cysB gene product by two-dimensional protein electrophoresis. J Bacteriol. 1982 Jul;151(1):495–499. doi: 10.1128/jb.151.1.495-499.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Engel H. W., Soedirman N., Rost J. A., van Leeuwen W. J., van Embden J. D. Transferability of macrolide, lincomycin, and streptogramin resistances between group A, B, and D streptococci, Streptococcus pneumoniae, and Staphylococcus aureus. J Bacteriol. 1980 May;142(2):407–413. doi: 10.1128/jb.142.2.407-413.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Payne W. J. The regulation of arylsulphatase formation in Pseudomonas C 12 B. Microbios. 1972 Sep-Oct;6(22):147–156. [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Hochstrasser D. F., Harrington M. G., Hochstrasser A. C., Miller M. J., Merril C. R. Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal Biochem. 1988 Sep;173(2):424–435. doi: 10.1016/0003-2697(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. The nature of the pleiotropic cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):597–602. doi: 10.1042/bj1100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- Laudenbach D. E., Grossman A. R. Characterization and mutagenesis of sulfur-regulated genes in a cyanobacterium: evidence for function in sulfate transport. J Bacteriol. 1991 May;173(9):2739–2750. doi: 10.1128/jb.173.9.2739-2750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas D. M., Yudkin M. D. Identification of a positive regulatory protein in Escherichia coli: the product of the cysB gene. Mol Gen Genet. 1980 Feb;177(3):535–539. doi: 10.1007/BF00271494. [DOI] [PubMed] [Google Scholar]
- Ostrowski J., Kredich N. M. In vitro interactions of CysB protein with the cysJIH promoter of Salmonella typhimurium: inhibitory effects of sulfide. J Bacteriol. 1990 Feb;172(2):779–785. doi: 10.1128/jb.172.2.779-785.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASTERNAK C. A., ELLIS R. J., JONES-MORTIMER M. C., CRICHTON C. E. THE CONTROL OF SULPHATE REDUCTION IN BACTERIA. Biochem J. 1965 Jul;96:270–275. doi: 10.1042/bj0960270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S., Whipple M. B., Dreyfuss J. A binding site for sulfate and its relation to sulfate transport into Salmonella typhimurium. J Biol Chem. 1966 Sep 10;241(17):3962–3969. [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Neidhardt F. C. The gene-protein database of Escherichia coli: edition 4. Electrophoresis. 1991 Nov;12(11):955–994. doi: 10.1002/elps.1150121114. [DOI] [PubMed] [Google Scholar]
- Wackett L. P., Shames S. L., Venditti C. P., Walsh C. T. Bacterial carbon-phosphorus lyase: products, rates, and regulation of phosphonic and phosphinic acid metabolism. J Bacteriol. 1987 Feb;169(2):710–717. doi: 10.1128/jb.169.2.710-717.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürrer D., Cook A. M., Leisinger T. Microbial desulfonation of substituted naphthalenesulfonic acids and benzenesulfonic acids. Appl Environ Microbiol. 1987 Jul;53(7):1459–1463. doi: 10.1128/aem.53.7.1459-1463.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]