Abstract
The pattern of responding on a peak-interval timing task allows one to make inferences regarding the sources of variation that contribute to interval-timing behavior. Non-temporal factors such as impulsivity may impact the validity of these inferences. Rats were trained on a 15-s peak-interval procedure (PI) or a mixed 15-s behaviorally-dependent variable-interval, 15-s peak-interval procedure (bdVIPI) for an extended number of sessions. Extended training on the PI revealed a bi-modal distribution in the times at which subjects started responding for temporally predictable reinforcement, suggesting that multiple processes contribute to the behavioral pattern obtained in this procedure. Training on the bdVIPI eliminated the early mode of this bi-modal distribution, thereby decreasing the variation in start times. These results suggest that alternative response options can modulate the influence of impulsivity in timing tasks.
Keywords: impulsivity, internal clock, peak procedure, time perception
1. Introduction
Interval timing, the perception and production of durations in the seconds to minutes range, is critical to organizing behavior in an efficient manner (Buhusi & Meck, 2005; Gallistel, 1990), and has been hypothesized to underlie optimal foraging decisions (Bateson, 2003; Brunner, Kacelnik, & Gibbon, 1992) as well as associative learning (Gallistel & Gibbon, 2000; Kirkpatrick & Church, 2000). Various psychological models of interval timing (Church & Broadbent, 1991; Gibbon, 1977; Grossberg & Schmajuk, 1989; Killeen & Fetterman, 1988; Matell & Meck, 2000, 2004; Staddon & Higa, 1999) have been developed that have been successful in explaining the average response form obtained from a variety of timing tasks. These models can be described in a very general manner (Church, 1997) in which three information-processing stages (clock, memory, and decision) interact to provide temporal control over behavior. The clock stage is composed of some internal signal that is mapped to time (note that in some models, this signal may not be dedicated to timing). One example of such a temporal signal is the linear summation of pacemaker pulses by an accumulator, as proposed by scalar expectancy theory (SET - Gibbon, 1977; Gibbon, Church, & Meck, 1984). The memory stage incorporates the long-term storage of the current temporal signal value at the time of a biologically meaningful event (e.g., food reinforcement). In future opportunities to predict the time of occurrence of a similar event, the clock-stage processes are initiated and decision-stage comparison processes compute the similarity between the current value of the temporal signal and the value or values sampled from temporal memory. When the current and remembered times are similar enough, the organism behaves as though it expects the timed event to occur.
In order to investigate the precise mechanisms of these information processing stages, as well as their neurobiological underpinnings, it is necessary to make inferences about these processes based upon the subject’s behavior in procedures designed to assess the subject’s perception of time. While a variety of tasks have been developed to investigate timing behavior, none of them is able to elicit behavior that is solely a reflection of the animal’s timing capacities, but rather they elicit a mix of timing behavior and behavior resulting from other, frequently unspecified, processes. As such, tasks are developed in which these other processes are minimized so that the behavior is as reflective as possible of the underlying timing system. For instance, one commonly used operant task, the peak-interval (PI) procedure (Buhusi et al., 2002; Catania, 1970; Church et al., 1991; Drew et al., 2005; Meck, 1996), has been demonstrated to reveal temporal control of behavior that is independent of motivational factors, such as reinforcer size (Roberts, 1981). In this variant of a fixed-interval schedule of reinforcement, the average response rate as a function of signal duration on long, non-reinforced probe trials is Gaussian or peak-shaped. The time of maximal responding on these probe trials, the peak time, falls very close to the criterion duration, while the standard deviation of this function, the peak spread, grows in direct proportion to the interval being timed. The peak time is typically interpreted as a subject’s estimate of the expected time of reinforcement, and the peak spread as a measure of confidence in the accuracy of the temporal estimate.
Further inferences regarding the functioning of the interval timing system are made following the analysis of behavior on single trials of the PI procedure. While the average response-rate function obtained from the PI procedure is peak-shaped, the pattern of responding on individual trials is well described by a two-state response pattern (Cheng & Westwood, 1993; Cheng, Westwood, & Crystal, 1993; Church, Meck, & Gibbon, 1994; Gibbon & Church, 1990; Rakitin et al., 1998). Generally, the subject switches from a low rate of responding to a high rate of responding at some point prior to the criterion time (start time), and then switches back to a low rate of responding at some point following the criterion time (stop time). According to SET, these response rate switch times (i.e., start and stop) correspond to the times at which the relative difference between the currently accumulating clock reading and a sample drawn from temporal memory fall below a similarity threshold (Gibbon & Church, 1984; Gibbon & Church, 1992; Meck & Church, 1984). By analyzing the means, variances, and covariances of these switch times, as well as the computed width and midpoint of the high state, Church and colleagues (Church et al., 1994; Gibbon & Church, 1990) were able to infer that clock speed (or the memory sample) varies between trials, and that the decisions to start and stop responding are computed independently (Cheng & Westwood, 1993; Cheng et al., 1993; Church et al., 1994; Gallistel, King, & McDonald, 2004).
As suggested above, behavior on the PI procedure is unlikely to reflect only the operation of the interval timing system, but other processes as well. One of these processes may be the multi-factor construct of impulsivity, or “actions that are poorly conceived, prematurely expressed, unduly risky, or inappropriate to the situation and that often result in undesirable outcomes” (Daruna & Barnes, 1993). With respect to the interval timing system, impulsivity will engender responses that are insensitive to the current value of the clock or decision processes. Within the PI procedure, these impulsive responses would occur in either of the low states that surround the high response rate period. These impulsive responses would likely be uniformly distributed throughout the low states, and coupled with variation in the placement of the high state, there will be a number of trials in which the high state incorporates these impulsive responses within it. For example, the subject could begin responding prematurely (i.e., before the decision stage similarity threshold has been crossed), but then, as the clock continues to run, switch into the temporally controlled high state, thereby producing a longer than normal high state. Likewise, the high state might extend longer than normal due to the inclusion of temporally-uncontrolled responses that occur early in the second low state. Indeed, the frequently reported finding that the coefficient of variation (CV) of the start time is substantially greater than the CV of the stop time (Cheng et al., 1993; Church et al., 1994; Gallistel et al., 2004; Gibbon & Church, 1990) may be the result of premature impulsive responding.
To address this question, we evaluated the temporal structure of responding for temporally predictable food (i.e., using a standard PI procedure) with and without the addition of a behaviorally-dependent variable-interval (bdVI) schedule programmed on a second response manipulandum. In the bdVI schedule used here, the constant probabilistic availability of reinforcement was only computed during periods of time in which the rat had its snout in the bdVI nosepoke aperture. We reasoned that by exposing the rats to a reinforcement schedule in which there was the possibility that they could earn food on another nosepoke aperture at an unknown time in the trial, i.e., by imposing a bdVI schedule on this second nosepoke aperture, the impact of impulsive responding on the PI nosepoke aperture might be substantially decreased.
2. Materials and Methods
2.1. Subjects
Twenty adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) approximately 60 days of age at the beginning of the experiment were used. Rats were housed in pairs with a 12 hr light:dark cycle (lights on at 8:00 AM). All behavioral testing was conducted during the light phase. The rats had ad libitum access to drinking water, but were kept on a restricted feeding schedule (Harlan 2019 Rat Diet) to maintain their body weights at 85–90% of free-feeding levels, adjusted for growth. Rodent chow was provided immediately following the daily session.
2.2. Apparatus
All behavioral data were obtained using 10 standard operant-conditioning chambers (30.5 x 25.4 x 30.5 cm – Coulbourn Instruments). The sides of the chamber were ventilated Plexiglas, and the front and back walls and ceiling were aluminum. The floor was composed of stainless steel bars. A pellet dispenser delivered 45-mg sucrose pellets (Formula F; Noyes Precision, Lancaster, NH) to a food magazine on the back wall of the chamber. Three nosepoke response apertures (2.5 cm opening diameter) with photobeam detection circuits were placed on the front wall of the chamber, and had yellow and green LED cue lights in their interior. Aluminum “hallways” (30.5 cm high x 8.2 cm deep) were attached to the front wall in between the nosepoke apertures to limit the quantity and rapidity of behavioral switching between nosepoke apertures. The operant chambers were also equipped with a houselight and a seven-tone audio generator for auditory cues. Stimulus control and data acquisition were achieved using a standard operant-conditioning control program (Graphic State, Coulbourn Instruments), with a temporal resolution of 20 msec.
2.3. Procedure
The rats were randomly assigned to one of two groups of 10 rats each, with one group trained on the Peak-Interval (PI) procedure, and the other group trained on a mixed behaviorally-dependent Variable Interval/Peak-Interval (bdVIPI) procedure after achieving threshold performance on the standard PI procedure. Rats were run five days per week at the same time each day. For clarity, the description of the procedures is broken down into “Pre-Training Procedures” which are procedures related to nosepoke acquisition, and “Temporal Training Procedures” which are procedures related to the primary data of interest.
Pre-Training Procedures
Magazine Training
Rats were given a single session of magazine training, in which a sucrose pellet was delivered once per min for 60 min, independent of the rat’s behavior.
Nosepoke Autoshaping (2–3 sessions)
Following magazine training, rats were trained to make nosepoke responses. A sucrose pellet was delivered, independent of responding, once per min for the 60 min session. Prior to pellet delivery, the center nosepoke aperture cue light was turned on for 1 s. Any responses made by the rat into the center nosepoke aperture resulted in reinforcement. Rats met the autoshaping criterion upon making 60 nosepokes before the session ended for two consecutive days.
Nosepoke Discrimination Training (1 session)
Rats were trained to respond on all three nosepoke apertures for reinforcement. Trials began with the onset of a 1 kHz pulsating tone (40 ms on/40 ms off). In addition to the tone, a randomly selected nosepoke aperture light was illuminated. When the rat made a nose-poke response in the illuminated nosepoke aperture, a sucrose pellet was delivered and the tone terminated, ending the trial. Responses on non-illuminated nosepoke apertures had no consequence.
Trials in this procedure and all subsequent procedures were separated by a uniformly distributed, randomly chosen, 20 to 40 s inter-trial interval (ITI). All rats were required to respond 20 times on each nosepoke aperture in 1 session to advance to the next phase of training.
Temporal Training Procedures
Fixed-Interval (FI) Training (8–15 sessions)
Rats were trained on a discrete-trials 15-s FI schedule. At the start of a trial, a 1 kHz pulsating tone commenced, and the cue light of the left nosepoke aperture was illuminated. Rats were then able to self-initiate the onset of the discriminative stimulus by making a nosepoke response on the left nosepoke aperture. This trial-initiation requirement was also in place for all subsequent procedures. Upon trial onset, the 1 kHz tone and left nosepoke aperture cue light were terminated, and a 4 kHz tone and the center FI nosepoke aperture cue light were turned on. The rats were reinforced for the first response made on the center FI nosepoke aperture that occurred after 15 s had elapsed from onset of the center nosepoke aperture cue light. Rats were also reinforced if their snout was inside the FI nosepoke aperture when the criterion duration (15 s) was reached. The tone and cue light were terminated at the time of reinforcement. Rats were advanced to the next phase of training once their response pattern resembled a typical FI scallop, and the snout was in the nosepoke aperture at the criterion duration (15 s) on at least 80% of the trials in a session.
Peak-Interval (PI) Training (bdVIPI group: range = 6–11 initial sessions; PI group: range = 6–8 initial sessions, 27 additional sessions for extended training in PI group)
Following FI training, rats were trained on a PI procedure. The procedure was identical to FI training, except that a proportion of trials (50%) were unreinforced probe trials, in which the 4 kHz tone and the cue light in the FI nosepoke aperture remained on for 2.5 – 3.5 times the criterion duration (38–53 s), and all responses had no programmed consequence. No indication was given to the rats as to which trial type had been selected. Average “responding” (i.e., the average proportion of time in the nosepoke aperture as a function of time in the trial) was peak-shaped. These peak functions were fit with a modified Gaussian curve to derive statistics characterizing the subjects’ performance.
Rats in the bdVIPI group were transferred to the next phase of training once the CV of the fitted function was ≤ 0.50, and the fit quality (i.e., R2) was ≥ 0.90 for 5 consecutive sessions. Rats in the PI group continued to be trained in this procedure for 27 sessions after achieving this performance threshold.
Behaviorally-dependent Variable-Interval (bdVI) Training (range = 0–18 sessions)
Rats in the bdVIPI group were trained to respond on the left nosepoke aperture under a bdVI 15-s schedule of reinforcement. The behaviorally-dependent aspect of this reinforcement schedule was created by sampling for reinforcement at a constant probability, but only during periods of time in which the rat had its snout inside the bdVI nosepoke aperture. Using this design, reinforcement would not be any more likely to be obtained for the first poke following a prolonged period away from the bdVI nosepoke aperture than if the rat kept its nose within the bdVI nosepoke aperture continuously. Specifically, reinforcement could be earned with a 2% probability, sampled at a temporal resolution of 300msec during the periods that the bdVI nosepoke aperture photobeam was broken (the slower temporal resolution of this sampling was required to compensate for the inability to sample with less than 1% probability using the operant control software). This probability of reinforcement resulted in a mean delay to reinforcement of 15 s if the rat kept its snout in the bdVI nosepoke aperture continuously.
Following trial initiation, the 4 kHz tone was activated and the cue light of the bdVI nosepoke aperture (left nosepoke aperture) remained on. Upon reinforcement, the trial was terminated. Responses made on the FI nosepoke aperture had no consequence. Rats in the bdVIPI group were transferred to the next phase of training once their mean proportion of time in the bdVI nosepoke aperture exceeded 50% during each of the first 5 s of a trial. One rat was inadvertently switched to the mixed bdVIPI procedure without bdVI training. Rats were given one additional session of PI training before advancing to the final phase of training.
Mixed behaviorally-dependent Variable-Interval/Peak-Interval Training (bdVIPI) (27 sessions)
In the final phase of the training, rats in the bdVIPI group were trained on a procedure that integrated the bdVI and PI phases of training. One of three possible trial types was selected on each trial: a FI 15-s trial, an unreinforced probe trial (38–50 s in duration), or a bdVI 15-s trial. Following trial initiation, the auditory stimulus switched to a steady 4 kHz tone, the bdVI nosepoke aperture remained illuminated and the FI nosepoke aperture cue light was illuminated as well. Each trial type had a 33% probability of being selected, and no external cues were provided to indicate which type of trial was in effect.
2.4. Data Analysis
Mean Functions
Mean peak functions relating the probability of “responding” as a function of time in the trial were constructed in the standard manner. Because we allowed reinforcement to be earned without requiring a discrete response (i.e., reinforcement was provided as long as the rat’s snout was in the appropriate nosepoke aperture at the appropriate moment), the proportion of time that the rat had its snout in the bdVI and FI nosepoke apertures as a function of time in the trial was the behavior of primary interest, rather than discrete response rate. Specifically, the times at which the rat’s snout entered and exited each nosepoke aperture were recorded with a temporal resolution of 20 msec and analyzed on both PI probe trials and bdVI trials that lasted longer than the shortest probe trial (≥38 s). In order to produce mean peak functions, the proportion of time (using a 1 sec bin width) that the rat had its snout in the nosepoke aperture as a function of time in the trial was computed and averaged across trials. In order to obtain quantitative indices of temporal responding, a Gaussian + linear ramp function (Buhusi, Perera, & Meck, 2005) was fit to the peak function. The addition of the linear ramp was performed in order to minimize the contribution of responding that sometimes occurs in anticipation of trial end from influencing the statistical measures reflecting temporally controlled responding in expectation of reinforcement. The peak time (mean), peak spread (standard deviation), CV (peak spread/peak time) and quality of fit (R2) from this function were recorded and used as indices of temporal accuracy (peak time) and precision (peak spread).
Single Trials
Single trial analyses were performed in the standard manner (i.e., iteratively fitting a single step function to the single trial “responses” until the discrepancy between step function and data was minimized). Specifically, an “out-in-out” step function was fit to the periods of time in which the rat’s snout went from primarily being outside the FI nosepoke aperture to primarily being inside the FI nosepoke aperture, and then returned to primarily being outside the FI nosepoke aperture (see Fig 1b). To this end, the data on each trial were characterized as a series of 0s and 1s representing whether the rat was out (0) or in (1) the nosepoke aperture during each 20 msec bin. These binomial sequences were then iteratively fit with a horizontal step function, in which each possible step function that started with an “out-to-in” bin and ended with an “in-to-out” bin was evaluated for its capacity to characterize the data (as judged by maximizing the fit). The fit quality was determined by computing the difference between the proportion of time spent in the nosepoke aperture during the entire “in” state and the total proportion of time spent in the nosepoke aperture during the two “out” states. If the data were perfectly represented by the rat abruptly switching from being wholly out of the nosepoke aperture to being wholly in the nosepoke aperture and then back out again, this value would compute to 1. On the contrary, if the rat was uniformly in and out of the nosepoke aperture throughout the trial, this value would compute to 0. The bin at which the best-fitting step function went from “out-to-in” was designated the start time, and the bin at which this step function went from “in-to-out” was designated the stop time. The width (stop time-start time) and the midpoint (start time + width/2) were also computed. The mean, variation, and covariation of these statistics were then analyzed. As has been done in previous single-trial analyses (Church et al., 1994; Gallistel et al., 2004), in order to avoid directly “forcing” the correlations, as well as to ensure good temporal control of behavior, only trials in which the rat entered the FI nosepoke aperture prior to the criterion time, and exited the FI nosepoke aperture after the criterion time were analyzed (89% of the trials, brief PI; 77% of the trials, extended PI; 65% of the trials, bdVIPI). The decrease in proportion of trials analyzed results from the decrease in spread of the “in” state with the increased training on the PI or bdVIPI procedure. Importantly, the pattern of data obtained from those rats (n = 6 PI, 4 bdVIPI) that had more than 80% of their trials remaining following this performance constraint were indistinguishable from the dataset with all rats included, suggesting that the performance constraint is not responsible for the obtained effects.
Figure 1.
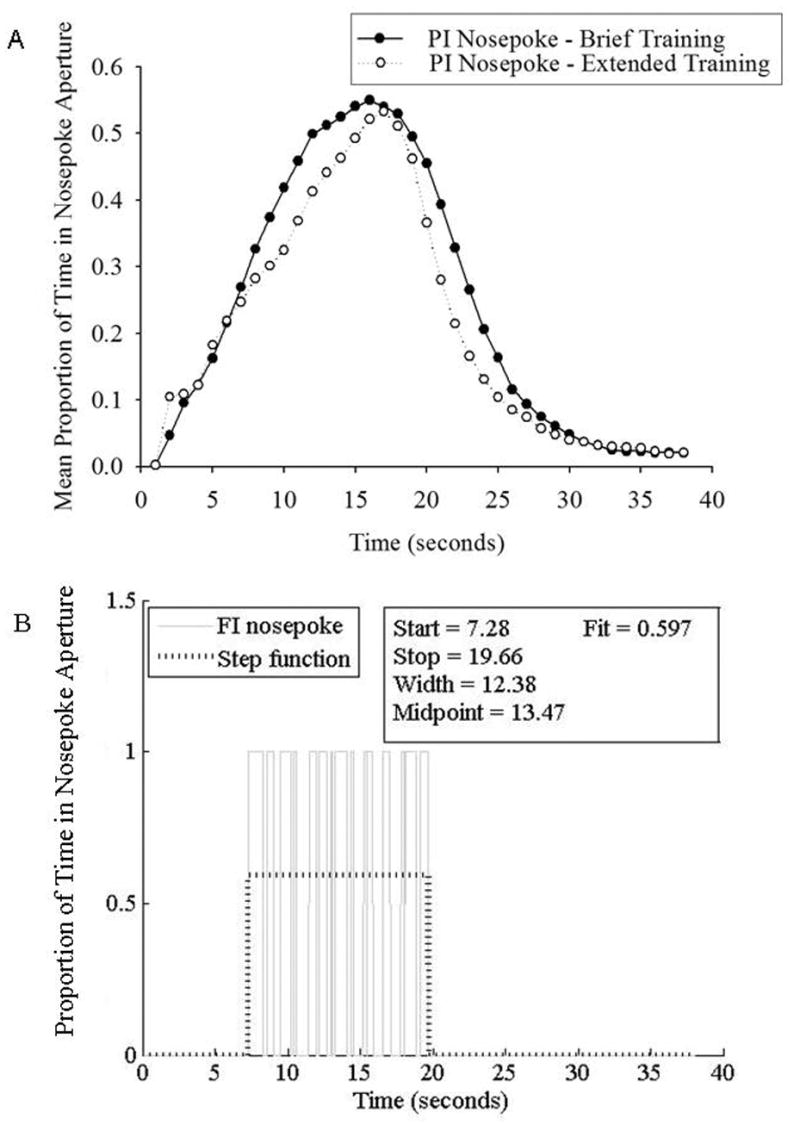
(A). Average proportion of probe trials in which the rats’ snouts were in the FI nosepoke aperture as a function of time on the 15-s peak interval procedure. (B).A representative pattern of responding from a single probe trial in this procedure during the early training period, with the best fitting step function overlaid on the raw response times.
Forward/Backward functions
To further evaluate the pattern of responding on single trials of this procedure without discrete response requirements, we graphically plotted the average proportion of time in the FI nosepoke aperture during the 1st “out” state, the “in” state, and the 2nd “out” state. These plots are similar to the backward functions used to justify the step function for lever pressing rates (see Figure 6, Church et al., 1994), and are in the spirit of traditional backward learning curves. Briefly, trials were lined up with respect to the start and stop times, and the average proportion of time in the nosepoke was plotted before and after these transitions. In order to allow all trials to contribute equally, the data before, during, and after the step were plotted into proportional bins of 1/15 of the width of the current state of the trial being analyzed. For example, if the start time occurred 15 seconds into the trial such that the 1st “out” state was 15 seconds long, the “in” state lasted 7.5 seconds, such that the stop time occurred at 22.5 s, and the second “out” state was 15.5 seconds long (as trials lasted a minimum of 38 seconds), the width of the bins would be: 1st “out” state, 15s/15 = 1s, “in” state, 7.5s/15 = 0.5s, 2nd “out” 15.5s/15 = 1.033s. As all states in this analysis are normalized into 15 bins, there is no need to replot the curves for both the start time and stop time, and the entire structure of the single trial response pattern can be seen in a single plot of 45 bins.
Statistics
Data from the last 3 PI sessions before the rats were switched to the bdVI procedure (mean = 7.2 PI sessions), and data from the 3 PI sessions in which rats in the PI group met an equivalent performance criterion (mean = 6.6 sessions) were pooled and designated as “brief” training for both groups. Rats in the PI group were given 27 additional sessions, and data from the last 3 of these sessions was pooled and designated “extended” PI training (mean total PI sessions = 33.6). Rats in the bdVIPI group were given 0–18 sessions of bdVI training (mean = 4.4), 1 session of PI training and then 27 sessions of mixed bdVIPI training. The data from the last 3 of these bdVIPI sessions was pooled and designated “extended” bdVIPI training (mean total PI and bdVIPI sessions = 38.6). Repeated measures (training duration: brief vs. extended) or between subjects (PI, bdVIPI) ANOVAs were conducted separately on statistics derived from the mean functions and the single trial analyses. The alpha level was set at 0.05 for all analyses.
3. Results
PI Procedure
Mean Function
The mean proportion of time in which rats had their snouts in the FI nosepoke aperture as a function of time in the trial from both groups at the brief training transition point of the PI procedure is shown in Figure 1a.
Like previous timing data from the PI procedure using a discrete response requirement, the probability that the rats were engaged in a potentially reinforced behavior (i.e., having their snout in the nosepoke aperture) rose as a function of signal duration, reached a maximum close to the FI criterion duration, and then returned to baseline in a nearly symmetrical manner. As there were no significant differences between the two groups of rats (which had received identical training), their data were pooled for quantitative characterization. The average peak time, peak spread, and CV were 15.2 (+/− 1.2) s, 6.1 (+/− 1.2) s, and 0.41 (+/− 0.09), respectively.
Rats in the PI group continued training on the PI procedure for another 27 sessions. As can be seen in Figure 1a, the mean PI function has an unusual shape with a notable leftward skew, rather than the typical rightward skew seen in the brief PI data. The mean peak time, peak spread, and CV from this extended training dataset was 16.4 (+/− 3.5) s, 5.3 (+/− 2.0) s, and 0.33 (+/− 0.13), respectively. A repeated measures ANOVA comparing these statistics to those from the brief training data demonstrated no significant change in peak time or peak spread, although it did show a trend for a decreased CV [F(1,9) = 4.36, p = 0.07].
The peak proportion of time in the nosepoke aperture during the brief PI phase was 0.57 (+/− 0.11), indicating that the rats spent more than half their time across all trials in the nosepoke aperture at the criterion duration. However, due to the pooling of data across trials, it is unclear whether the difference between this proportion of time and unity is due to rapid movement of the snout in the nosepoke aperture, thereby leading to the photobeam being repeatedly broken/unbroken despite the rat being “in the nosepoke aperture” or whether this difference reflects variation in the times at which the rats were at the nosepoke aperture across trials (but with their snouts in continuously during these times).
Single Trials
To this end, and to verify that the rats’ behavior could be characterized by a single step function, we fit the data with an out-in-out step function in order to evaluate the structure of nosepoking on individual trials. Figure 1a shows a representative single trial. As can be seen in the averaged “forward/backward curves” in Figure 2, early in the trial, rats were almost completely out of the FI nosepoke aperture (mean proportion of time spent in nosepoke aperture = 0.002). At some point in the trial, the rats abruptly switched to a state of being predominantly in the FI nosepoke aperture, i.e., the “in” state (mean proportion of time spent in nosepoke aperture = 0.628). At some later point in the trial, the rats abruptly switched back to being predominantly out of the FI nosepoke aperture (mean proportion of time spent in nosepoke aperture = 0.031).
Figure 2.
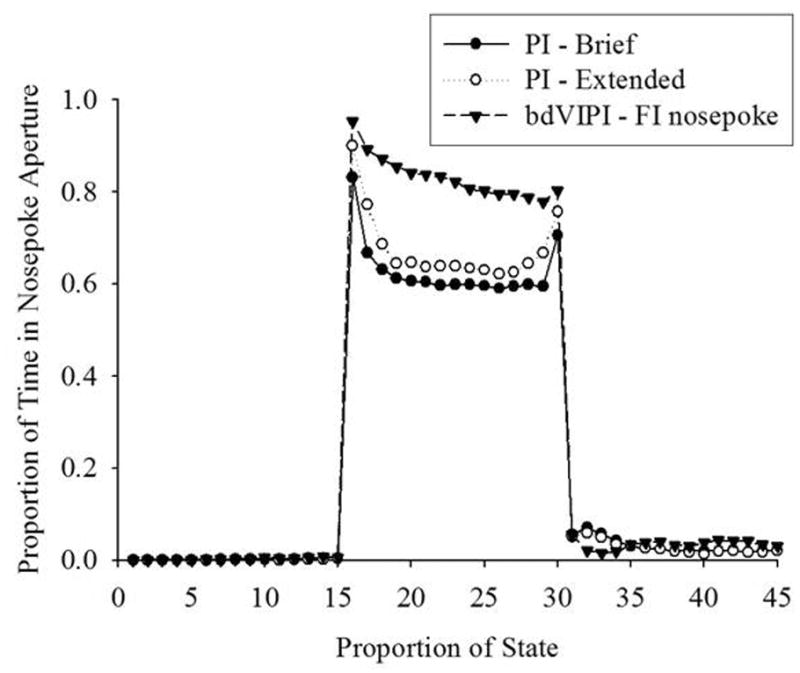
Average proportion of time spend in the FI nosepoke aperture on the PI and bdVIPI procedures during the 1st “out” state, the “in” state, and the 2nd “out” state, normalized as a function of the length of each state. On each trial, each state was binned into 15 bins, thereby allowing trials with different length states to be averaged for presentation.
The primary aspect to note in this figure is the general flatness of the proportion of time in the FI nosepoke aperture before, during, and after the “in” state. The higher proportion of time in the FI nosepoke aperture at the edges of the “in” state reflect the fact that the algorithm is going to categorize the edges of the “in’ state according to whether the rat is actually in the nosepoke aperture, not just predominantly in the nosepoke aperture. Also shown in Figure 2 are the data from the PI group following extended training. These data are very similar to the brief-training PI data, with the exception that the proportion of time in the nosepoke aperture during the “in” state is slightly higher, leading to a significantly better overall fit [early fit = 0.573, late fit = 0.662, F(1,9) = 13.88, p < 0.01]. It should be noted that changes in the average time at which rats started or stopped would not be visible in this figure due to the fact that each state is binned into 15 bins, irrespective of its width or placement.
Table 1a/b provides the statistics derived from these single-trial fits from the brief and extended training datasets, respectively. The single trial statistics from the brief PI data are very similar to previously reported results using a discrete lever or key press requirement (Cheng & Westwood, 1993; Cheng et al., 1993; Church et al., 1994; Gibbon & Church, 1990), and we would like to emphasize several points. First, all correlations between step function indices were in the same direction as obtained in these previous reports with a discrete response requirement and all were significantly different from zero (all p<0.05). Second, the magnitude of the positive start-stop correlation (0.22 +/− .23), the negative start-width correlation (−0.51 +/− .17), and the CVs of the start and stop times should be noted. A repeated measures ANOVA comparing brief versus extended single trial statistics demonstrated that additional training caused the stop times to move closer to the criterion time [F(1,9) = 6.82, p < 0.05], whereas there was no significant changes in start time, resulting in a significant decrease in width [F(1,9) = 11.23, p < 0.01], but no change in the midpoint. Furthermore, the start time vs. width correlation, and the width vs. midpoint correlation were significantly shifted towards more negative values following extended training [start-width, F(1,9) = 5.11, p = 0.05; width-midpoint, F(1,9) = 8.02, p < 0.05]. The remaining correlations were not significantly different as a function of the length of training. However, the extended training start-stop correlation and width-midpoint correlations decreased to the point that they were no longer significantly different from zero. Finally, the CV of the start time remained 2.4 times greater than the CV of the stop time.
Table 1.
Descriptive statistics and correlations derived from single trial analyses following early training on the peak-interval procedure (A), late training on the peak-interval procedure (B), or training on a mixed behaviorally-dependent variable-interval/peak-interval procedure (C).
| A) PI early training | Start (s1) | Stop (s2) | Width | Midpoint | rs1,s2 | rs1,width | rs1,mid | rs2,width | rs2,mid | rwidth,mid |
| Mean | 7.90 | 22.05 | 14.15 | 14.97 | 0.22 | −0.51 | 0.73 | 0.71 | 0.82 | 0.19 |
| Standard Deviation | 2.61 | 3.13 | 3.61 | 2.24 | 0.23 | 0.17 | 0.09 | 0.10 | 0.10 | 0.20 |
| Coefficient of Variation | 0.35 | 0.14 | 0.26 | 0.15 | ||||||
| B) PI late training | Start (s1) | Stop (s2) | Width | Midpoint | rs1,s2 | rs1,width | rs1,mid | rs2,width | rs2,mid | rwidth,mid |
| Mean | 9.02 | 20.62 | 11.59 | 14.82 | 0.12 | −0.64 | 0.75 | 0.67 | 0.73 | 0.01 |
| Standard Deviation | 2.62 | 2.69 | 3.54 | 1.99 | 0.20 | 0.15 | 0.08 | 0.09 | 0.13 | 0.24 |
| Coefficient of Variation | 0.31 | 0.13 | 0.33 | 0.13 | ||||||
| C) bdVIPI training | Start (s1) | Stop (s2) | Width | Midpoint | rs1,s2 | rs1,width | rs1,mid | rs2,width | rs2,mid | rwidth,mid |
| Mean | 11.29 | 19.86 | 8.57 | 15.57 | 0.44 | −0.33 | 0.80 | 0.67 | 0.88 | 0.26 |
| Standard Deviation | 2.01 | 2.49 | 2.41 | 1.89 | 0.32 | 0.23 | 0.13 | 0.13 | 0.09 | 0.17 |
| Coefficient of Variation | 0.18 | 0.13 | 0.30 | 0.12 |
bdVIPI Procedure
Mean Function
Rats trained in the bdVIPI procedure initiated the trial by responding on the bdVI nosepoke aperture, and stayed in this nosepoke aperture for approximately 5 s, at which point they switched to primarily having their snouts in the FI nosepoke aperture. After poking in the FI nosepoke aperture for approximately 10 s, the rats switched back to the bdVI nosepoke aperture and continued responding in this nosepoke aperture for the remainder of the trial. The average PI and bdVI functions from rats on this procedure are shown in Figure 3a.
Figure 3.
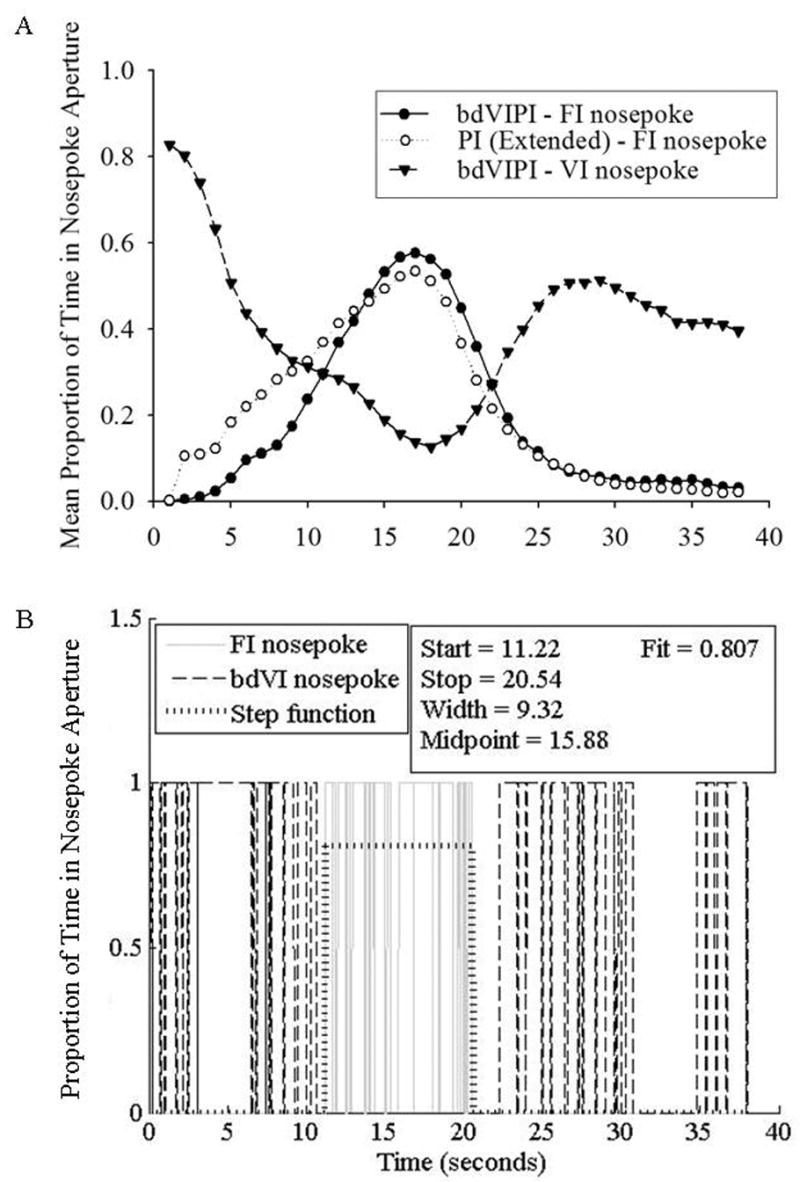
(A). Average proportion of probe trials during which the rat’s snout was in the bdVI or FI nosepoke aperture as a function of time on either the peak-interval procedure following extended training or the mixed 15-s behaviorally-dependent variable-interval/ 15-s peak-interval procedure . (B). A representative pattern of responding from a single probe trial in the bdVIPI procedure, with the best fitting step function overlaid on the raw response times.
As can be seen in both the mean function and the single trial data, the rat responded on the VI nosepoke aperture at the outset of the trial, switched to the PI nosepoke aperture as time approached the fixed interval duration of 15-s, and then returned to the VI nosepoke aperture for the remainder of the trial. There was a robust decrease in the width of the peak-interval timing function as a result of extended training on this concurrent variable-interval/peak-interval timing procedure.
The mean peak time, peak spread, and CV for these rats was 16.8 (+/− 1.2) s, 4.0 (+/− 0.9) s, and 0.24 (+/− 0.06), respectively. Average responding on the FI nosepoke aperture became sharper and slightly later as compared to these rats’ brief PI data, as indicated by a repeated measures ANOVA [peak time: F(1,9) = 16.16, p < 0.005; peak spread: F(1,9) = 37.15, p < 0.001; CV: F(1,9) = 40.29, p < 0.001]. Figure 3a also replots the mean peak function from the PI group following extended training. Between subjects ANOVAs comparing the PI functions from these two groups following equivalent amounts of training show strong trends toward better performance on the bdVIPI, with a narrower peak spread [F(1,18) = 3.74, p = 0.069] and smaller CV [F(1,18) = 4.10, p = 0.058].
Single Trials
Figure 3b shows a representative single trial from a rat on the bdVIPI procedure. The average pattern of responding in the FI nosepoke aperture on single trials of the bdVIPI procedure (Figure 2) differed from that seen following extended training on the PI procedure in that the proportion of time in the FI nosepoke aperture during the “in” state increased significantly [quality of fit, PI = 0.662, bdVIPI = 0.813, [F(1,18) = 14.97, p < 0.001]. Furthermore, there was a change in the shape of the response pattern, such that while the flat step function captured the majority of the variability of the data, the rats spent progressively less time in the FI nosepoke aperture as the “in” state progressed.
Statistics from the single-trial analyses performed on these data are provided in Table 1C. Repeated measures ANOVAs on these statistics as compared to these rats’ brief PI data demonstrated that both the start time [F(1,9) = 25.70, p < 0.001] and stop time [F(1,9) = 30.74, p < 0.001] moved closer to the criterion time, leading to a decrease in width [F(1,9) = 44.08, p < 0.001]. Furthermore, comparing these data to the extended training PI group revealed that the start time was later [F(1,18) = 5.55, p < 0.05] and the width narrower [F(1,18) = 4.94, p < 0.05]. All correlations between these statistics remained significantly different from zero. While none of the correlations was significantly different compared to these rats’ brief PI statistics, when compared to the extended training PI group, the start time vs. stop time correlation was more positive [F(1,18) = 7.29, p < 0.05], the start time vs. width correlation was less negative [F(1,18) = 12.79, p < 0.005], the stop time vs. midpoint correlation was more positive [F(1,18) = 8.99, p < 0.01], and the width vs. midpoint correlation was more positive [F(1,18) = 7.66, p < 0.05]. Finally, while the CV of the stop time remained virtually unchanged, the CV of the start time decreased dramatically, becoming only 1.4 times greater than the stop time [comparing start time CVs between extended PI and bdVIPI training, F(1,18) = 12.13, p < 0.005; comparing CVs between brief PI and bdVIPI training, F(1,9) = 24.45, p < 0.001].
Distribution of Single Trial Measures
As the CV of the start time changed following training on the bdVIPI procedure, we evaluated whether the distribution of start and stop times were equivalent in shape across the two procedures. Figures 4a and 4b show the distribution of start and stop times, respectively, from the PI and bdVIPI groups following brief and extended training. As can be seen in Figure 4a, rats in the extended PI group had a bi-modal distribution of start times, of which the later mode substantially overlapped with the distribution of start times from rats in the bdVIPI group. In contrast, the distributions of stop times in the two groups were highly similar (Figure 4b).
Figure 4.
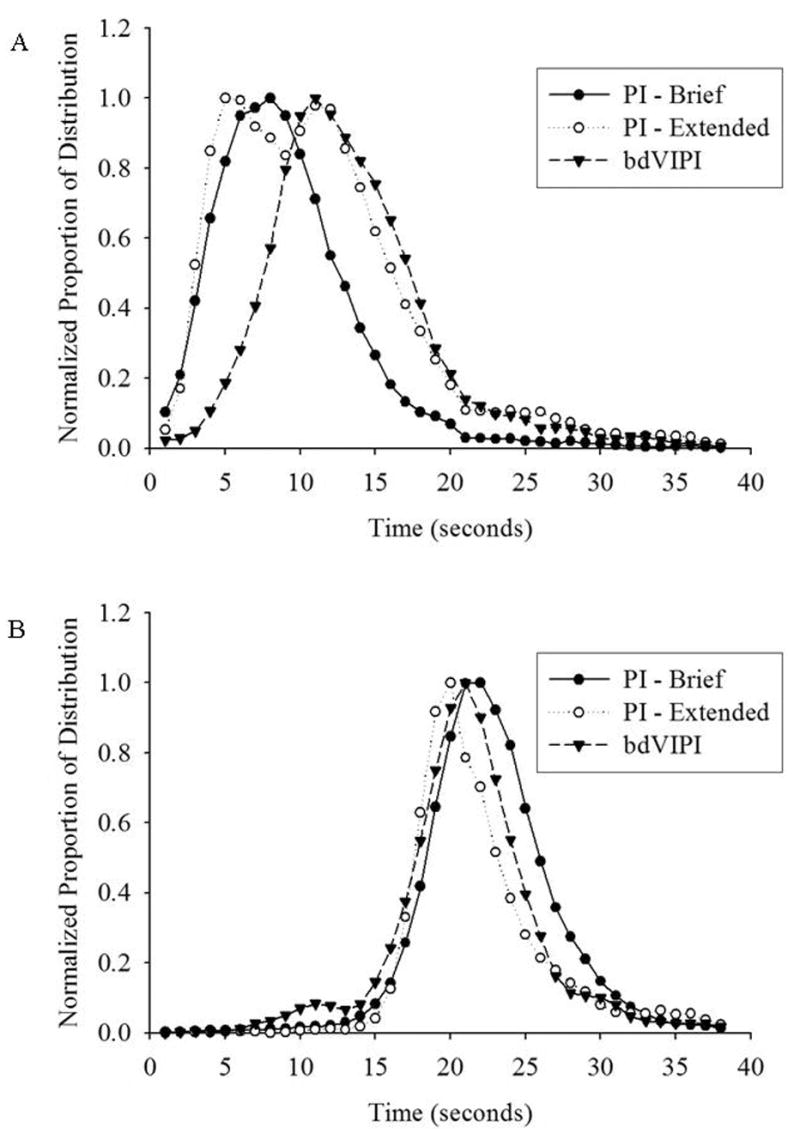
Normalized distributions of start times (A) and stop times (B) in the PI and bdVIPI procedures.
One possibility for the change in these statistics is that the effects of response competition from the bdVI schedule caused rats in the bdVIPI procedure to earn FI reinforcement at a later time than rats on the PI procedure. To evaluate this possibility, we compared the times at which reinforcement was earned from responding on the FI nosepoke aperture. A repeated measures (Brief, Extended), between-subjects (PI, bdVIPI) ANOVA on the mean time of FI reinforcement revealed a significant main effect of length of training in which the time of reinforcement got later [brief = 15.75, extended = 16.55; F(1,9) = 6.60, p < 0.05], but no effect of procedure, nor a training by procedure interaction.
4. Discussion
Rats trained in a PI procedure in which reinforcement could be earned simply by being in the appropriate place at the appropriate time (i.e., having their snout in a nosepoke aperture) behaved very similarly to that seen in previous reports in which discrete responses (e.g., lever presses or key pecks) were required for reinforcement (Cheng & Westwood, 1993; Church et al., 1994). The shape of the mean functions were approximately Gaussian, with the peak times occurring very close to the criterion duration (15.2 s), with an average CV of 0.41, which is typical of rats in a PI procedure (e.g., Roberts, 1981). This similarity also held for the pattern of responding on individual trials, as well as the correlations between measures of single trial responding. Specifically, as in previous reports, once the rats’ mean functions showed temporal control, responding was well characterized by a single step function in which the proportion of time making the operant response abruptly switched from a low value to a high value before the criterion duration, and then abruptly returned to a low value following the criterion duration. The fact that the maximal proportion of time in the FI nosepoke in the mean function (0.57) was very close to the mean proportion of time in the nosepoke during the “in” state in the single trials analysis (0.63) indicates that by and large, the rats had their snouts at the nosepoke aperture at the criterion time on almost every trial. The difference between the maximal proportion of time in the nosepoke aperture at the criterion time and unity reflects triggering of the nosepoke aperture photobeam while the rat was at the nosepoke. Anecdotal observation of the rats showed that this rapid triggering of the photobeam resulted from the rats making overt movements into and out of the nosepoke aperture, rather than from micro-movements of the rats’ snouts within the nosepoke aperture. Thus, the rats were not maintaining a rigid posture throughout their operant response, despite the fact that this would have led to the shortest delay of reinforcement. Nevertheless, responding during the “in” state was stable, in the sense that the proportion of time in the nosepoke was predominantly flat throughout the “in” state, with the exception of the edges which were artifactually higher due to the algorithmic selection of the onset or end of a nosepoke at the onset or end of the “in” state.
After receiving an additional 27 sessions of training on the PI procedure, responding became slightly more precise, such that there was a strong trend for the relative duration of responding (i.e., CV) to decrease. Such a decrease in the width of responding as a function of length of training is similar to previous reports showing a decrease in the width of responding following continued training on a fixed-interval procedure (Machado & Cevik, 1998). However, in contrast to those fixed-interval data, single-trials analyses of the current PI procedure revealed that the decrease in the relative width of the mean function was due to a decrease in the time at which rats stopped responding, rather than an increase in the time at which they started responding, as seen previously. As there was no change in the mean start time, the change in the stop time is unlikely to have been brought about through an increase in clock speed, which would cause both the start and stop times to get earlier. Rather, it was likely brought about by an increase in the similarity threshold needed to engage in the operant response. While a change in the threshold to stop responding, without a change in the threshold to start responding is consistent with the notion of two independently computed thresholds (Cheng & Westwood, 1993; Church et al., 1994), Cheng (1992) demonstrated that punishment for responding too early in a PI procedure led to a change in both the start time and stop time, and hypothesized that this indicated a linked set of response thresholds.
While the failure to see changes in both thresholds may indicate that the threshold linking is asymmetrical (i.e., changes in the start threshold lead to changes in the stop threshold, but not vice-versa), an alternative explanation that can account for both of these datasets is that a rightward shift in the start threshold is masked by the contribution of impulsive, premature responding. Consistent with this idea is the fact that the distribution of start times was bimodal, suggesting that multiple processes contributed to the onset of responding. We hypothesize that these two modes of response initiation are related to impulsive responding (i.e., responding that is insensitive to the functioning of the “clock”) and temporally controlled responding, respectively. Indeed, the unusual leftward skew in the peak function following extended training on the PI procedure as seen in Figure 1a is suggestive of a rightward shift in the start time, but with a continued influence of premature, impulsive responding on some, or all, of the trials. This premature responding would have been reduced by the punishment resulting from early responding in the Cheng (1992) study, whereas we had no such contingencies to decrease this behavior. Furthermore, these premature responses would be masked by the greater variability or looser thresholds seen in the brief training. Extended training on the PI procedure also caused the start time vs. stop time correlation to disappear, and the start time vs. width correlation to become even more negative. Both of these changes are consistent with a substantial proportion of the detected start times including non-temporally controlled “impulsive” responses, as this would diminish the relationship with the stop time, thereby weakening this correlation, and increase the width following early start times thereby strengthening this negative correlation. These changes are indicative of an insensitivity to the current clock value when initiating responding. In other words, on a subset of trials, the rats initiate responding in an impulsive manner (i.e., before they cross the similarity threshold to begin responding based upon their current clock reading). However, during this period of impulsive responding, the similarity threshold is then crossed (due to continued “accumulation” of the clock), and therefore responding continues for a longer than normal duration. Finally, the CV of the start time was 2.5 times greater than the CV of the stop time. Although these differential CVs have been seen before, and have been interpreted as indicating that the computational processes underlying the temporal control of response initiation are more variable than those underlying the temporal control of response termination (Church et al., 1994), we offer the possibility that this difference in relative variability is related to the inclusion of these premature responses as part of the “in” state.
Rats trained on the bdVIPI procedure showed an even greater sharpening of their mean peak functions than that seen following extended training on the PI. This enhanced sharpening was due to both the start time and the stop time moving closer to the criterion time. As there were no changes in the placement of the midpoint, these changes indicate that the thresholds for both initiating and terminating responding moved closer to the criterion duration. Thus, training with a second response alternative was equivalent in effect to training with a penalty for early responding (Cheng, 1992). One possible explanation for this rightward shift in the start time in the bdVIPI group is simply that there is response competition from the bdVI schedule, such that the rat could not begin responding on the FI nosepoke aperture until it terminated responding on the VI nosepoke aperture. Such an influence of an alternate behavior has been seen in a variety of tasks (Frank & Staddon, 1974; Matell, Bateson, & Meck, 2006; Matell, King, & Meck, 2004). However, while simply “preventing” the response would shift the start time rightward, it would not necessarily be expected to tighten the coefficient of variation of the start times, which decreased by 50%. In fact, it is conceivable that the impact of bdVI response competition would be to increase the CV for starting responding on the FI nosepoke aperture by sometimes preventing the initial response beyond the normal start time distribution. Further, simply preventing the initiation of responding on the FI nosepoke aperture would be expected to decrease the correlation between the start and stop times, as the start time would no longer be primarily controlled by the interval timing system. Instead, the average correlation between the start and stop times was twice as strong. Taken together, these data suggest that allowing the subject to potentially earn reinforcement on another nosepoke aperture decreased the likelihood that the rats would begin responding on the FI nosepoke aperture as a purely impulsive response, thereby permitting greater temporal control of their behavior. While others have found that the influence of responding in a temporally controlled manner for one duration tends to decrease the CV of the start time of another later duration being timed on that trial (Cheng et al., 1993; Crystal & Miller, 2002; Leak & Gibbon, 1995), it is likely that these previous findings are due to the start times simply reflecting the earlier durations’ stop times (i.e., the sharpening of the 2nd duration’s start CV may be viewed as being a consequence of the 1st duration’s tighter stop CV). In contrast, because the time of reinforcement on the “first” duration (i.e., the bdVIPI) in the current experiment was highly variable, it would not be expected to have a sharp CV for response stopping, and the timing of the switch to the FI nosepoke aperture would necessarily be predominantly based upon the decision to start responding on the FI nosepoke aperture. Thus the current data are unique in providing evidence regarding the impact of an alternative response option for decreasing premature responding.
Given the findings in the present report that premature responding can have substantive impacts on measures of temporal control in the PI procedure, the contribution of impulsivity should be taken into account in subsequent investigations of interval timing. Impulsive behavior in the form of premature responding, as seen here, will also be a particularly strong contributor to poor performance in delayed reinforcement of low rates (DRL) procedures, which are often used to investigate temporal control (Bayley, Bentley, & Dawson, 1998; Liao & Cheng, 2005; Neil & Herndon Jr., 1978; Niki & Watanabe, 1979; Young & McNaughton, 2000). Impulsivity as a concept has been proposed to include such behaviors as premature responding, response switching, perseveration, and response inhibition. Why then do the current data demonstrate a particularly strong influence of premature responding, but little evidence of perseveration, response switching (in the case of the bdVIPI), or response inhibition? While we don’t have a conclusive answer to this question, we offer the following suggestion. Impulsivity encompasses a variety of behaviors (i.e., premature responding, perseveration, etc.) which are all indicative of an insensitivity to task contingencies. However, these different impulsive behaviors have been suggested to be under the control of different neurological systems (Evenden, 1999b), and these different neural systems may interact with the variable task requirements such that they are each expressed to different degrees in different tasks (Evenden, 2002; Evenden & Ko, 2005; Evenden, 1998a, 1998b, 1998c, 1999a; Evenden & Robbins, 1983). For instance, the administration of haloperidol increases impulsivity in motor execution tasks, decreases impulsivity in motor preparation tasks, and has no effect in outcome assessment tasks. Amphetamine also increases impulsivity in motor execution tasks, does not alter impulsivity in motor preparation tasks (though see Amalric, Moukhles, Nieoullon, & Daszuta, 1995), but increases impulsivity in outcome assessment tasks. In contrast, ritanserin, a 5-HT2 antagonist, increases impulsivity in motor preparation tasks, but has no effect on motor execution or outcome assessment impulsivity. In a similar vein, Bradshaw and Szabadi and their colleagues have demonstrated in a series of papers that pharmacological and anatomical manipulations to the serotonergic system produce different effects depending on the timing task used (Al-Ruwaitea et al., 1999; Asgari, Body, Bak et al., 2006; Asgari et al., 2005; Asgari, Body, Zhang et al., 2006; Body et al., 2006; Body, Chiang et al., 2002; Body et al., 2004; Body, Kheramin et al., 2002; Ho et al., 1996; Ho, Velazquez-Martinez, Bradshaw, & Szabadi, 2002), and many of these effects are thought to be related to serotonergic mediated changes in impulsivity. Furthermore, given the typical rightward skew seen in the peak procedure due to scalar variability, as well as the increased responding in expectation of trial end (which is strong enough to warrant addition of a linear slope to the Guassian curves used to characterize the peak functions), post-high state, or even perseverative, impulsive responses may have less of an impact on performance measures. In summary, the present results suggest that non-temporal factors such as impulsivity can make a substantive contribution to the pattern and timing of behavior in temporal perception tasks.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Ruwaitea AS, Chiang TJ, Al-Zahrani SS, Ho MY, Bradshaw CM, Szabadi E. Effect of central 5-hydroxytryptamine depletion on tolerance of delay of reinforcement: Evidence from performance in a discrete-trials "Time-left" Procedure. Psychopharmacology. 1999;141:22–29. doi: 10.1007/s002130050802. [DOI] [PubMed] [Google Scholar]
- Amalric M, Moukhles H, Nieoullon A, Daszuta A. Complex deficits on reaction time performance following bilateral intrastriatal 6-ohda infusion in the rat. The European journal of neuroscience. 1995;7:972–980. doi: 10.1111/j.1460-9568.1995.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Asgari K, Body S, Bak VK, Zhang ZQ, Rickard JF, Glennon JC, Fone KC, Bradshaw CM, Szabadi E. Effects of 5-ht2a receptor stimulation on the discrimination of durations by rats. Behavioural pharmacology. 2006;17:51–59. doi: 10.1097/01.fbp.0000189810.69425.89. [DOI] [PubMed] [Google Scholar]
- Asgari K, Body S, Rickard JF, Zhang Z, Fone KC, Bradshaw CM, Szabadi E. Effects of quipazine and m-chlorophenylbiguanide (m-cpbg) on the discrimination of durations: Evidence for the involvement of 5-ht2a but not 5-ht3 receptors. Behavioural pharmacology. 2005;16:43–51. doi: 10.1097/00008877-200502000-00005. [DOI] [PubMed] [Google Scholar]
- Asgari K, Body S, Zhang Z, Fone KC, Bradshaw CM, Szabadi E. Effects of 5-ht1a and 5-ht2a receptor stimulation on temporal differentiation performance in the fixed-interval peak procedure. Behavioural processes. 2006;71:250–257. doi: 10.1016/j.beproc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bateson M. Interval timing and optimal foraging. In: Meck WH, editor. Functional and neural mechanisms of interval timing. CRC Press; Boca Raton, FL: 2003. pp. 113–141. [Google Scholar]
- Bayley PJ, Bentley GD, Dawson GR. The effects of selected antidepressant drugs on timing behaviour in rats. Psychopharmacology. 1998;136:114–122. doi: 10.1007/s002130050546. [DOI] [PubMed] [Google Scholar]
- Body S, Asgari K, Cheung TH, Bezzina G, Fone KF, Glennon JC, Bradshaw CM, Szabadi E. Evidence that the effect of 5-ht2 receptor stimulation on temporal differentiation is not mediated by receptors in the dorsal striatum. Behavioural processes. 2006;71:258–267. doi: 10.1016/j.beproc.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Body S, Chiang TJ, Mobini S, Ho MY, Bradshaw CM, Szabadi E. Effect of 8-oh-dpat on temporal discrimination following central 5-hydroxytryptamine depletion. Pharmacology, biochemistry, and behavior. 2002;71:787–793. doi: 10.1016/s0091-3057(01)00674-8. [DOI] [PubMed] [Google Scholar]
- Body S, Kheramin S, Ho MY, Miranda Herrera F, Bradshaw CM, Szabadi E. Effects of fenfluramine on free-operant timing behaviour: Evidence for involvement of 5-ht2a receptors. Psychopharmacology. 2004;176:154–165. doi: 10.1007/s00213-004-1871-1. [DOI] [PubMed] [Google Scholar]
- Body S, Kheramin S, Mobini S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E. Antagonism by way-100635 of the effects of 8-oh-dpat on performance on a free-operant timing schedule in intact and 5-ht-depleted rats. Behavioural pharmacology. 2002;13:603–614. doi: 10.1097/00008877-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Brunner D, Kacelnik A, Gibbon J. Optimal foraging and timing processes in the starling sturnus vulgaris: Effect of intercapture interval. Animal Behaviour. 1992;44:597–613. [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005 doi: 10.1038/nrn1764. in press. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Perera D, Meck WH. Memory for timing visual and auditory signals in albino and pigmented rats. J Exp Psychol Anim Behav Process. 2005;31:18–30. doi: 10.1037/0097-7403.31.1.18. [DOI] [PubMed] [Google Scholar]
- Cheng K. The form of timing distributions in pigeons under penalties for responding early. Animal Learning & Behavior. 1992;20:112–120. [Google Scholar]
- Cheng K, Westwood R. Analysis of single trials in pigeons timing performance. Journal of Experimental Psychology-Animal Behavior Processes. 1993;19:56–67. [Google Scholar]
- Cheng K, Westwood R, Crystal J. Memory variance in the peak procedure of timing in pigeons. Journal of Experimental Psychology. 1993;19:68–76. [Google Scholar]
- Church RM. Timing and temporal search. In: Bradshaw CM, Szabadi E, editors. Time and behaviour: Psychological and neurobehavioural analyses. Vol. 120. Elsevier; Amsterdam: 1997. pp. 41–78. [Google Scholar]
- Church RM, Broadbent HA. A connectionist model of timing. In: Michael SGJERS, Commons L, editors. Neural network models of conditioning and action. Quantitative analyses of behavior series. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ, US: 1991. pp. 225–240. [Google Scholar]
- Church RM, Meck WH, Gibbon J. Application of scalar timing theory to individual trials. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:135–155. doi: 10.1037//0097-7403.20.2.135. [DOI] [PubMed] [Google Scholar]
- Crystal JD, Miller BJ. Simultaneous temporal and spatial processing. Animal Learning and Behavior. 2002;30:53–65. doi: 10.3758/bf03192909. [DOI] [PubMed] [Google Scholar]
- Daruna J, Barnes P. A neurodevelopmental view of impulsivity. In: McCown W, Johnson J, Shure M, editors. The impulsive client: Theory, research and treatment. American Psychological Association; Washington, D.C.: 1993. [Google Scholar]
- Evenden J. The effects of psychotomimetics and psychomotor stimulants on two schedules promoting response switching in the rat. Psychopharmacology. 2002;163:381–390. doi: 10.1007/s00213-002-1184-1. [DOI] [PubMed] [Google Scholar]
- Evenden J, Ko T. The psychopharmacology of impulsive behaviour in rats viii: Effects of amphetamine, methylphenidate, and other drugs on responding maintained by a fixed consecutive number avoidance schedule. Psychopharmacology. 2005;180:294–305. doi: 10.1007/s00213-005-2163-0. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats ii: The effects of amphetamine, haloperidol, imipramine, chlordiazepoxide and other drugs on fixed consecutive number schedules (fcn 8 and fcn 32) Psychopharmacology. 1998a;138:283–294. doi: 10.1007/s002130050673. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats iii: The effects of amphetamine, haloperidol, imipramine, chlordiazepoxide and ethanol on a paced fixed consecutive number schedule. Psychopharmacology. 1998b;138:295–304. doi: 10.1007/s002130050674. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats iv: The effects of selective serotonergic agents on a paced fixed consecutive number schedule. Psychopharmacology. 1998c;140:319–330. doi: 10.1007/s002130050773. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats vii: The effects of serotonergic agonists and antagonists on responding under a discrimination task using unreliable visual stimuli. Psychopharmacology. 1999a;146:422–431. doi: 10.1007/pl00005487. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999b;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Robbins TW. Increased response switching, perseveration and perseverative switching following d-amphetamine in the rat. Psychopharmacology. 1983;80:67–73. doi: 10.1007/BF00427498. [DOI] [PubMed] [Google Scholar]
- Frank J, Staddon JE. Effects of restraint on temporal discrimination behavior. Psychological Record 1974 [Google Scholar]
- Gallistel CR. The organization of learning. Cambridge, Mass: MIT Press; 1990. [Google Scholar]
- Gallistel CR, Gibbon J. Time, rate, and conditioning. Psychological Review. 2000;107:289–344. doi: 10.1037/0033-295x.107.2.289. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, King A, McDonald R. Sources of variability and systematic error in mouse timing behavior. J Exp Psychol Anim Behav Process. 2004;30:3–16. doi: 10.1037/0097-7403.30.1.3. [DOI] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and weber's law in animal timing. Psychological Review. 1977;84:279–325. [Google Scholar]
- Gibbon J, Church RM. Sources of variance in an information processing theory of timing. In: Roitblat HL, Bever TG, Terrace HS, editors. Animal cognition. Erlbaum; Hillsdale, NJ: 1984. [Google Scholar]
- Gibbon J, Church RM. Representation of time. Cognition. 1990;37:23–54. doi: 10.1016/0010-0277(90)90017-e. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Church RM. Comparison of variance and covariance patterns in parallel and serial theories of timing. Journal of the Experimental Analysis of Behavior. 1992;57:393–406. doi: 10.1901/jeab.1992.57-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Annals of the New York Academy of Sciences. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Grossberg S, Schmajuk NA. Neural dynamics of adaptive timing and temporal discrimination during associative learning. Neural Networks. 1989;2:79–102. [Google Scholar]
- Ho MY, al-Zahrani SS, Velazquez Martinez DN, Lopez Cabrera M, Bradshaw CM, Szabadi E. Effects of desipramine and fluvoxamine on timing behavior investigated with the fixed-interval peak procedure and the interval bisection task. Psychopharmacology. 1996;125:274–284. doi: 10.1007/BF02247339. [DOI] [PubMed] [Google Scholar]
- Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E. 5-hydroxytryptamine and interval timing behaviour. Pharmacology, biochemistry, and behavior. 2002;71:773–785. doi: 10.1016/s0091-3057(01)00672-4. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Fetterman JG. A behavioral theory of timing. Psychological Review. 1988;95:274–295. doi: 10.1037/0033-295x.95.2.274. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Church RM. Stimulus and temporal cues in classical conditioning. Journal of Experimental Psychology-Animal Behavior Processes. 2000;26:206–219. doi: 10.1037//0097-7403.26.2.206. [DOI] [PubMed] [Google Scholar]
- Leak TM, Gibbon J. Simultaneous timing of multiple intervals: Implications of the scalar property. Journal of Experimental Psychology: Animal Behavior Processes. 1995;21:3–19. [PubMed] [Google Scholar]
- Liao RM, Cheng RK. Acute effects of d-amphetamine on the differential reinforcement of low-rate (drl) schedule behavior in the rat: Comparison with selective dopamine receptor antagonists. Chin J Physiol. 2005;48:41–50. [PubMed] [Google Scholar]
- Machado A, Cevik M. Acquisition and extinction under periodic reinforcement. Behavioural processes. 1998;44:237–262. doi: 10.1016/s0376-6357(98)00052-7. [DOI] [PubMed] [Google Scholar]
- Matell MS, Bateson M, Meck WH. Single-trials analyses demonstrate that increases in clock speed contribute to the methamphetamine-induced horizontal shifts in peak-interval timing functions. Psychopharmacology. 2006 doi: 10.1007/s00213-006-0489-x. in press. [DOI] [PubMed] [Google Scholar]
- Matell MS, King GR, Meck WH. Differential modulation of clock speed by the administration of intermittent versus continuous cocaine. Behav Neurosci. 2004;118:150–156. doi: 10.1037/0735-7044.118.1.150. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Neuropsychological mechanisms of interval timing behavior. Bioessays. 2000;22:94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: Coincidence detection of oscillatory processes. Brain Res Cogn Brain Res. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM. Simultaneous temporal processing. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:1–29. [PubMed] [Google Scholar]
- Neil DB, Herndon JG., Jr Anatomical specificity within rat striatum for the dopaminergic modulation of drl responding and activity. Brain research. 1978;153:529–538. doi: 10.1016/0006-8993(78)90337-2. [DOI] [PubMed] [Google Scholar]
- Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain research. 1979;171:213–224. doi: 10.1016/0006-8993(79)90328-7. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Gibbon J, Penney TB, Malapani C, Hinton SC, Meck WH. Scalar expectancy theory and peak-interval timing in humans. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:15–33. doi: 10.1037//0097-7403.24.1.15. [DOI] [PubMed] [Google Scholar]
- Roberts S. Isolation of an internal clock. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:242–268. [PubMed] [Google Scholar]
- Staddon JER, Higa JJ. Time and memory: Towards a pacemaker-free theory of interval timing. Journal of the Experimental Analysis of Behavior. 1999;71:215–251. doi: 10.1901/jeab.1999.71-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B, McNaughton N. Common firing patterns of hippocampal cells in a differential reinforcement of low rates of response schedule. Journal of Neuroscience. 2000;20:7043–7051. doi: 10.1523/JNEUROSCI.20-18-07043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


