Abstract
High-affinity binding of a set of proteins with specificity for the 5′ untranslated region (UTR) of the Chlamydomonas reinhardtii chloroplast psbA mRNA correlates with light-regulated translational activation of this message. We have isolated a cDNA encoding the main psbA RNA binding protein, RB47, and identified this protein as a member of the poly(A) binding protein family. Poly(A) binding proteins are a family of eukaryotic, cytoplasmic proteins thought to bind poly(A) tails of mRNAs and play a role in translational regulation. In vitro translation of RNA transcribed from the RB47 cDNA produces a precursor protein that is efficiently transported into the chloroplast and processed to the mature 47-kDa protein. RB47 expressed and purified from Escherichia coli binds to the psbA 5′ UTR with similar specificity and affinity as RB47 isolated from C. reinhardtii chloroplasts. The identification of a normally cytoplasmic translation factor in the chloroplast suggests that the prokaryotic-like chloroplast translation machinery utilizes a eukaryotic-like initiation factor to regulate the translation of a key chloroplast mRNA. These data also suggest that poly(A) binding proteins may play a wider role in translation regulation than previously appreciated.
Translation plays a key role in the regulation of gene expression across the spectrum of organisms (1, 2). The majority of regulatory schemes characterized to date involve translational repression often involving proteins binding to mRNA to limit ribosome association (3, 4). Translational activation has also been observed (5), but few of the underlying molecular mechanisms for this type of regulation have been identified. In plants, light activates the expression of many genes. Light has been shown to activate expression of specific chloroplast encoded mRNAs by increasing translation initiation (6, 7). Genetic evidence in higher plants and algae has shown that nuclear-encoded factors are required for translational activation of specific chloroplast-encoded mRNAs (8–12). In the green algae Chlamydomonas reinhardtii, a number of nuclear mutants have been identified that affect translation of single specific mRNAs in the chloroplast, often acting at translation initiation (ref. 7 and C.B.Y. and S.P.M., unpublished results). Mutational analysis of chloroplast mRNAs has identified sequence elements within the 5′ untranslated region (UTR) of mRNAs that are required for translational activation (6, 13, 14), and the 5′ UTR of a chloroplast mRNA can confer a specific translation phenotype on a reporter gene in vivo (15, 16).
We previously identified putative translational activator proteins by purifying a complex of four proteins that binds with high affinity and specificity to the 5′ UTR of the chloroplast encoded psbA mRNA (encoding the D1 protein, a major component of photosystem II) (17). Binding of these proteins to the 5′ UTR of psbA mRNA correlates with translation of this mRNA under a variety of physiological (17) and biochemical conditions (18, 19), and in different genetic backgrounds (7). The binding of this complex to the psbA mRNA can be regulated in vitro in response to both redox potential (18, 20) and phosphorylation (19, 20), both of which are thought to transduce the light signal to activate translation of psbA mRNA. The 47-kDa member of the psbA RNA binding complex (RB47) is in close contact with the RNA, and antisera specific to this protein inhibits binding to the psbA mRNA in vitro (17). This combination of genetic and biochemical data has allowed the proteins that make up the psbA mRNA specific binding complex to be identified as putative message-specific translational activators.
We have cloned a cDNA encoding the RB47 protein and show here that the encoded protein is homologous to poly(A) binding protein (PABP). PABPs are generally thought to associate with the poly(A) tail of cytoplasmic mRNAs (21) and have been shown to interact with translation initiation factor 4G in the presence of poly(A) (22), suggesting a role for PABPs in translation initiation. The fact that RB47 is a PABP that specifically binds the psbA message in the chloroplast suggests that in the chloroplast PABPs can bind with high specificity to the 5′ UTR of an mRNA and act directly in translation initiation.
MATERIALS AND METHODS
Cell Growth Conditions.
C. reinhardtii strains were grown in complete media (Tris/acetate/phosphate; ref. 23) to a density of 5 × 106 cells/ml under constant light. Cells were harvested by centrifugation at 4°C for 5 min at 4,000 × g. Cells were either used immediately or frozen in liquid N2 for storage at −70°C.
Chromatography.
Approximately 5 × 109 cells were resuspended in low-salt extraction buffer (10 mM Tris, pH 7.5/10 mM NaCl/10 mM MgCl2/5 mM 2-mercaptoethanol). After passage through a cell disruption bomb (Parr Instruments, Moline, IL), the soluble cell extract was applied to a 5-ml Econo-Pac heparin cartridge (Bio-Rad) as described in Danon and Mayfield (17).
Cloning of the RB47 cDNA.
After purification of RB47 by published procedures (17), the proteins were digested with proteinase Lys-C or trypsin, run on HPLC and microsequenced (John Lesyk, Worcester Foundation for Experimental Biology, Worcester, MA, and Arie Admon, The Protein Center, Department of Biology, Technion, Haifa, Israel). Two peptide sequences were obtained (QYGFVHFEDQAAADR and GFGFINFKDAESAA) and degenerate oligos were designed based on the reverse translation of these peptides. A C. reinhardtii cDNA phage library was screened with these oligos by using standard methods (24). One set of duplicate filter lifts was probed with each oligo, and plaques that hybridized to both were isolated. One of several 2.6-kb inserts was subcloned and sequenced.
Heterologous Expression.
A full-length RB47 cDNA was cloned into the E. coli expression vector pET3A (25) and transformed into BL21 E. coli cells. The cells were grown to a density of 0.4 (OD600), then induced with 0.5 mM isopropyl β-d-thiogalactoside. Cells were then allowed to grow for an additional 4 h, at which point they were pelleted and frozen.
The RB47 protein expressed in E. coli was purified by using a protocol similar to that used previously for purification of RB47 from C. reinhardtii. Approximately 5 g of E. coli cells grown as described above were resuspended in low-salt extraction buffer and disrupted by sonication. The soluble cell extract was applied to a 5-ml Econo-Pac heparin cartridge (Bio-Rad) that was washed prior to elution of the RB47 protein (17).
GMS and UV-Crosslinking.
Gel mobility shift was performed with heparin agarose fractions essentially as described in Danon and Mayfield (17). Equal quantities of protein lysate were incubated with 0.05 pmol of a 32P-labeled, T7-transcribed RNA containing the first 133 bases of the 5′ end of psbA, 20 μg tRNA, and 0.17 μg of Fud7 total RNA (Fud7 lacks psbA mRNA) for 15 min. Where applicable, 200-fold unlabeled competitor RNA was added to the reaction mixture. Equal RB47 protein load was based on immunoblots of endogenous and recombinant protein. Electrophoresis was carried out on a 1× TBE, 5% native polyacrylamide gel. RNA-protein complexes were detected by autoradiography.
For UV-crosslinking, binding reactions were irradiated with short-wave UV light for 1 h on ice, digested with RNase A for 30 min at 55°C and separated by SDS/PAGE as described in Danon and Mayfield (17). Proteins labeled by crosslinked 32P-labeled nucleotides were detected by autoradiography.
In Vitro Translation and Chloroplast Import.
RNA was transcribed from 0.5 μg of an E. coli plasmid (pBS) containing either the coding region of the C. reinhardtii RB47 cDNA cloned downstream of the T7 promoter or the coding region of the pea small subunit cDNA of ribulose-1,5-bisphosphate carboxylase/oxygenase cloned downstream of the SP6 promoter (26). Each reaction contained 40 mM Tris⋅HCl (pH 7.5), 50 mM NaCl, 8 mM MgCl2, 8 mM DTT, 1 mM NTPs, and 10 units T7 or SP6 RNA polymerase in a 10-μl total volume. The reaction was incubated at 37°C for 1 h and the resulting RNAs, in addition to luciferase RNA (Promega), were used in a rabbit reticulocyte in vitro translation (IVT) system (Promega) following the manufacturer’s directions with a slight modification: translation reactions were placed at 37°C for 50 min.
Pea seedlings (Pisum sativum cv. Little Marvel) were grown according to the procedures of Cline et al. (27). Chloroplasts were isolated from 10 day old seedlings according to the procedures described by Bartlett et al. (28) and Cline et al. (27) with slight modifications. Briefly, 50 g of pea leaves were ground in a Waring blender in the presence of 200 ml G-R buffer (50 mM Hepes–KOH, pH 7.5/0.33 M sorbitol/1 mM MgCl2/1 mM MnCl2/5 mM sodium ascorbate/1% BSA). A crude chloroplast preparation was obtained by filtering the homogenate through two layers of Miracloth and centrifugation at 3,000 × g for 3 min at 4°C. Intact chloroplasts were obtained by layering of the crude chloroplast preparation resuspended in G-R buffer over 40% Percoll in G-R buffer (1:1 vol/vol), and centrifugation at 2,500 × g for 7 min at 4°C. The chloroplast pellets were washed in 1× import buffer (0.33 M sorbitol/50 mM Hepes–KOH, pH 8.0) and then resuspended to a concentration of 1 mg chlorophyll/ml (29).
Import assays were performed by incubating 8–10 μl of the RB47, small subunit, or luciferase IVT mix in a reaction containing 0.7 mg/ml chloroplasts, 100 mM Mg/ATP and 25 mM methionine in 1× import buffer for 30 min at room temperature in the presence of light, mixing every 5 min. After import, chloroplasts were repurified over 40% Percoll cushions in 1× import buffer or treated with 1 mg/ml thermolysin (Sigma) for 40 min on ice to degrade any proteins remaining outside the chloroplasts. EDTA was added to a final concentration of 50 mM to suppress protease activity, and the thermolysin-treated chloroplasts were repurified over 40% Percoll containing 5 mM EDTA. Purified chloroplast proteins were separated by SDS/PAGE and imported proteins were detected by autoradiography after treatment of the gel with Enhance (Dupont/NEN) according to the manufacturers instructions.
RESULTS
The psbA mRNA Binding Protein (RB47) Is a PABP.
The psbA mRNA specific RNA binding protein (RB47) was purified from wild-type C. reinhardtii cells by previously described methods (17) and blotted to polyvinylidene difluoride membrane for subsequent protease digestion and reverse phase HPLC. Several peaks were chosen for protein microsequence. Two independent peptide sequences were obtained. Degenerate oligos were designed based on the reverse translation of these two peptides. A C. reinhardtii cDNA library in lambda phage was screened and several cDNA clones that hybridized to oligos deduced from both peptides were identified. Four of these clones were 2.6 kb in length, the predicted full length of the mRNA as determined by subsequent Northern blot analysis (data not shown). One of the cDNAs was subcloned into an E. coli plasmid for sequence determination. As shown in Fig. 1, the predicted protein sequence from the cloned cDNA contained both of the derived peptide sequences of RB47 (wavy lines above sequence) and is highly homologous to PABP from a variety of eukaryotic organisms. Under high-stringency hybridization conditions a probe corresponding to the entire RB47 cDNA hybridized to a unique band on a genomic Southern blot of wild-type DNA, while under lower stringency a number of bands were detected (data not shown). This suggests that PABPs are a family of proteins in C. reinhardtii, as they are in other organisms (30).
Figure 1.
Predicted amino acid sequence of RB47 and comparison with five other PABPs. The amino-terminal two-thirds of RB47 contains the four highly conserved RRMs common to all members of the PABP family (underline), with each RRM containing conserved ribonucleoprotein octapeptides and hexapeptides (boxed). There is also a carboxyl-terminal conserved (CTC, crosshatched underline) domain that is highly conserved in known PABPs, including RB47. Amino acids that are identical to the consensus of all six sequences are shaded. Peptides from RB47 obtained by microsequence and used for cloning are indicated by a wavy overline. A single mismatch occurred in the first peptide (Q in the peptide; G in the predicted sequence), indicated by a ∗. Amino acids are indicated by single letter code. PABP sequences are from Arabidopsis thaliana (30), Drosophila melanogaster (36), Homo sapiens (33), Saccharomyces cerevisiae (31), and Xenopus laevis (34).
Members of the PABP family of proteins show high sequence conservation in organisms ranging from yeast to plants to mammals (30–36), but to date PABPs have not been identified in prokaryotic organisms. Fig. 1 shows a comparison between the predicted RB47 amino acid sequence and PABPs from five other representative species. The amino-terminal two thirds of RB47 contains four highly conserved RNA recognition motifs (RRMs) (37) common to all members of the PABP family, with each RRM containing conserved ribonucleoprotein octapeptides (ribonucleoprotein 1) and hexapeptides (ribonucleoprotein 2) (38). While pair-wise comparisons between each of RB47’s RRMs with the corresponding RRM from other organisms give a percent identity range of 39–67%, all four of RB47’s RRMs have an average of 57% identity relative to the other PABP’s RRMs. The ribonucleoprotein 1 and ribonucleoprotein 2 domains in RB47 are 95% identical to the consensus sequence for PABPs. There is also a carboxy- terminal conserved domain (30) that is highly conserved in known PABPs, including RB47 (56% identical to other PABPs). The region between the 4 RRMs and the carboxyl-terminal conserved domain varies in both size and sequence between species. Despite this variation, this region is generally rich in proline and glutamic acid, and contains a stretch of poly-alanine. RB47 contains each of these signature domains, and the level of conservation between RB47 and any other member of the PABP family is generally as high as between any two PABPs from different species. These data clearly show that RB47 is a PABP containing all of the domains normally associated with eukaryotic cytoplasmic PABPs.
Heterologously Expressed and Endogenous RB47 Proteins Have Similar Properties.
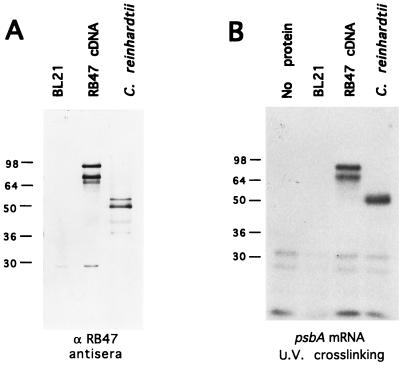
Confirmation of the identity of the cloned cDNA as encoding the authentic RB47 protein was initially accomplished by examining protein expressed from the cDNA by immunoblot analysis and by RNA binding activity assay. As shown in Fig. 2A, the protein produced when the RB47 cDNA was expressed from an E. coli expression vector (pET3A, ref. 25) was recognized by antisera raised against the C. reinhardtii RB47 protein. The E. coli expressed protein migrates at 80 kDa on SDS/PAGE, but the protein is actually 69 kDa, as determined by mass spectrometry of the E. coli expressed protein (data not shown). This mass agrees with the mass predicted for RB47 from the cDNA sequence. A 60-kDa product is also produced in E. coli, and recognized by the antisera against the C. reinhardtii protein, which is most likely a degradation or early termination product of the RB47 cDNA. The protein expressed from the RB47 cDNA is recognized by the antisera raised against the C. reinhardtii protein at levels similar to the recognition of the authentic C. reinhardtii RB47 protein (Fig. 2A), demonstrating that the cloned cDNA produces a protein product that is immunologically related to the authentic RB47 protein. Peptide mapping by mass spectrometry has shown that the endogenous RB47 protein corresponds primarily to the RNA recognition motifs contained within the N-terminal region of the predicted precursor protein (data not shown), suggesting that a cleavage event is necessary to produce the mature 47-kDa protein.
Figure 2.
Characteristics of E. coli expressed RB47 protein. (A) Proteins from whole cell extract of untransformed E. coli strain (BL21), from BL21 cells transformed with the RB47 cDNA in a pET expression vector (RB47 cDNA), and from heparin agarose-purified proteins from C. reinhardtii, were detected with antisera against C. reinhardtii RB47 protein. (B) UV crosslinking of radiolabeled psbA RNA to E. coli expressed RB47 cDNA. Lane 1 contains the radiolabeled RNA only (no protein). Lane 2 contains whole cell extract from untransformed E. coli (BL21). Lane 3 contains heparin agarose purified proteins from E. coli transformed with the RB47 cDNA (RB47 cDNA), and lane 4 contains heparin agarose purified proteins from C. reinhardtii. Molecular mass is indicated at left.
To determine if the heterologously expressed RB47 protein was capable of binding the psbA RNA, the E. coli expressed protein was purified by heparin agarose chromatography. The E. coli expressed protein that bound to the heparin agarose matrix was eluted from the column at the same salt concentration as used to elute the authentic C. reinhardtii RB47 protein. This protein fraction, which contained almost exclusively recombinant RB47 protein, was used in in vitro binding assays with the psbA 5′ UTR. As shown in Fig. 2B, both the 69 and 60-kDa E. coli expressed proteins crosslink to the radiolabeled psbA 5′ UTR at levels similar to crosslinking of the endogenous RB47 protein, when the RNA/protein complex is subjected to UV irradiation.
Binding of the psbA UTR to the Heterologously Expressed and Endogenous RB47 Proteins Shows Similar Specificity and Affinity.
Heparin agarose purified proteins, both from the E. coli expressed RB47 cDNA and from C. reinhardtii cells, were used in an RNA gel mobility shift assay to determine the relative affinity and specificity of these proteins for the 5′ UTR of the psbA mRNA. As shown in Fig. 3, the E. coli expressed proteins bind to the psbA 5′ UTR in vitro with properties that are similar to those of the endogenous RB47 protein purified from C. reinhardtii. Different forms of the RB47 protein (47-kDa endogenous protein vs. the 69-kDa E. coli expressed protein) likely accounts for the slight differences in mobility observed when comparing the binding profiles of purified C. reinhardtii protein to heterologously expressed RB47. RNA binding to both the E. coli expressed and the endogenous RB47 protein can be competed by using either 200-fold excess of unlabeled psbA RNA (lanes 3 and 9) or 200-fold excess of poly(A) RNA (lanes 4 and 10). RNA binding to either of these proteins is poorly competed by using 200-fold excess of total RNA (lanes 7 and 13) or 200-fold excess of the 5′ UTR of the psbD (lanes 5 and 11) or psbC (lanes 6 and 12) RNAs. Recombinant RB47 protein containing a poly-histidine tag and purified by Ni2+ column chromatography showed identical binding characteristics (data not shown).
Figure 3.
Comparison of the binding specificity of endogenous and heterologously expressed RB47 proteins by gel mobility shift. Proteins were purified by heparin agarose chromatography from wild-type C. reinhardtii (lanes 2–7) and from E. coli expressing the RB47 cDNA (lanes 8–13). A radiolabeled psbA RNA fragment containing the first 133 bases of the 5′ end of the RNA was incubated with proteins and a 200-fold excess of unlabeled RNA as indicated above the lane.
The Precursor Protein Translated from the RB47 cDNA Is Imported into Chloroplasts and Processed to 47 kDa.
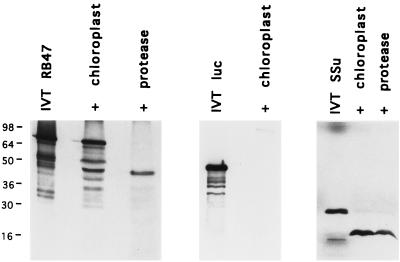
To demonstrate that the PABP that we have identified is localized to the chloroplast, isolated pea chloroplasts were used for in vitro protein import assays. Pea chloroplasts were utilized because C. reinhardtii chloroplasts are recalcitrant to purification, and thus more likely to produce aberrant results. For import assays, the RB47 cDNA was transcribed in vitro by using T7 RNA polymerase and the RB47 RNA was translated in a rabbit reticulocyte IVT system (Promega). The RNA was transcribed from a construct lacking the normal RB47 5′ UTR, as the expressed precursor protein binds to its own 5′ UTR, interfering with in vitro translation and chloroplast import (data not shown). Binding of other PABPs to their own 5′ UTRs has been shown to block in vitro translation, and has therefore been implicated in regulation of protein expression in vivo (39, 40). As shown in Fig. 4 Left, the major product of the in vitro translation reaction (IVT RB47) was a single protein that migrates at the same position on SDS/PAGE as the 69-kDa protein from E. coli expression. When this precursor protein was incubated with isolated pea chloroplasts in vitro, the protein bound to the chloroplast envelope (Fig. 4, + chloroplast), and some of the protein was transported into the chloroplast and processed to give a mature protein of 47 kDa, consistent with the endogenous C. reinhardtii RB47 protein. Proteolytic processing to produce a mature protein, in addition to the removal of the transit peptide, has been observed for several chloroplast imported proteins (41). The processed protein was resistant to exogenously added thermolysin (Fig. 4, + protease), as expected of a protein contained within the plastid, while the precursor protein was degraded, as expected of a protein bound to the chloroplast envelope. The processed protein could be degraded by protease if the chloroplasts were lysed by sonication prior to the addition of thermolysin, while sonication without the addition of thermolysin had no impact on the imported protein (data not shown). In vitro translated firefly luciferase (IVT luc) protein did not bind to the chloroplast envelope, nor was it imported into chloroplasts in vitro (Fig. 4 Center). In vitro translated pea ribulose-1,5-bisphosphate carboxylase/oxygenase was imported efficiently into pea chloroplasts (Fig. 4 Right), as expected.
Figure 4.
SDS/PAGE of proteins following import of in vitro synthesized RB47 protein into isolated pea chloroplast. 35S-Met-labeled RB47 produced by in vitro translation (IVT RB47) was incubated with ATP and isolated pea chloroplasts. Chloroplasts were repurified by gradient centrifugation following incubation (+ chloroplast). Treatment with thermolysin prior to repurification (+ protease) degraded proteins not imported into the chloroplasts resulting in the loss of larger molecular mass proteins, but not in the loss of the 47-kDa protein. IVT of luciferase mRNA resulted in the production of a 48-kDa protein (IVT luc) that did not import or associate with repurified pea chloroplasts (+ chloroplast). IVT of RuBPCase small subunit mRNA produced a protein (IVT SSu) that imported efficiently in isolated pea chloroplast (+ chloroplast) and was protected from protease treatment (+ protease). Molecular mass is indicated at left.
DISCUSSION
The identification of RB47 as a PABP is somewhat unexpected given that translation in the chloroplast is generally considered prokaryotic like, and PABPs have not been identified as components of the prokaryotic translation apparatus. The chloroplast has 70S ribosomes (as in prokaryotes) and the mRNAs encoded by the chloroplast genome do not, in general, have poly(A) tails, and often contain prokaryotic consensus ribosome binding sequences (42, 43). The addition of A-rich sequences to the 3′ end of endonucleolytic cleavage products of some chloroplast mRNAs has recently been described (44, 45), and this seems to play a role in degradation of the RNA, as in prokaryotes. The identification of a PABP in the chloroplast suggests that components of the cytoplasmic translation machinery may have been appropriated by the chloroplast for a function in the chloroplast similar to the one performed in the cytoplasm. These data also suggest that PABPs may function in translational regulation in the chloroplast in a manner not previously described for cytoplasmic mRNAs, although the role of RB47 in psbA translation seems to fit with the limited information known about the function of PABPs in other systems. While limited specific biochemical functions have been identified for any member of the PABP family, these proteins have been defined as specific RNA binding proteins with a role in translational regulation. In yeast, PABP is essential for viability (21, 46), and a temperature-sensitive allele of PABP shows that depletion of PABP in yeast results in inhibition of translation initiation and poly(A) tail shortening (47). Further, revertants of this temperature-sensitive mutation mapped to a ribosomal protein, suggesting that PABP interacts with the ribosome to mediate translation initiation (47). In addition, PABPs have been shown to physically interact with ribosomes (48), and with eukaryotic initiation factors (eIF4G) (22).
The role of RB47 in psbA translation is consistent with the general function of PABPs as defined in other systems. A C. reinhardtii nuclear mutant lacking RB47 shows no psbA translation or psbA mRNA binding activity, but has otherwise normal chloroplast translation (C.B.Y. and S.P.M., unpublished results). This mutant demonstrates that the RB47 protein is required in vivo for psbA translation. Our observation that poly(A) RNA is able to compete RB47 binding to the psbA UTR (Fig. 3B) further supports the hypothesis that RB47 is a true member of the PABP family. Another protein component of the psbA RNA binding complex (RB60) can influence RB47 binding in vitro (20), while the other components’ influence on binding or specificity is not yet known. However, as shown in Fig. 3B, RB47 alone is capable of recognizing the psbA 5′ UTR. The exact nature of the RNA element contained within the psbA 5′ UTR has yet to be determined in C. reinhardtii, though site-directed mutagenesis has shown that secondary structure in the 5′ UTR is an essential element of both RB47 binding and light-regulated translation of the psbA mRNA (13). In addition, by using an IVT system from tobacco chloroplasts, Hirose and Sugiura (49) identified a short stretch of adenosine residues in the psbA 5′ UTR as a critical element for translation of that mRNA. Studies in other systems suggest that stretches of 4–5 adenosine residues followed by a G or C may be sufficient for RNA recognition by PABPs (50, 51), and the 5′ UTR of psbA contains this target sequence three times.
RB47 fits the general role predicted for PABPs, with two important exceptions. First, RB47 shows specific binding to the 5′ UTR of a single chloroplast encoded mRNA. Second, RB47 is acting in the chloroplast, which in general has translational machinery similar to that of prokaryotes, where PABPs have not previously been identified. The fact that this nuclear encoded, eukaryotic protein has been exploited for use in the chloroplast may not be surprising given the bidirectional exchange of genetic information between the chloroplast and nucleus (52). However, the basic mechanisms of translation in the eukaryotic cytoplasm compared with the prokaryotic-like chloroplast have traditionally been viewed as divergent. Determining the precise mechanism of translational activation associated with RB47 will help us to further define the role of PABPs in translational regulation. This in turn may provide a perspective on the underlying molecular mechanism used to regulate translation in two distinct translation systems using a single type of RNA binding protein.
Acknowledgments
We thank Josh Bliesath for technical assistance and Ken Cline for help with the protein import assays. C.B.Y. is the recipient of a National Science Foundation predoctoral fellowship and a National Science Foundation graduate research traineeship. A.C. is the recipient of National Institutes of Health postdoctoral fellowship GM15639-03. This work supported by the U.S. Department of Energy Grant DE-FG03-93ER20116 to S.P.M.
ABBREVIATIONS
- IVT
in vitro translation
- PABP
poly(A) binding protein
- RRM
RNA recognition motif
- UTR
untranslated region
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF043297).
References
- 1.Kozak M. Annu Rev Cell Biol. 1992;8:197–225. doi: 10.1146/annurev.cb.08.110192.001213. [DOI] [PubMed] [Google Scholar]
- 2.de Smit M H, van Duin J. Prog Nucleic Acid Res Mol Biol. 1990;38:1–35. doi: 10.1016/s0079-6603(08)60707-2. [DOI] [PubMed] [Google Scholar]
- 3.Winter R B, Morrissey L, Gauss P, Gold L, Hsu T, Karam J. Proc Natl Acad Sci USA. 1987;84:7822–7826. doi: 10.1073/pnas.84.22.7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang C K, Draper D E. Biochemistry. 1990;29:4434–4439. doi: 10.1021/bi00470a025. [DOI] [PubMed] [Google Scholar]
- 5.Wulczyn F G, Kahmann R. Cell. 1991;65:259–269. doi: 10.1016/0092-8674(91)90160-z. [DOI] [PubMed] [Google Scholar]
- 6.Mayfield S P, Yohn C B, Cohen A, Danon A. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:147–166. [Google Scholar]
- 7.Yohn C B, Cohen A, Danon A, Mayfield S P. Mol Cell Biol. 1996;16:3560–3566. doi: 10.1128/mcb.16.7.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochaix J D, Kuchka M, Mayfield S, Schirmer-Rahire M, Girard-Bascou J, Bennoun P. EMBO J. 1989;8:1013–1021. doi: 10.1002/j.1460-2075.1989.tb03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuchka M R, Goldschmidt-Clermont M, van Dillewijn J, Rochaix J D. Cell. 1989;58:869–876. doi: 10.1016/0092-8674(89)90939-2. [DOI] [PubMed] [Google Scholar]
- 10.Girard-Bascou J, Pierre Y, Drapier D. Curr Genet. 1992;22:47–52. doi: 10.1007/BF00351741. [DOI] [PubMed] [Google Scholar]
- 11.Barkan A, Walker M, Nolasco M, Johnson D. EMBO J. 1994;13:3170–3181. doi: 10.1002/j.1460-2075.1994.tb06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Klein P G, Mullet J E. Plant Mol Biol. 1994;25:459–467. doi: 10.1007/BF00043874. [DOI] [PubMed] [Google Scholar]
- 13.Mayfield S P, Cohen A, Danon A, Yohn C B. J Cell Biol. 1994;127:1537–1545. doi: 10.1083/jcb.127.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rochaix J D. Annu Rev Cell Biol. 1992;8:1–28. doi: 10.1146/annurev.cb.08.110192.000245. [DOI] [PubMed] [Google Scholar]
- 15.Zerges W, Rochaix J D. Mol Cell Biol. 1994;14:5268–5277. doi: 10.1128/mcb.14.8.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staub J M, Maliga P. EMBO J. 1993;12:601–606. doi: 10.1002/j.1460-2075.1993.tb05692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danon A, Mayfield S P. EMBO J. 1991;10:3993–4001. doi: 10.1002/j.1460-2075.1991.tb04974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danon A, Mayfield S P. Science. 1994;266:1717–1719. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- 19.Danon A, Mayfield S P. EMBO J. 1994;13:2227–2235. doi: 10.1002/j.1460-2075.1994.tb06500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Mayfield S P. Science. 1997;278:1954–1957. doi: 10.1126/science.278.5345.1954. [DOI] [PubMed] [Google Scholar]
- 21.Sachs A B, Davis R W, Kornberg R D. Mol Cell Biol. 1987;7:3268–3276. doi: 10.1128/mcb.7.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarun S Z, Jr, Sachs A B. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- 23.Harris E H. The Chlamydomonas Sourcebook. San Diego: Academic; 1989. [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 25.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 26.Anderson S, Smith S M. Biochem J. 1986;240:709–715. doi: 10.1042/bj2400709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cline K, Werner-Washburne M, Lubben T H, Keegstra K. J Biol Chem. 1985;260:3691–3696. [PubMed] [Google Scholar]
- 28.Bartlett S G, Grossman A R, Chua N-H. In: Methods in Chloroplast Molecular Biology. Edelman M, Hallick R B, Chua N-H, editors. Amsterdam: Elsevier/North–Holland; 1982. pp. 1081–1092. [Google Scholar]
- 29.Robinson C. In: Plant Cell Biology: A Practical Approach. Harris N, Oparka K J, editors. Oxford: Oxford Univ. Press; 1994. pp. 273–282. [Google Scholar]
- 30.Hilson P, Carroll K L, Masson P H. Plant Physiol. 1993;103:525–533. doi: 10.1104/pp.103.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adam S A, Nakagawa T, Swanson M S, Woodruff T K, Dreyfuss G. Mol Cell Biol. 1986;6:2932–2943. doi: 10.1128/mcb.6.8.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belostotsky D A, Meagher R B. Proc Natl Acad Sci USA. 1993;90:6686–6690. doi: 10.1073/pnas.90.14.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grange T, de Sa C M, Oddos J, Pictet R. Nucleic Acids Res. 1987;15:4771–4787. doi: 10.1093/nar/15.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zelus B D, Giebelhaus D H, Eib D W, Kenner K A, Moon R T. Mol Cell Biol. 1989;9:2756–2760. doi: 10.1128/mcb.9.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nietfeld W, Mentzel H, Pieler T. EMBO J. 1990;9:3699–3705. doi: 10.1002/j.1460-2075.1990.tb07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefrere V, Vincent A, Amalric F. Gene. 1993;126:295–296. doi: 10.1016/0378-1119(93)90387-i. [DOI] [PubMed] [Google Scholar]
- 37.Bandziulis R J, Swanson M S, Dreyfuss G. Genes Dev. 1989;3:431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- 38.Kenan D J, Query C C, Keene J D. Trends Biochem Sci. 1991;16:214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- 39.Bag J, Wu J. Eur J Biochem. 1996;237:143–152. doi: 10.1111/j.1432-1033.1996.0143n.x. [DOI] [PubMed] [Google Scholar]
- 40.de Melo Neto O P, Standart N, Martins de Sa C. Nucleic Acids Res. 1995;23:2198–2205. doi: 10.1093/nar/23.12.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Douwe de Boer A, Weisbeek P J. Biochim Biophys Acta. 1991;1071:221–253. doi: 10.1016/0304-4157(91)90015-o. [DOI] [PubMed] [Google Scholar]
- 42.Gillham N W, Boynton J E, Hauser C R. Annu Rev Genet. 1994;28:71–93. doi: 10.1146/annurev.ge.28.120194.000443. [DOI] [PubMed] [Google Scholar]
- 43.Harris E H, Boynton J E, Gillham N W. Microbiol Rev. 1994;58:700–754. doi: 10.1128/mr.58.4.700-754.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kudla J, Hayes R, Gruissem W. EMBO J. 1996;15:7137–7146. [PMC free article] [PubMed] [Google Scholar]
- 45.Lisitsky I, Klaff P, Schuster G. Proc Natl Acad Sci USA. 1996;93:13398–13403. doi: 10.1073/pnas.93.23.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sachs A B, Bond M W, Kornberg R D. Cell. 1986;45:827–835. doi: 10.1016/0092-8674(86)90557-x. [DOI] [PubMed] [Google Scholar]
- 47.Sachs A B, Davis R W. Cell. 1989;58:857–867. doi: 10.1016/0092-8674(89)90938-0. [DOI] [PubMed] [Google Scholar]
- 48.Proweller A, Butler J S. J Biol Chem. 1996;271:10859–10865. doi: 10.1074/jbc.271.18.10859. [DOI] [PubMed] [Google Scholar]
- 49.Hirose T, Sugiura M. EMBO J. 1996;15:5268–5277. [PMC free article] [PubMed] [Google Scholar]
- 50.Gorlach M, Burd C G, Dreyfuss G. Exp Cell Res. 1994;211:400–407. doi: 10.1006/excr.1994.1104. [DOI] [PubMed] [Google Scholar]
- 51.Kuhn U, Pieler T. J Mol Biol. 1996;256:20–30. doi: 10.1006/jmbi.1996.0065. [DOI] [PubMed] [Google Scholar]
- 52.Morden C W, Delwiche C F, Kuhsel M, Palmer J D. Biosystems. 1992;28:75–90. doi: 10.1016/0303-2647(92)90010-v. [DOI] [PubMed] [Google Scholar]