Abstract
Previous research has found that exposure to unpredictable stress can augment anxiety in humans and animals. The appearance of anxiety symptoms in humans frequently develop after stress exposure has terminated, but few rodent studies have systematically examined the delayed anxiogenic effects of unpredictable stress. Therefore, the current study investigated whether anxiety-like behaviors in rats would increase at several time intervals following exposure to chronic unpredictable stress (CUS). Unconditioned and conditioned response tasks were used to assess anxiety in male rats 1, 7 or 14 days following exposure to 10 days of a variety of stressors. Rats exposed to CUS showed increased burying behaviors and immobility during the defensive burying test, a conditioned anxiety test. The effects on burying behavior were apparent 7 and 14 days after the termination of the unpredictable stress procedure, but not when tested 1 day after CUS. Total time immobile in the defensive burying test also increased 14 days after termination of the last stressor. In contrast, there were no significant effects of CUS on behavioral measures in the unconditioned response tasks, the elevated plus-maze or light-dark box, at any time point following exposure to CUS. The current findings suggest that CUS may be a useful model of human conditioned anxiety that develops subsequent to chronic stress exposure.
Keywords: Chronic stress, Rats, Anxiety, Elevated Plus-Maze, Defensive Burying, Light-Dark Test, Unpredictable Stress, Long Lasting
I. Introduction
A large number of individuals report high levels of chronic stress in their lives [22,49]. One potential negative outcome associated with chronic stress exposure is the development of mental illnesses, including anxiety or affective disorders. The increased risk of mental illness has been reported in individuals with high life stress due to low income and education levels [29,35,42] or high risk occupations [46]. In addition, high levels of self-reported stress in the workplace or academic setting predicts a greater risk of mental illness [17,20]. While the human data clearly support a correlative relationship between stress exposure and mental illness, it is difficult to isolate the contribution of stress to the etiology or the severity of a mental illness due to the limitations associated with human studies [16].
Animal studies have also demonstrated that exposure to stress increases behaviors associated with anxiety or depression, while providing greater experimental control of variables. Exposure of rodents to unpredictable stress has resulted in behavioral profiles indicative of human psychopathology [7,14,61,63]. Several chronic stress procedures have been published that use different stressors, 1-2 times per day for 7-54 days to model long-term human stress exposure [14,33,50,62,63]. Rodents exposed to these unpredictable stress procedures have shown increased anxiety-like behavior on a number of behavioral tasks, including the elevated plus-maze, unconditioned and conditioned freezing tests, the social interaction test and the passive avoidance task [33,50,63].
Frequently, the detrimental effects of stress reported by humans are not experienced until after the stress exposure has ended. The most common example of this situation is posttraumatic stress disorder, in which the symptoms and disruption in daily living may not occur for several weeks following exposure to the stressor. Although the unpredictable stress procedures have been suggested as appropriate animal models of anxiety disorders due to their behavioral effects, these behavioral effects have not been measured after the cessation of the stress procedure [14,58]. Previous research with chronic predictable or single stress exposure reported an increase in anxiety measures 1-3 weeks following the last stress exposure [4,7,19,30,32,54,55,59]. Therefore, to systematically investigate the delayed effects of chronic unpredictable stress (CUS), the current study exposed rats to 10 days of unpredictable stress and then assessed anxiety on several behavioral measures 1, 7 or 14 days following the last stress exposure.
There are many tests that have been used to assess anxiety behavior in rodents to screen potential pharmacological treatments or model particular aspects of anxiety or emotional behavior in humans [15,43,44,51]. These behavioral tests can be separated into 2 broad categories, unconditioned and conditioned responses, based on the type of responding measured and the test situation [43,44]. Unconditioned response tests make use of rodents’ innate response to anxiogenic situations, such as an open space or light conditions [for reviews see 10,36,44,60]. The elevated plus-maze and light-dark box are examples of unconditioned response tests that are frequently used to assess anxiety, most analogous to Generalized Anxiety Disorder [53]. In contrast to unconditioned tests, conditioned response tasks require the animal to learn that a particular stimulus is aversive through experience, such as defensive burying [for review see 15]. Previous studies have found unpredictable stress to increase or have no effect on anxiety-like behaviors in unconditioned response tasks [14,25,33,58,63], while anxiety behaviors were shown to increase in a conditioned response task [63]. The current experiment was conducted to clarify the effects of CUS on both unconditioned and conditioned response tasks. Identifying the effects of unpredictable stress on specific behavioral tasks related to anxiety may lead to a greater understanding of the utility of the stress procedures in modeling specific anxiety disorders.
2. Materials and methods
2.1 Animals and housing
Male Sprague-Dawley rats weighing 250-425g were obtained from the Psychology Department’s rat colony at Northern Illinois University. The subjects were housed in white opaque polycarbonate cages (46 × 25 × 21cm) on a 12:12-hr light/dark cycle (lights on at 6:00) with food and water available ad libitum. All subjects were weighed daily to monitor general health. Rats were paired housed from weaning (postnatal day 21) until euthanasia, unless specified by the stress protocol as isolation housing. The morning following isolation, all rats were returned to a cage with their original cage mate. The current procedures complied with National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996). All procedures were approved by the Northern Illinois University’s Institutional Animal Care and Use Committee.
Rats were randomly assigned to 1 of 4 groups: control, stress-1, stress-7, and stress-14. The animals in the 3 stress groups were exposed to the CUS protocol [21,24,37] as follows: Day 1 11:00 a.m. 50 min cold room (4°C), and 12:00 p.m. 60 min cage rotation; Day 2 1:00 p.m. 4 h wet bedding (400 ml tap water in home cage), and 6:00 p.m. lights on overnight; Day 3 12:00 p.m. 3 h lights off, and 3:00 p.m. 60 min restraint stress (6 × 21.6 cm; Harvard Apparatus, Inc., Holliston, MA); Day 4 6:00 p.m. 50 min cage rotation, and food and water deprivation overnight (15 h); Day 5 3:00 p.m. 15 min cold room isolation, and 4:00 p.m. isolation housing overnight (17 h); Day 6 11:00 a.m. 4 h wet bedding, and 3:00 p.m. 2 h lights off; Day 7 1:00 p.m. 30 min cage rotation, and 6:00 p.m. 1 h lights on; Day 8 10:00 a.m. 20 min cage rotation, and 3:00 p.m. 60 min restraint stress; Day 9 10:00 a.m. 4 h wet bedding, and 6:00 p.m. food and water deprivation; and Day 10 6:00 p.m. isolation housing and lights on overnight.
Control animals were left undisturbed until the time of the experiment with the exception of daily weighing. The stress groups differed by the number of days between the last stressor and the behavioral tests as follows: stress-1 rats were tested 1 day after, stress-7 rats were tested 7 days after, and stress-14 rats were tested 14 days after cessation of the stress protocol. The same rats were tested for elevated plus-maze and then defensive burying, while separate subjects were used for the light-dark box test. A total of 80 rats were tested in the elevated plus-maze and defensive burying tests, divided into the following groups: 39 non-stressed controls, 16 stress-1 rats, 14 stress-7 rats, and 11 stress-14 rats. A total of 68 rats were tested in the light-dark box, divided into the following groups: 29 controls, 11 stress-1 rats, 10 stress-7 rats, and 18 stress-14 rats. Rats assigned to the control group were matched to a specific stress delay group, such that both stress and control rats were introduced into the colony, handled and weighed for the same number of days.
2.2. Procedures
2.2.1. Defensive Burying
The defensive burying task was used to measure anxiety-like behavior following a single shock from a novel object, a wood dowel. The defensive burying apparatus was a modified home cage (46 × 25 × 21 cm) with 4 cm of wood chip bedding material evenly distributed throughout the cage. One end of the cage contained a 0.75 cm hole through which a shock probe was inserted, extending 6 cm into the cage. The shock probe was made in the laboratory by using a wooden dowel 0.5 cm in diameter wrapped with 2 uninsulated copper wires, such that the two wires spiraled to the end of the probe. A constant current generator (Lafayette Instrument Company, Lafayatte, IN) was connected to the shock probe and delivered a shock of approximately 1.0 mA upon contact with the probe [52].
On the test day, animals were placed in a holding room 3 hours prior to testing. The holding room and adjacent testing room were dimly lit with red lighting and contained an exhaust fan to provide background noise. For the defensive burying test, each animal was placed individually into the testing apparatus facing away from the shock probe for a 15 min test. When the animal received a shock by making contact with the shock probe, the current was terminated so as not to provide additional shocks upon contact. At the end of the 15 min session, the animal was removed from the testing apparatus and returned to his home cage. The testing apparatus was cleaned and new bedding was placed into the test cage for the next rat.
All behavioral testing sessions were videotaped for later analysis for the latency to contact the shock probe, latency to begin burying after contact with the shock probe and duration of burying. Burying was defined as any spraying or throwing of the bedding with the head or forepaws in the direction of the shock probe [41]. Two independent raters scored each behavioral test with a reliability correlation of 0.987 for duration of defensive burying, similar to a previous study [52]. The height of the bedding pile against the wall with the shock probe was measured immediately at the end of the each test. In addition, the duration of immobility and frequency of immobility episodes were recorded with immobility assessed when the rat was standing on 4 feet, sitting or lying with body and head motionless. An immobility episode was any period of time greater than 1 sec during which the rat was immobile.
2.2.2. Elevated Plus-Maze
The elevated plus-maze was used to measure anxiety-like behaviors by utilizing an animal’s natural fear of elevated, open spaces. The apparatus consisted of 4 arms, 2 open arms (11 × 50 cm) with 0.5 cm ledges and two enclosed arms of the same size with 50 cm high walls. The arms were attached to a central square (10 cm2) and shaped as a plus sign. The entire apparatus was elevated 48 cm above the floor.
The testing room was dimly lit with red lighting. For testing, each rat was placed individually on the apparatus with half of their body in a closed arm facing the central square. The rat was allowed to explore the plus-maze for 5 minutes with the test being videotaped for later analysis. After each rat, the maze was cleaned with a diluted cleaning solution containing a citrus odor and 3.95% hydrogen peroxide. The following behaviors were scored for the 5 min trial: latency to enter the open arm, time spent with all four paws in the open arms and the frequency of entries into each arm.
2.2.3. Light-Dark Box
The light-dark box was used to measure anxiety-like behavior by utilizing the rat’s natural preference for dark spaces. The light-dark box was a Plexiglas box (80 × 40 × 20cm) divided into two compartments of equal size [6]. The light compartment had a clear Plexiglas ceiling with the surrounding walls and floor painted white. Four black lines divided the floor of the light compartment into nine 13.3 × 13.3 cm2 squares. The dark compartment also had a Plexiglas ceiling with the surrounding walls, floor and ceiling painted black. The rat could cross between the 2 compartments through an opening of 10 × 8 cm in the dividing wall (transition). Two 60-W fluorescent bulbs were placed above the light compartment to provide illumination.
Each experimental session lasted for 10 minutes. The rat was placed in the center of the light compartment with its head facing the opening in the dividing wall. The behavior of each animal was then videotaped for later analysis. After each animal the cage was cleaned with a diluted citrus cleaner with 3.95% hydrogen peroxide. Videotapes were scored for the number of transitions between the light and dark compartments, initial latency to enter the dark compartment and total time spent in the light compartment.
2.3 Statistics
Multiple analysis of variance (MANOVA) tests were conducted for the behavioral measures associated with each behavioral test. These analyses compared the dependent measures between control and stressed rats for each delay interval (0, 7 and 14 days post stress). Statistically significant MANOVA findings were followed by Dunnett’s t-test to compare each stress group to the control group and by Tukey’s post hoc test to compare the 3 stress groups. The scores for non-stressed control rats in all conditions (matched to each stress delay group) were initially compared, but there were no statistically significant differences. Therefore, the measures of all control rats were combined for statistical and graphical purposes.
Pearson’s correlational analyses were used to compare the behavioral measures on the elevated plus-maze and defensive burying to determine if there was a relationship between the measures assessed in the 2 tasks. The light-dark box measures were taken from separate groups of rats and therefore a comparison of their measures on different tasks could not be made. Pearson’s correlational analyses were also used to compare measures within each task separately. Chi-square calculations were used to assess the proportion of rats for the following measures: entry into any open arm in the elevated plus-maze, falling off the maze, and failure to touch the shock probe or bury the probe in the defensive burying task.
Repeated measures analysis of variance was used to compare the body weight of CUS and control rats during the 10 days of the stress procedure. Independent t-tests were used to compare the total weight gain (day 1 - day 11 difference) of the 2 groups for all rats used in all experiments. Additional independent t-tests were used to compare the body weight differences for control and stress rats following the stress exposure period for the rats tested after a delay (day 11 - 17 difference and day 17 - 24 difference).
3. Results
3.1. Defensive Burying
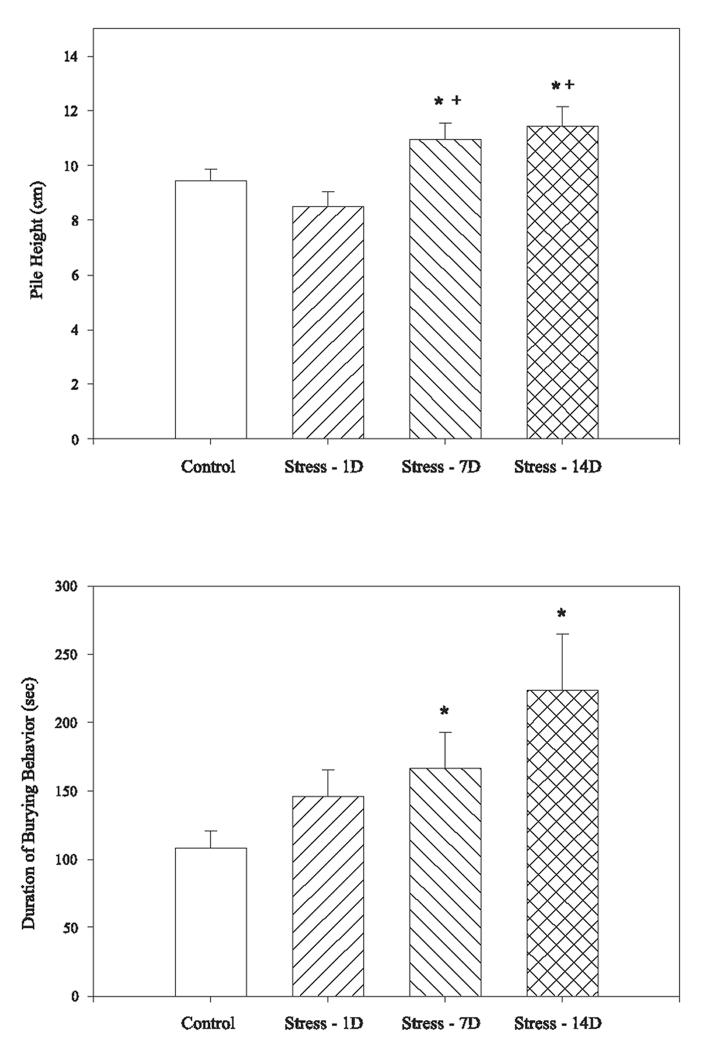
Overall, there was a significant effect of group on burying behavior in rats. Chronically stressed rats had increased pile heights when tested 7 or 14 days following the last stressor compared to non-stressed controls (F(3,65)=4.87, p< .01; Figure 1a). Rats exposed to CUS and tested 7 (Stress-7D) or 14 days (Stress-14D) later also had greater pile heights than CUS rats tested 1 day later (Stress-1D, p<.05). There was a significant difference in the duration of time spent burying between the groups as well (F(3,65)=4.66, p<.01, Figure 1b). When compared with the Dunnett t-test, chronically stressed rats tested 7 or 14 days after the last stressor buried the probe for a greater duration compared to the control group (p<.05).
Fig 1.
Exposure to CUS increased anxiety measures in the defensive burying task. Rats exposed to CUS and tested 7 or 14 days later demonstrated increased burying behaviors as indicated by the height of the bedding pile (top panel) and the duration of burying behavior in the 15 min test (bottom panel). Bars represent mean ± SEM. * indicates a significant difference from non-stressed control group (p<.05), + indicates a significant different from CUS rats tested 1 day after stress ended (p<.05).
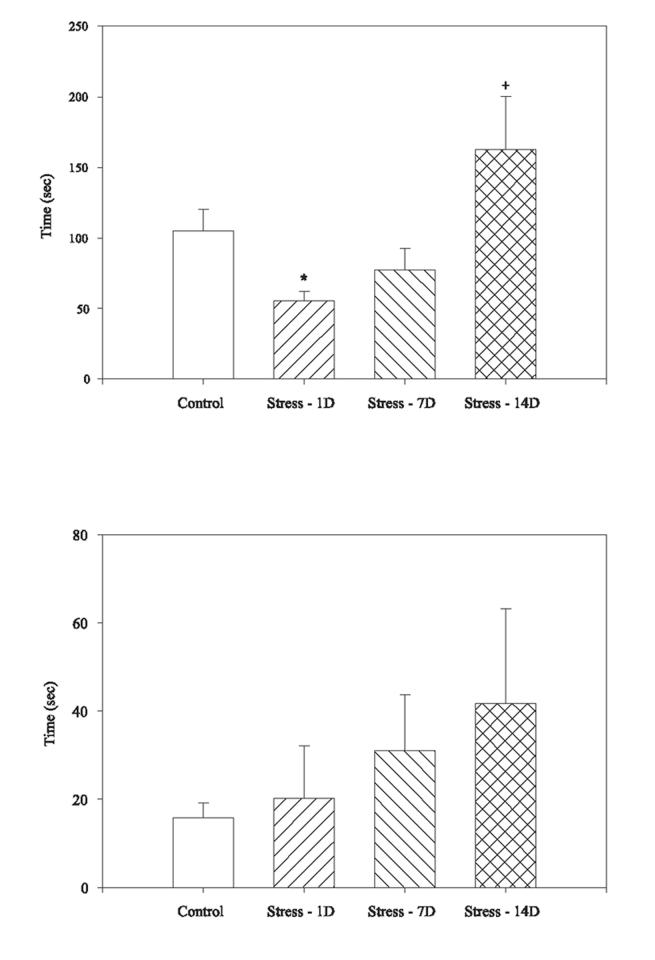
There was a statistically significant effect of group on latencies to begin burying the probe (F(3,65)=4.14, p<.01, Figure 2a). Rats exposed to CUS and then tested 1 day later initiated burying the probe more quickly than controls (p<.05). Rats exposed to CUS and tested 14 days later showed significantly longer latencies to bury than CUS rats tested 1 day later (p<.05) and tended to show longer latencies than CUS rats tested 7 days after stress (p=.056). There were no differences observed in shock latencies between groups (F(3,65)=1.26, n.s.; Figure 2b). A similar proportion of rats failed to bury at all during the test in both groups (12.5% of controls and 10.5% of CUS rats) when compared across groups (χ2(3)=3.25, n.s.). Likewise, there was no difference in the proportion of rats that contacted the shock probe (χ2(3)=2.82, n.s.).
Fig. 2.
Exposure to CUS altered the latency to initiate burying in the defensive burying task. The rats exposed to CUS and tested 1 day later initiated burying faster than non-stressed controls (top panel). There were no differences in the time to initiate shock between groups (bottom panel). Bars represent mean ± SEM. * indicates a significant difference from non-stressed control group (p<.05), + indicates a significant different from CUS rats tested 1 day after stress ended (p<.05).
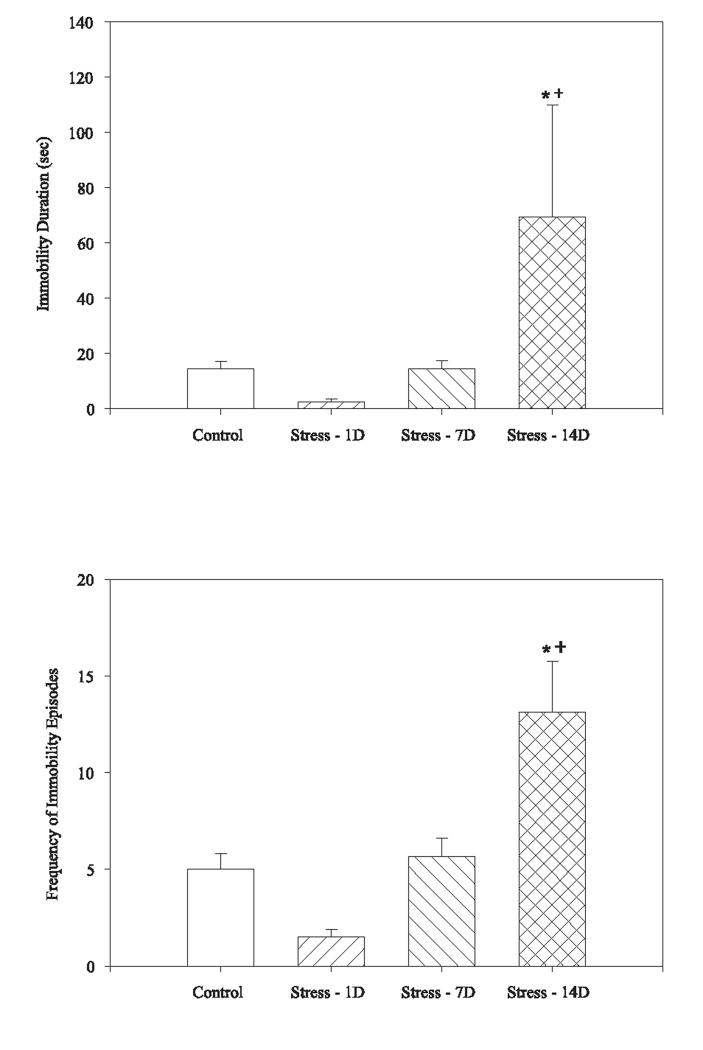
Immobility measures in the defensive burying task also differed by group. There was a significant effect of group on the total time immobile (F(3,45)=4.212, p<.05; Figure 3a) and the frequency of immobility episodes (F(3,45)=11.124, p<.01; Figure 3b) within the defensive burying task. Rats tested 14 days after the last stressor showed greater immobility than all other groups (p<.05).
Fig. 3.
Exposure to CUS altered immobility measures in the defensive burying task. Rats exposed to CUS and tested 14 days later showed increased immobility behavior as indicated by the duration of immobility in the 15 min test (top panel) and the frequency of immobility episodes (bottom panel). Bars represent mean ± SEM. * indicates a significant difference from non-stressed control group (p<.05), + indicates a significant different from CUS rats tested 1 or 7 days after stress ended (p<.05).
3.2 Elevated Plus-Maze
There were no significant effects of exposure to CUS on behavioral measures in the elevated plus-maze. The duration of time spent on the open arm and latency to enter the open arm were similar across groups (duration: F(3,55)=1.93, n.s.; latency: F(3,43)=1.96, n.s., Table 1). Likewise, the frequency of entries into open arms was similar between groups (F(3,37)=1.16, n.s.), as was the total number of arm entries (F(3,54)=1.47, n.s.).
Table 1.
Behavioral measures from the elevated plus-maze and the light-dark box for controls rats and CUS rats tested at 3 delay periods.
| Non-Stressed | CUS Rats | CUS Rats | CUS Rats | |
|---|---|---|---|---|
| Rats | 1 D Delay | 7 D Delay | 14 D Delay | |
| Elevated Plus Maze | ||||
| Duration in Open Arm (sec) | 60.71 ± 8.16 | 78.21 ± 16.23 | 51.70 + 8.70 | 30.71 + 7.97 |
| Latency to Open Arm (sec) | 24.65 ± 5.27 | 51.33 ± 19.24 | 48.80 + 4.64 | 16.43 + 4.78 |
| Frequency of Entries into All Arms | 8.89 ± 0.63 | 6.93 ± 0.93 | 8.30 + 0.73 | 9.29 + 0.89 |
| Proportion of Rats that did not | ||||
| Enter the Open Arm | 20.50% | 0 | 21.40% | 36.36% |
| Proportion of Rats that Fell | 5.13% | 9.52% | 12.50% | 0.00% |
| Light-Dark Box | ||||
| Duration in Light Box (sec) | 203.31 + 18.94 | 165.27 + 33.55 | 217.44 + 31.62 | 161.50 + 18.28 |
| Latency to Dark Box (sec) | 48.89 + 6.23 | 22.73 + 3.24 | 65.33 + 25.96 | 43.89 + 14.64 |
| Frequency of Transitions | 12.48 + 0.98 | 12.91 + 2.00 | 11.90 + 2.21 | 11.94 + 1.29 |
There were no differences between groups on the proportion of rats that remained in the closed arms for the entire test (χ2(3)=6.07, n.s.) or fell off the platform (χ2(3)=1.86, n.s.). However, rats exposed to CUS and then tested 14 days later were less likely to enter the open arm (36.6%) compared to control rats (20.5%; Table 1).
3.3 Correlational Analysis
Eight dependent variables were selected for comparison of behavioral measures from the elevated plus-maze and defensive burying tasks. The variables included 5 measures from defensive burying—duration of burying, height of bedding pile, burying latency, duration of immobility and frequency of immobility episodes—and 3 measures from elevated plus-maze— duration in the open arm, frequency of arms entered and latency to enter the open arm.
An overall comparison of all rats showed a significant correlation between the duration of burying and height of bedding pile in the defensive burying task (r=0.752, p<.01) and immobility frequency and duration (r=.577, p< .01). When the measures were compared within control and CUS rats, the correlation for both groups separately between duration of burying and height of bedding pile was significant (controls: r=0.913, p<.01; CUS: r=0.680, p<.01; Table 2) and between immobility frequency and duration (controls: r=.814, p<.01; CUS: r=.573, p<.01). Rats tested 14 days after CUS exposure had significant negative correlations between latency to bury and duration of burying (r=-0.866, p<.05) and pile height (r=-0.874, p<.05). In rats exposed to CUS only, there was a significant positive correlation between immobility duration and burying latency (r=.716, p<.01). However, the negative correlation between immobility and burying durations was not significant (r=-.04, n.s.).
Table 2.
Correlation coefficients for behavioral measures of the defensive burying test and the elevated plus-maze.
| Non-Stressed | CUS | |
|---|---|---|
| Dependent Measures | Rats | Rats |
| Pile Height - Bury Duration | 0.913 ** | 0.680 ** |
| Bury Latency - Bury Duration | -0.026 | -0.217 |
| Bury Duration - Immobility Duration | 0.246 | -0.138 |
| Bury Latency - Immobility Duration | 0.235 | 0.716 ** |
| Immobility Frequency - Immobility Duration | 0.814 ** | 0.573 ** |
| Duration EPM - Bury Duration | -0.214 | -0.274 |
| Duration EPM - Total Arms Entered | 0.472 * | -0.158 |
indicates significant correlation at p<.05
indicates significant correlation at p< .01.
For control rats, the correlation in the elevated plus-maze between the duration in the open arm and frequency of arms entered was also significant (r=.472, p<.05). There were no significant correlations between any measures of the elevated plus-maze and the defensive burying task.
3.4 Light-Dark Box
Rats exposed to CUS showed similar behavioral responses in the light-dark box to non-stressed control rats. None of the behavioral measures were statistically significant: duration of time in the light box (F(3,63)=1.17, n.s., Table 1); latency to enter the dark box (F(3,63)=1.37, n.s.); or frequency of transitions (F(3,63)=0.09, n.s.).
3.5 Body Weights
Overall, rats exposed to CUS for 10 days showed less body weight gain than control rats when compared immediately following the stress procedure (t(144)=12.15, p<.001). When body weights were compared over all days of CUS exposure, there was a statistically significant interaction between group and day (9,1224)=109.64, p<.001; Figure 4). There were no differences when weight gain was compared from day 11 to 17 between control and CUS rats (t(41)=1.02, n.s.). Interestingly, when weight gain was compared between control and CUS rats from day 18 to 24, there was almost a statistical difference between the groups (t(49)=1.99, p=0.052) with the CUS rats gaining more weight than control rats (CUS: 28.8 ± 1.54 g; control: 24.2 ± 1.73 g).
Fig. 4.
Exposure to CUS decreased body weight gain. During the stress exposure (days 2-11), CUS rats gained less weight over time than non-stressed control rats. Symbols represent mean + SEM. Each arrow indicates when behavioral tests were conducted. The 1st arrow also indicates the end of the CUS procedure for all stressed rats.
4.0 Discussion
Overall, the current study found that exposure to 10 days of unpredictable stress augmented the expression of anxiety-like behavior in rats when assessed 1 and 2 weeks following the termination of the stress procedures. The increased anxiety-like behavior was observed in the defensive burying test through two types of behavioral strategies, the active behavior associated with burying the shock probe and the passive behavior associated with immobility or freezing. Rats exposed to CUS and tested 7 or 14 days later showed an increase in measures associated with burying the shock probe (Figure 1) and an increase in immobility compared to non-stressed control rats (Figure 3). Moreover, CUS rats tested 7 or 14 days following the last stressor showed increased immobility and buried the probe to a greater extent than CUS rats tested 1 day following the last stressor. This later finding indicates that the delay between stress exposure and testing sensitizes the anxiety behavior in rats exposed to CUS when tested in a conditioned response task.
The current results are consistent with previous research testing the sensitized effects of stress exposure on anxiety-like behavior in rodents. Our findings in the defensive burying task are similar to a study in which rodents were exposed to 7 days of unpredictable stress and subsequently showed increased freezing behavior in a conditioned freezing test [63]. This study by Zurita and colleagues (2000) did not systematically examine anxiety measures at different time points following stress exposure, but the increase in freezing behavior occurred at 7 days, which supports the importance of the delay between stress exposure and potentiation of anxiety-like behaviors. The delayed effects following stress exposure have also been reported after single stress exposure in rodents. Rats exposed to inescapable shock demonstrated an increase of anxiety-like behaviors in a shuttle box, 14 days after the shock application [30]. Likewise, a single tail shock session produced increased startle response 7 days later, while 3 days of tail shock increased startle 10 days after shock exposure [47]. The current study provides additional evidence that chronic unpredictable stress, similar to acute stress, potentiates anxiety-like responses after the stress exposure has ceased and may be an appropriate model for understanding human anxiety.
There were no significant effects of CUS on anxiety-like behaviors when assessed through 2 unconditioned responses tasks, the elevated plus-maze and light-dark box (Table 1). In general, the published studies examining the immediate effects of unpredictable stress procedures on anxiety-like behaviors support the current findings. Vyas and colleagues (2002) reported no significant effects of CUS exposure on behavioral measures assessed in the elevated plus-maze when testing occurred immediately after stress exposure. Likewise, exposure to longer unpredictable stress procedures (3 or 9 weeks) had no effect on or decreased anxiety measures when assessed in the elevated plus-maze [14,25]. However stress-induced arterial hypertensive rats showed increased anxiety in the elevated plus-maze following 12 days of unpredictable stress, although the same stress procedures did not affect anxiety measures in Wistar rats [33]. Based on the above chronic unpredictable stress studies, it appears that a more sensitive rat strain may be important for detecting changes in anxiety-like behaviors immediately following unpredictable stress exposure.
On the other hand, long-lasting anxiogenic effects of chronic or single stress exposure on measures in unconditioned response tasks have been reported. Rats exposed to 7 days of unpredictable stress and tested 7 days later in the elevated plus-maze or a novel environment showed increased anxiety-like behaviors [13]. Single or multiple experiences of exposure to a predator, predator odor or social defeat augmented anxiogenic responses in rodents when tested 1 to 21 days following the exposure episode [1,2,4,5,7,8,11,45]. The sustained effects of predator stress have been reported in several unconditioned response tasks, including the elevated plusmaze and light-dark box [1-3,5,8]. Similarly, a single session of foot shocks increased immobility and freezing behaviors in a novel environment when assessed 1-4 weeks after the stress exposure [54-56]. The greatest increases in anxiety-like behavior following foot shock occurred 7-14 days following the stress exposure [56], similar to the time interval observed in the defensive burying task for the current study. Thus, acute or chronic stress exposures result in an increase of anxiety-like behaviors on unconditioned response tasks that lasts for several weeks. Although the elevated plus-maze measures did not reach statistical significance in the current study following CUS exposure when compared across all stress groups, the CUS rats 14 days after stress tended to spend less time in the open arm than non-stressed control rats or CUS rats tested immediately (Table 1). Likewise, the CUS rats tested at 14 days were less likely to enter the open arm anytime during the test compared to non-stressed rats or rats exposed to CUS and tested immediately. However, a similar pattern did not emerge on the elevated plus-maze with rats tested 7 days after CUS or CUS rats tested in the light-dark box.
The inconsistency between the behavioral measures on conditioned and unconditioned response tests observed in the current paper have been reported previously and may suggest that such tests assess different components of anxiety or types of responses [15]. Although the behavioral measures within defensive burying associated with anxiety were highly correlated as expected, no behavioral measures correlated between the defensive burying test and the elevated plus-maze (Table 2). Unfortunately, the same rats were not tested in both of the unconditioned response tests. Several studies have used factor analysis to identify the dependent measures assessed in unconditioned response tasks that contribute to specific behavioral components, such as anxiety, locomotion, reactivity, and exploration [1,9,12,18,43,60]. The papers assessing 2 or more behavioral tasks tended only to find significant correlations between parameters on 2 tasks if the rat was genetically engineered for increased anxiety [15,18,40].
The current data suggests that CUS selectively alters behavioral responses to a conditioned stimulus, but not unconditioned anxiety. In the conditioned response task, CUS exposure appears to influence both active and passive behaviors associated with anxiety for both burying and immobility increased in CUS rats 14 days following the last stressor. This result was surprising given previous research that reported a negative correlation between the two types of behaviors in the same task [15]. It is possible that CUS rats shift their behavioral expression of anxiety throughout the 15 min defensive burying task from passive to active behaviors. There was a significant positive correlation between the latency to bury and the duration of immobility in rats exposed to CUS, suggesting that the initial behavioral response following the shock stimulus may be passive as indicated by immobility, but then more active behavioral strategies may dominate such as burying behavior. Certain characteristics of the defensive burying task may optimize the sensitivity of the task to the effects of CUS. For example, the shock used to elicit the anxiety-like behavior is an acutely stressful stimulus. The stressful stimulus may be necessary to potentiate passive and active anxiety-like behaviors in rats exposed previously to chronic stress, as seen with other behavioral or neurochemical responses [e.g. 13,23,26,38,39,48].
Behavioral research, such as the current study, demonstrates that the potentiated anxiety response after the cessation of stress exposure in humans can be modeled in rodents. The rodent model then can provide an opportunity to understand the neural mechanisms underlying the behavioral change and suggest potential treatment opportunities. Of particular interest would be to understand how chronic unpredictable stress alters brain regions and neurotransmitters thought to participate in stress activation and anxiety behaviors. Previous studies have found that structural remodeling of neurons in the dorsal hippocampus and basolateral amygdala persists for 3 weeks following termination of chronic restraint stress, as does increased anxiety-like behaviors [7,31,57,59]. Unpredictable stress exposure did not result in similar structural or immediate behavioral changes, which may suggest that different neural substrates mediate the increase in anxiety behaviors following predictable compared to unpredictable stress [57,58]. We have previously found that CUS alters serotonin receptor mediated responses for several months following termination of the last stressor [34]. The increase in anxiety responses following unpredictable stress exposure may be related to alterations in the serotonin receptor system, although glutamatergic alterations have also been reported [27,28]. Future studies will be aimed at determining the neuorochemical substrates mediating the effects of CUS and reversing those effects with behavioral or pharmacological interventions.
Acknowledgements
The experiments conducted in the paper were supported by a grant from the National Institutes of Health in the United States to L. Matuszewich (DA016947) and through funds from the Psychology Department at Northern Illinois University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: Sex differences in vulnerability to stress and predicting severity of anxiogenic responses from the stress experience. Physiol Behav. 2006;88:12–29. doi: 10.1016/j.physbeh.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [2].Adamec R, Walling S, Burton P. Long-lasting, selective, anxiogenic effects of feline predator stress in mice. Physiol Behav. 2004;83:401–410. doi: 10.1016/j.physbeh.2004.08.029. [DOI] [PubMed] [Google Scholar]
- [3].Adamec RE. Stress effects on limbic function and behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1173–1175. doi: 10.1016/j.pnpbp.2003.09.011. [DOI] [PubMed] [Google Scholar]
- [4].Adamec RE, Blundell J, Collins A. Neural plasticity and stress induced changes in defense in the rat. Neurosci Biobehav Rev. 2001;25:721–744. doi: 10.1016/s0149-7634(01)00053-7. [DOI] [PubMed] [Google Scholar]
- [5].Adamec RE, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiol Behav. 1993;54:101–109. doi: 10.1016/0031-9384(93)90050-p. [DOI] [PubMed] [Google Scholar]
- [6].Andrade TGCS, Graeff FG. Effect of electrolytic and neurotoxic lesions of the median raphe on anxiety and stress. Pharmacol Biochem Behav. 2001;70:1–14. doi: 10.1016/s0091-3057(01)00512-3. [DOI] [PubMed] [Google Scholar]
- [7].Buwalda B, Kole MHP, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. Long term effects of social stress on brain and behavior: A focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- [8].Calvo-Torrent A, Brain PF, Martinez M. Effect of predatory stress on sucrose intake and behavior on the plus-maze in male mice. Physiol Behav. 1999;67:189–196. doi: 10.1016/s0031-9384(99)00051-7. [DOI] [PubMed] [Google Scholar]
- [9].Campbell T, Lin S, DeVries C, Lambert K. Coping strategies in male and female rats exposed to multiple stressors. Physiol Behav. 2003;78:495–504. doi: 10.1016/s0031-9384(03)00033-7. [DOI] [PubMed] [Google Scholar]
- [10].Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- [11].Cohen H, Zohar J, Matar M. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2003;53:463–473. doi: 10.1016/s0006-3223(02)01909-1. [DOI] [PubMed] [Google Scholar]
- [12].Cruz APM, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- [13].Cuadra G, Zurita A, Gioino G, Molina VA. Influence of different antidepressant drugs on the effect of chronic variate stress on restraint-induced dopamine release in frontal cortex. Neuropsychopharmacol. 2001;25:384–394. doi: 10.1016/S0893-133X(01)00234-2. [DOI] [PubMed] [Google Scholar]
- [14].D′Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–867. doi: 10.1016/0031-9384(94)90316-6. [DOI] [PubMed] [Google Scholar]
- [15].De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- [16].Dohrenwend BS, Dohrenwend BP, Dodson M, Shrout PE. Symptoms, hassles social supports, and life events: the problem of confounded measures. Journal of Abnormal Psychology. 1984;93:222–230. doi: 10.1037//0021-843x.93.2.222. [DOI] [PubMed] [Google Scholar]
- [17].Dusselier L, Dunn B, Wang Y, Shelley MC, Whalen DF. Personal, health, academic, and environmental predictors of stress for residence hall students. Journal of American College Health. 2003;54:15–24. doi: 10.3200/JACH.54.1.15-24. [DOI] [PubMed] [Google Scholar]
- [18].File SE. Behavioural detection of anxiolytic action. In: Elliott JM, Heal DJ, Marsden CA, editors. Experimental Approaches to Anxiety and Depression. John Wiley & Sons Ltd.; Chichester: 1992. pp. 25–44. [Google Scholar]
- [19].Gameiro GH, Gameiro PH, Andrade AS, Pereira LF, Arthuri MT, Marcondes FK, Veiga MCFA. Nociceptive- and anxiety-like behavior in rats submitted to different periods of restraint stress. Physiol Behav. 2006;87:643–649. doi: 10.1016/j.physbeh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- [20].Godin I, Kittel F, Coppieters Y, Siegrist J. A prospective study of cumulative job stress in relation to mental health. BMC Public Health. 2005:5. doi: 10.1186/1471-2458-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gouirand AM, Matuszewich L. The effects of chronic unpredictable stress on male rats in the water maze. Physiol Behav. 2005;86:21–31. doi: 10.1016/j.physbeh.2005.06.027. [DOI] [PubMed] [Google Scholar]
- [22].Greenberg A, Berktold J. Stress and mind/body health. Greenberg Quinlan Rosner; 2006. pp. 1–23. [Google Scholar]
- [23].Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem. 1994;63:575–583. doi: 10.1046/j.1471-4159.1994.63020575.x. [DOI] [PubMed] [Google Scholar]
- [24].Haile CN, GrandPre T, Kosten TA. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacol. 2001;154:213–20. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- [25].Hill MN, Gorzalka BB. Enhancement of anxiety-like responsiveness to the cannabinoid CB1 receptor agonist HU-210 following chronic stress. Eur J Pharmacol. 2004;499:291–295. doi: 10.1016/j.ejphar.2004.06.069. [DOI] [PubMed] [Google Scholar]
- [26].Irwin J, Ahluwalia P, Anisman H. Sensitization of norepinephrine activity following acute and chronic footshock. Brain Res. 1986;379:98–103. doi: 10.1016/0006-8993(86)90260-x. [DOI] [PubMed] [Google Scholar]
- [27].Joels M, Karst H, Alfarez D, Heine VM, Qin Y, Van Riel E, Verkuyl JM, Lucassen PJ, Krugers HJ. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- [28].Joels M, Verkuyl JM, Van Riel E. Hippocampal and hypothalamic function after chronic stress. Ann NY Acad Sci. 2003;1007:367–378. doi: 10.1196/annals.1286.036. [DOI] [PubMed] [Google Scholar]
- [29].Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the national comorbidity survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- [30].Koba T, Kodama Y, Shimizu K, Soichiro N, Sugawara M, Kobayashi Y, Ogasawara T. Persistent behavioural changes in rats following inescapable shock stress: a potential model of posttraumatic stress disorder. World J Biol Psychiatry. 2001;2:34–37. doi: 10.3109/15622970109039982. [DOI] [PubMed] [Google Scholar]
- [31].Kole MHP, Costoli T, Koolhaas JM, Fuchs E. Bi-directional shift in the Cornu Ammnois 3 pyramidal dendritic orgnaization following brief stress. Neurosci. 2004;125:337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- [32].Koolhaas JM, Meerlo P, de Boer SF, Strubbe JH, Bohus B. The temporal dynamics of the stress response. Neurosci Biobehav Rev. 1997;21:775–782. doi: 10.1016/s0149-7634(96)00057-7. [DOI] [PubMed] [Google Scholar]
- [33].Maslova LN, Bulygina VV, Markel AL. Chronic stress during prepubertal development: immediate and long-lasting effects on arterial blood pressure and anxiety-related behavior. Psychoneuroendocrin. 2002;27:549–561. doi: 10.1016/s0306-4530(01)00092-0. [DOI] [PubMed] [Google Scholar]
- [34].Matuszewich L, Yamamoto BK. Long-lasting effects of chronic stress on DOI-induced hyperthermia in male rats. Psychopharmacol. 2003;169:169–175. doi: 10.1007/s00213-003-1498-7. [DOI] [PubMed] [Google Scholar]
- [35].McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacol. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- [36].Ohl F, Toschi N, Wigger A, Henniger MSH, Landgraf R. Dimensions of emotionality in a rat model of innate anxiety. Behav Neurosci. 2001;115:429–436. [PubMed] [Google Scholar]
- [37].Ortiz J, Fitzgerald LW, Lane S, Terwillinger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacol. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- [38].Overmier JB, Murison R. Trauma and resulting sensitization effects are modulated by psychological factors. Psychoneuroendocrin. 2005;30:965–973. doi: 10.1016/j.psyneuen.2005.04.012. [DOI] [PubMed] [Google Scholar]
- [39].Pardon M-C, Ma S, Morilak DA. Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Res. 2003;971:55–65. doi: 10.1016/s0006-8993(03)02355-2. [DOI] [PubMed] [Google Scholar]
- [40].Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- [41].Pinel JPJ, Treit D. Burying as a defensive response in rats. J Comp Physiol Psychol. 1978;92:708–712. [Google Scholar]
- [42].Regier DA, Farmer ME, Rae DS, Myers JK, Kramer M, Robins LN, George L, Karno M, Locke BZ. One-month prevalence of mental disorders in the United States and sociodemographic characteristics: The Epidemiologic Catchment Area study. Acta Psychiatrica Scand. 1993;88:35–47. doi: 10.1111/j.1600-0447.1993.tb03411.x. [DOI] [PubMed] [Google Scholar]
- [43].Rodgers RJ. Animal models of ‘anxiety’: where next. Behav Pharmacol. 1997;8:477–496. doi: 10.1097/00008877-199711000-00003. [DOI] [PubMed] [Google Scholar]
- [44].Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- [45].Ruis MA, te Brake JH, Buwalda B, de Boer SF, Meerlo P, Korte SM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrin. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- [46].Sauter S, Murphy L, Colligan M, Swanson N, Hurrell JJ, Schart FJ, Sinclair R, Grubb P, Goldenhar L, Alterman T, Johnston J, Hamilton A, Tisdale J. Stressed at work. Vol. 2006. National Institute for Occupational Safety and Health; 1999. [Google Scholar]
- [47].Servatius RJ, Ottenweller JE, Natelson BH. Delated startle sensitization distinguishes rats exposed to one or three stress sessions: further evidence toward an animal model of PTSD. Biol Psychiatry. 1995;38:539–46. doi: 10.1016/0006-3223(94)00369-E. [DOI] [PubMed] [Google Scholar]
- [48].Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the medial prefrontal cortex. Neurosci. 1993;53:695–703. doi: 10.1016/0306-4522(93)90617-o. [DOI] [PubMed] [Google Scholar]
- [49].Stambor Z. Stressed out nation. Monitor on Psychology. 2006;37:28. [Google Scholar]
- [50].Tannenbaum B, Tannenbaum GS, Sudom K, Anisman H. Neurochemical and behavioral alterations elicited by a chronic intermittent stressor regimen: Implications for allostatic load. Brain Res. 2002;953:82–92. doi: 10.1016/s0006-8993(02)03273-0. [DOI] [PubMed] [Google Scholar]
- [51].Treit D. Animal models for the study of anti-anxiety agents: a review. Neurosci Biobehav Rev. 1985;9:203–222. doi: 10.1016/0149-7634(85)90046-6. [DOI] [PubMed] [Google Scholar]
- [52].Treit D, Pinel JPJ, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- [53].Uys JDK, Stein DJ, Daniels WMU, Harvey BH. Animal models of anxiety disorders. Current Psychiatry Reports. 2003;5:274–281. doi: 10.1007/s11920-003-0056-7. [DOI] [PubMed] [Google Scholar]
- [54].Van Dijken HH, Mos J, van der Heyden JAM, Tilders FJH. Characterization of stress-induced long-term behavioural changes in rats: evidence in favor of anxiety. Physiol Behav. 1992;52:945–951. doi: 10.1016/0031-9384(92)90375-c. [DOI] [PubMed] [Google Scholar]
- [55].Van Dijken HH, Tilders FJH, Olivier B, Mos J. Effects of anxiolytic and antidepressant drugs on long-lasting behavioural deficits resulting from one short stress experience in male rats. Psychopharmacol. 1992;109:395–402. doi: 10.1007/BF02247714. [DOI] [PubMed] [Google Scholar]
- [56].Van Dijken HH, van der Heyden JAM, Mos J, Tilders FJH. Inescapable footshocks induce progressive and long-lasting behavioural changes in male rats. Physiol Behav. 1992;51:787–794. doi: 10.1016/0031-9384(92)90117-k. [DOI] [PubMed] [Google Scholar]
- [57].Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–4. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- [58].Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neurosci. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- [60].Wall PM, Messier C. Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci Biobehav Rev. 2001;25:275–286. doi: 10.1016/s0149-7634(01)00013-6. [DOI] [PubMed] [Google Scholar]
- [61].Willner P. The validity of animal models of depression. Psychopharmacol. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- [62].Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: A realistic model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- [63].Zurita A, Marinelli M, Cuadra G, Brandao ML, Molina VA. Early exposure to chronic variate stress facilitates the occurrence of anhedonia and enhanced emotional reactions to novel stressors: reversal by naltrexone pretreatment. Behav Brain Res. 2000;117:163–171. doi: 10.1016/s0166-4328(00)00302-8. [DOI] [PubMed] [Google Scholar]