SUMMARY
Our laboratory has identified plasma membrane oestrogen receptors on a GH3/B6 rat pituitary tumour cell line and several sublines which produce rapid (within minutes), non-genomic responses to oestrogens. Oestrogen receptors have been identified by their binding to nine different antibodies (Abs) which together recognize at least seven epitopes on the oestrogen receptor-α. GH3/B6/F10 cells, a membrane oestrogen receptor-enriched subline, elevate intracellular calcium levels in response to 10 nM oestradiol. Prolactin release in these cells is triggered by both 1 pM and 1 nM oestradiol and diethylstilbestrol (DES). A membrane oestrogen receptor-α immunocyto-chemical signal rapidly disappears (at 3 min) and reappears (at 12–15 min) when 1 nM oestradiol, 10 nM diethylstilbestrol, or 10 nM nonylphenol is applied to the cells. This suggests that both oestrogens and xenoestrogens can utilize this alternative pathway for oestrogenic action. Xenoestrogens, which have so far shown weak effects in genomic assay systems, should now be retested for activity in eliciting membrane-initiated oestrogenic responses.
INTRODUCTION
The mechanisms of action of many environmental oestrogens have remained a conundrum, as attempts to explain their actions via standard laboratory tests for steroid action have always shown them to be very weak compared to physiological oestrogens. Therefore, attempts to explain and predict their in vivo activity have for the most part failed. This low in vitro potency is perplexing, since it cannot account for the potent endocrine-disrupting effects observed as a result of environmental exposures (Colborn et al. 1993; McLachlan, 1993). It is possible that currently employed laboratory tests, which almost always measure effects solely via the genomic mechanistic pathway (McLachlan, 1993; Ramamoorthy et al. 1997), are missing an alternative pathway through which these compounds could operate.
Rapid effects of steroids do not fit into the genomic mechanistic scheme largely accepted as the main (or only) mode of action for steroids (reviewed by us in Watson et al. 1998; Watson & Gametchu, 1999). Genomic mechanisms employing steroid receptors acting as transcription factors require many macromolecular syntheses, and thus relatively long periods of time, to culminate in the final hormone-induced outcome. Our laboratories have focused on functions associated with activation of membrane steroid receptors and the characterization of the receptor proteins which mediate these actions (Pappas et al. 1994, 1995a,Pappas et al. b; Watson et al. 1995; Gametchu et al. 1995; Gametchu & Watson, 1995; Norfleet et al. 1999a,b). The protein identity of such receptors has been a major source of controversy in the steroid hormone field. To identify these proteins we have used a tool developed relatively recently for steroid receptors, multiple antibodies to multiple epitopes of the intracellular receptors. In this paper we will summarize our immuno-identification studies of the membrane oestrogen receptor-α (mERα) and report our initial findings about the ability of xenoestrogens to utilize this alternative receptor pathway of action.
METHODS
Cell line origin and maintenance
GH3/B6 cells (Dufy et al. 1979) were a gift of Dr Bernard Dufy (Universitie de Bordeaux II, Bordeaux, France). These cells are a subclone of the rat pituitary tumour cell line, GH3, which produces prolactin (PRL) and growth hormone (Tashjian et al. 1968; Bancroft & Tashjian, 1971). GH3/B6/F10 cells are a subclone of GH3/B6 cells expressing high levels of mERα (Pappas et al. 1994). Cells were routinely propagated in serum-supplemented media composed of Ham’s F-10 (Gibco-BRL, Gaithersburg, MD, USA), 12.5% heat-inactivated horse serum (Gibco-BRL; Hyclone, Logan, UT, USA) and 2.5% heat-inactivated defined/supplemented bovine calf serum (Hyclone). Our defined medium used in some experiments contained DMEM (Gibco-BRL, phenol red-free), insulin-transferrin-selenium (Sigma, St Louis, MO, USA) and 0.1 % BSA (Sigma).
Antibodies to ERα
Characterization and affinity purification of the polyclonal anti-peptide Abs to ER (R3 and R4), have been described previously (Pappas et al. 1994). Monoclonal Abs H222 and H226 and polyclonal Ab ER21 were a gift of Dr Geoffrey Greene (Greene et al. 1984; King & Greene, 1984; Blaustein, 1992). Abs H151 (anti-human hinge region) and C542 (anti-human carboxy terminus) were from StressGen Biotechnologies Corp. (Victoria. BC, Canada). MC20 Ab was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The ER715 Ab is from the laboratory of Dr Jack Gorski (Furlow et al. 1990).
Fixed cell staining with enzyme-immunocytochemistry
GH3/B6/F10 pituitary tumour cells (Pappas et al. 1994) were cultured on glass coverslips that had been treated with poly-D-lysine (Sigma) for 72 h in the defined medium. Oestrogens or vehicle (0.01 % ethanol) premixed in medium were applied to the cells continuously for the times indicated. The cells were washed once in phosphate-buffered saline, pH 7.4 (PBS), prior to fixation. In order to render the cell membranes impermeable to Ab, a 30 min fixation period in 1 % glutaraldehyde at room temperature was employed. After fixation, the cells were washed three times in PBS followed by a reduction of aldehyde groups caused by the glutaraldehyde (treatment for 10 min with 70 mM Na2HPO4, 13 mM NaBH4, pH 7.4, followed by three more PBS rinses). The fixed cells were incubated overnight at 4 °C with Ab H151 anti-ERα primary Ab in 0.1 % BSA in PBS (elimination of the primary Ab, or pre-incubation of the Ab with the peptide to which it was raised, eliminates the signal; C. H. Campbell, unpublished observations). Subsequent incubations were carried out at room temperature, using reagents from a VECTASTAIN ABC-Alkaline Phosphatase kit in conjunction with the Vector Red substrate (Vector Labs, Burlingame, CA, USA). Cells were incubated for 60 min in the presence of a biotinylated ‘universal’ horse anti-mouse/anti-rabbit IgG (Vector Labs) diluted in PBS, then washed with PBS and exposed to an avidin-biotinylated alkaline phosphatase complex for 60 min. The cells were rinsed once with PBS and then incubated with VectorRed substrate and levamisole (Vector Labs), an inhibitor of endogenous alkaline phosphatase, for 10 min. The reaction was stopped with an excess volume of water, and methyl green was used to counterstain the cells during a 4 min incubation at 60 °C. The cells were dehydrated through a series of washes in ethanol and xylene, prior to mounting with Cytoseal 280 (Electron Microscopy Sciences, Ft Washington, PA, USA). For each treatment group multiple fields were examined and 200 cells for each condition were scored for the presence of mER antigen (mER positive). A one-way ANOVA comparing all treatment groups was performed using SigmaStat version 2.0 (Jandel, San Rafael. CA, USA); differences were analysed using Tukey’s test.
Earlier immunocytochemistry experiments (represented in Table 1 and Fig. 1) employed live cell antibody labelling techniques at 4°C with fluorescent tags on secondary antibodies (Pappas et al. 1994, 1995a,Pappas et al. b; Watson et al. 1995) or other methods of fixation (Norfleet et al. 1999a,b).
Table 1.
Characteristics of subcloned lines compared to wild-type GH3/B6 cells (WT)
| Characteristic | WT | F10 | D9 | mER+ | mER− | Reference |
|---|---|---|---|---|---|---|
| MERα presence | + | +++ | − | +++ | +/− | a, b, c, d, e, f |
| IERα presence | ++ | ++ | ++ | ++ | ++ | b, d, e |
| E2-BSA binding and function | ++ | n.d. | n.d. | +++ | n.d. | a, d,f |
| mER appearance | Punctate | Punctate | Not present | Punctate | Punctate | a, b, c, d, e, f |
| Cell shape | Elongated or round | Usually elongated | Cuboidal | Elongated or round | Elongated or round | a, b, c, d, e, f |
| Rapid response (PRL release) | + | +++ | − | ++ | − | a, c,e, f |
Limiting dilution subcloning produced a variety of lines varying in their expression of rnER, two of which are shown here (F10, D9). Other cells were immunopanned for (mER+) and against (mER−) the membrane ERα antigen, n.d., not done,
Fig. 1.
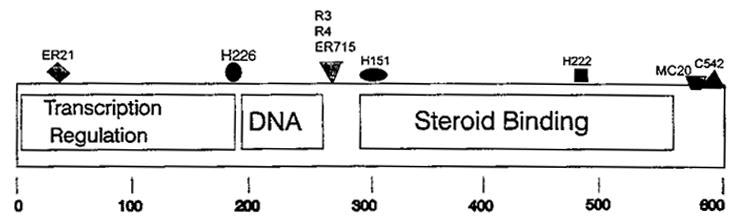
Epitopes of the nuclear ERα recognized by available anti-ERαAbs that also recognize mERα. These results are compiled from experiments on both unfixed and fixed cells which sometimes differed in their intensity of staining for a given Ab (Pappas et al. 1994, 1995a,Pappas et al. b; Watson et al. 1995; Norfleet et al. 1999a,b). The location of the epitopes are shown by the various symbols above the map of the peptide. Abs recognizing each epitope are listed above the symbol; their sources and descriptions are in the Methods section. The established functional domain structure of intracellular ERα is shown for reference.
Prolactin release assays – radioimmunoassay for prolactin
GH3/B6/F10 cells, plated in serum-containing medium, and then cultured in 24-well plates for 72 h in defined medium, were washed once and pre-incubated for 15 min in phenol red-free DMEM containing 20 mM Hepes and 0.1 % BSA (DHB). The cells were maintained at 37 °C for the pre- and the test incubations. The pre-incubation medium was removed and replaced at time zero with test (DHB ± 0.001 % ethanol ± test agents). The test agents were 1 pM and 1 nM oestradiol 17 β (E2) and diethylstilbestrol (DES). A stock solution of E2 (Sigma) in 95 % ethanol was diluted to give a final ethanol concentration of 0.001 %, which was also used in all control and test conditions. After 6 min, test medium was collected into pre-chilled tubes, which were immediately stored at 20 °C. Prolactin (PRL) concentrations in the test media were determined by radioimmunoassay using reagents provided by the National Institute of Diabetes and Digestive and Kidney Disease and the National Hormone and Pituitary Program. The intra-assay variability was 7 %. The PRL release observed in the vehicle control group was considered to be 100%. A one-way ANOVA comparing treatment groups to the control was performed using SigmaStat version 2.0 (Jandel, San Rafael, CA, USA); post hoc group differences were analysed using Dunnett’s test. Statistical significance was accepted at P < 0.05.
Analysis ofCa2+ by whole cell imaging
Intracelluiar Ca2+ measurements were performed on groups of cells grown on poly-D-lysine-coated coverslips, and loaded with fura-2 (Molecular Probes, Eugene, OR, USA) Ca2+-sensitive fluorescent dye. The responses to 10 nM E2 and 1 μM thyrotropin-releasing hormone (TRH; Martin, 1983; Ohmichi et al. 1994; Shorte et al. 1995) are shown over a 9.5 min time course via fluorescence imaging on a Nikon Diaphot inverted microscope coupled to a dual monochrometer system via a fibreoptic cable (Photon Technology International, Monmouth Junction, NJ, USA). Images were processed using ImageMaster software (Tsien & Harootunian, 1990).
RESULTS AND DISCUSSION
Cell lines and sublines useful in receptor identification and correlation to function
We previously reported that while all GH3/B6 rat pituitary tumour cells contain nuclear or iniracellular oestrogen receptors (iERs), only some cells have oestrogen receptors on their surface (Pappas et al. 1994). It was apparent that mER expression is quite variable or dynamic compared to that of iER. Therefore, we isolated cell populations (subcloned and immunoseparated cells) based on their expression of mER into ‘+’ and ‘−’ populations, in order to test whether the presence of the receptor was necessary for a rapid oestrogenic action – secretion of PRL – within minutes of hormone administration (Pappas et al. 1994, 1995a). Wild-type GH3/B6 cells (a subclone of the well-known GH3 cell line; Dufy et al. 1979) represent the parental mixed cell population, and are intermediate in characteristics and responses. Table 1 summarizes the characteristics of the different cell sublines that we obtained or isolated for these studies with references to the original studies provided. In general, cells bearing the highest amounts of mER on their surfaces gave more robust rapid responses to oestradiol 17 β (E2), and tended to contain cells which were more elongated in shape, while all cells contained equivalent amounts of iER. The reports summarized in Table 1 have allowed us to associate oestrogen’s action as a secretagogue with the presence of mER.
Identity of the protein which we call mER
The nature of the protein which mediates rapid responses to oestrogen is of great interest. There are those that have proposed that mER is a unique protein unrelated to the intracellular form of the receptor (reviewed in Watson et al. 1998; Watson & Gametchu, 1999). This hypothesis is based on the following arguments. One is that membrane responses exhibit some differences in pharmacological specificity or the hierarchy of sensitivity to their cognate class of steroids (reviewed in Watson & Gametchu, 1999). It is possible, however, that receptor residence in lipid membranes, as opposed to the aqueous environment of the cytosol or nucleus, may dictate a different protein conformation (Wu & Gorenstein, 1993), and consequently alter the geometry of the steroid binding pocket for both agonists and antagonists. Another argument for membrane-initiated functions being accomplished by unique proteins is that when membrane-isolated receptors have been identified, their size is different from that of the corresponding intracellular receptor (Wehling et al. 1992; Zheng & Ramirez, 1997). While this argument could be one reason to consider unique proteins, it does not fully consider the possibilities that proteolytic cleavage into fragments may occur during the course of protein isolation (a common occurrence with steroid receptors), or that membrane targeting may involve the addition or deletion of peptide sequence or other chemical moieties to a known protein. Nevertheless, there are recent examples of identification of unique membrane proteins associated with rapid steroid responses (Falkenstein et al. 1996).
The alternative hypothesis is that mER is related to iERα. We tested this hypothesis by using as many antibodies to the iERα as would adequately represent the different domains of the protein (Pappas et al. 1994, 1995a,Pappas et al. b; Watson et al. 1995; Norfleet et al. 1999a,b). Our results from these experiments are summarized in Fig. 1, which depicts recognition of seven epitopes by nine antibodies to iERα. It is likely that a protein with this many epitopes in common with iERα is actually a closely related protein, modified in some way for targeting to the plasma membrane. Other investigators have found identity of a membrane steroid receptor with the corresponding intracellular receptor via Ab or peptide sequence characterization (Gametchu et al. 1995; Gametchu & Watson, 1995; Kim et al. 1996; Sabeur el al. 1996).
How do we reconcile the findings that membrane steroid receptors are related to their nuclear counterparts with those that indicate that cell surface ‘steroid receptor’ proteins are distinct from their intracellular complements? It may be that the plasma membrane is a location which contains mixed binding systems for steroids, much as do other intra- and extracellular locations. Much of the data for membrane steroid receptors relies on labelled steroid or impeded ligand binding to membrane preparations or to the cell surface of intact cells, and the association of these observations with a rapid steroid-induced response. It is possible, indeed likely, that multiple proteins that bind steroids participate in the complete cellular response. These other participating proteins may be transporters (Madar et al. 1982; Feldman & Hilf, 1985; Lackner et al. 1998), enzymes (Tsai & Sapolsky, 1996; Ansonoff & Etgen, 1998) or other binding proteins of unknown function, like the blood-borne binding proteins whose functions are still being explored (Siiteri et al. 1982; Nakhla et al. 1994, 1997; Sonnenschein et al. 1996; Ding et al. 1998). Therefore, both themes can be accommodated.
Prolactin release by E2 and diethylstilbestrol – sensitivity of the reponse
For a response to oestrogens and xenobiotics to have physiological and environmental consequences, it is important to consider how sensitive the response is to the hormone or hormonal mimetic. Therefore, we have performed dose–response studies at concentrations likely to be of physiological importance. Table 2 shows a 6 min PRL release response to 1 nM or 1 pM E2 or diethylstilbestrol (DES). Such concentrations are well within physiological relevancy in the case of E2. DES has been shown to be equipotent with E2 in other measures of activity on pituitary cells (Jordan & Lieberman, 1984) and is of interest because its former uses as a pharmaceutical and as a food additive for agricultural animals (Colborn et al. 1993; McLachlan, 1993). Effects in the picomolar range (3 orders of magnitude below the sensitivity necessary to be physiologically relevant) demonstrate much greater potency than previously shown. Very recently a positive effect of 100 pM E2 on glucose-induced insulin secretion and intracellular Ca2+ levels in primary cultures of pancreatic islet cells has also been demonstrated (Nadal et al. 1998). Therefore these responses could be elicited in organisms exposed to minute quantities of hormone or mimetic. Such results suggest that it is imperative that the ability of other oestrogenic mimetics to elicit these and other non-genomic responses should be studied in more detail. One such analysis has recently been published. In this study, coronary vascular tone was rapidly affected by xenoestrogens via changes in intracellular Ca2+ levels in vascular smooth muscle cells (Ruehlmann et al. 1998).
Table 2.
Rapid (6 min) PRL release elicited by 1 pM and 1 nM E2 and DES expressed as percentage increase over basal levels
| 1 pM | 1 nM | |
|---|---|---|
| Percentage increase in E2 | 81.0 ± 32.9 | 176 ± 65.3 |
| Percentage increase in DES | 69.8 ± 22.0 | 73.1 ± 16.5 |
Effects of hormonal treatments were compared to a vehicle control (basal level) containing 0.001 % ethanol. Each point represents mean ± S.E.M. of at least 6 observations per concentration from 3 (DES) or 4 (E2) separate experiments. All values were significant increases in comparison to the vehicle control at P < 0.05.
The mechanistic pathway by which oestrogens cause prolactin secretion - Ca2+ levels
One potential means of studying the effects of xenoestrogens is to identify the signalling cascade(s) by which oestrogen-induced release of PRL is triggered. Since secretory responses are often associated with increases in intracellular Ca2+ levels, we investigated the rapid change in Ca2+ levels in GH3/B6/F10 cells. We reasoned that we should address this problem with a technique which allows assessment of individual cells, because even in the F10 subclone, mER expression is dynamic, and only a percentage of the population at any given time bears significant amounts of mER on the cell surface. We also compared the response to E2 to that of a well-characterized response to the peptide TRH (Shorte et al. 1995). Figure 2 shows the averaged profiles of cells demonstrating both TRH- and oestrogen-induced Ca2+ level increases. Other cells responded to E2 only, TRH only, or to neither agent (data not shown). Note the difference in the type of response to E2 (slow gradual rise in Ca2+) vs. TRH (rapid spike and return to baseline). The TRH pattern is similar to that observed previously (Shorte et al. 1995). In three experiments, we assessed 137 cells of which 51 (37%) responded to both hormones, 34 (25 %) responded to E2 alone, 38 (28 %) responded to TRH alone, and 14 (10%) either did not respond or were not categorizable. Future experiments will explore more mechanistic details of these responses.
Fig. 2.

Hormonal (1 μM TRH, 10 nM E2) stimulation of intracellular Ca2+ levels in individual cells by fura-2 imaging. Cells whose profiles are shown were from a single coverslip, ensuring synchronized hormonal application and response times. Means ± S.E.M. of data from 22 cells representing responders to both E2 and TRH.
Modulation of mER by oestrogens and xenoestrogens
Finally, we wondered how the expression of mER would be acutely affected by ligands and mimetics in the time frame of the observed functional responses. We have begun to explore the effects of several oestrogenic compounds on mER levels, assessed by immunocytochemistry (see Table 3). In these studies, 1 nM E2 caused the disappearance of the mER signal (binding of the H151 Ab) from GH3/B6/F10 cells by 3 min. The signal returned by 15 min. In comparison, 10 nM DES and 10 nM nonylphenol caused a similar time course of disappearance and reappearance. We are unsure of the meaning of the immunocytochemical signal disappearance at this point, as it could mean an actual removal of the receptor from the surface of the cell, a conformational change of the receptor which alters the H151 epitope and disrupts recognition by this antibody, or some other phenomenon. It is interesting to consider that the hormones are still present during the entire course of the experiment and it is unlikely that return of the signal is the result of hormonal removal by the metabolic activity of the cells over such short periods of time. Whatever the mechanism of the signal disappearance, it does suggest that xenoestrogens can mimic E2 in this response very rapidly and at very low concentrations, much lower than have been shown effective in gene expression assays for xenoestrogen activity (Ramamoorthy et al. 1997).
Table 3.
The disappearance and reappearance of Ab H151 labelling of mER after hormone or mimetic treatment
| Treatment | Duration | Percentage mER positive cells |
|---|---|---|
| Vehicle control | 3 min | 93.3 ± 9.8 |
| 1 nME2 | 3 min | 8.4 ± 10.6* |
| 1 nME2 | 15 min | 89.7 ± 8.7 |
| 10 nM DES | 3 min | 5.1 ± 6.3* |
| 10 nM DES | 15 min | 91.2 ± 5.4 |
| 10 nM NP | 3 min | 7.3 ± 4.5* |
| 10 nM NP | 15 min | 90.9 ± 5.2 |
Cells grown on coverslips and serum withdrawn were then treated with vehicle (0.01 % ethanol), E2, DES, or nonylphenol (NP) for the times and at the concentrations indicated. Fixed and stained cells for each condition were examined by microscopy; multiple fields were examined and 200 cells for each condition were scored for the presence of mER antigen (mER positive).
Significant difference from vehicle control at the P < 0.001 level. Vehicle treatment at 15 min and a group which had no treatment were not statistically different from the 3 min vehicle treatment results (data not shown).
Conclusions and future directions
Clearly, the emerging data on oestrogenic actions via the membrane-initiated pathway needs much more investigation, as we are only beginning to understand some of the major themes of this alternative path of action and the other oestrogen binding proteins which may participate. Perhaps we can look for guidance to recent examples of the structures and functions of members of the cytokine/growth hormone/prolactin receptor super family, where a single gene in this family can give rise to multiple membrane, cytoplasmic, and transcriptional activities (Sotiropoulos et al. 1994; Ferrag et al. 1997). Consequences for the functioning of many different tissues are implied, although only the pituitary, pancreatic and cardiovascular systems were specifically cited here. The patterns and pathways defined will undoubtedly greatly contribute to our ability to understand diseases of endocrine disruption, and develop preventive and therapeutic agents relevant for these conditions.
Acknowledgments
We gratefully acknowledge NICHD grant 32481 and the John Sealy Memorial Endowment Fund for support for this work. We are very grateful to Dr Mark Hellmich and Mr Kirk Ives for allowing us access to the Ca2+ imaging system housed in the UTMB Department of Surgery. Additional advice on the interpretation of measurements of intracellular Ca2+ levels was thoughtfully provided by Dr Aileen Ritchie of the UTMB Physiology Department. We also wish to thank Dr David Konkel for critically reading this manuscript while in preparation.
References
- Ansonoff MA, Etgen AM. Estradiol elevates protein kinase C catalytic activity in the preoptic area of female rats. Endocrinology. 1998;139:3050–3056. doi: 10.1210/endo.139.7.6088. [DOI] [PubMed] [Google Scholar]
- Bancroft FC, Tashjian AH., Jr Growth in suspension culture of rat pituitary cells which produce growth hormone and prolactin. Experimental Cell Research. 1971;64:125–128. doi: 10.1016/0014-4827(71)90201-1. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- Colborn T, Vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environmental Health Perspectives. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding VD, Moller DE, Feeney WP, Didolkar V, Nakhla AM, Rhodes L, Rosner W, Smith RG. Sex hormone-binding globulin mediates prostate androgen receptor action via a novel signaling pathway. Endocrinology. 1998;139:213–218. doi: 10.1210/endo.139.1.5681. [DOI] [PubMed] [Google Scholar]
- Dufy B, Vincent JD, Fleury H, Pasquier PD, Gourdji D, Vidal T. Membrane effects of thyrotropin-releasing hormone and estrogen shown by intracellular recording from pituitary cells. Science. 1979;204:509–511. doi: 10.1126/science.107590. [DOI] [PubMed] [Google Scholar]
- Falkenstein E, Meyer C, Eisen C, Scriba PC, Wehling M. Full-length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 1996;229:86–89. doi: 10.1006/bbrc.1996.1761. [DOI] [PubMed] [Google Scholar]
- Feldman JM, Hilf R. Effect of estradiol-17 beta on glucose and proline uptake in plasma membrane vesicles from the R3230AC rat mammary carcinoma. Biochimica et Biophysica Acta. 1985;845:265–271. doi: 10.1016/0167-4889(85)90186-7. [DOI] [PubMed] [Google Scholar]
- Ferrag F, Goffin V, Buteau H, Kelly PA. Immune function of prolactin (PRL) and signal transduction by PRL/GH/cytokine receptors: specificity, redundancy and lessons from chimaeras. Cytokines, Cellular and Molecular Therapy. 1997;3:197–213. [PubMed] [Google Scholar]
- Furlow JD, Ahrens H, Mueller GC, Gorski J. Antisera to a synthetic peptide recognize native and denatured rat estrogen receptors. Endocrinology. 1990;127:1028–1032. doi: 10.1210/endo-127-3-1028. [DOI] [PubMed] [Google Scholar]
- Gametchu B, Chen F, Watson CS. Intracellular and plasma membrane-resident glucocorticoid receptors in rodent leukemia models. In: Gametchu B, editor. Glucocorticoid Receptor Structure and Leukemic Cell Responses. R. G. Landes Co.: Austin, TX, USA; 1995. pp. 75–103. [Google Scholar]
- Gametchu B, Watson CS. Plasma membrane-associated glucocorticoid hormone receptor in human leukemic patients: Clinical implications. In: Gametchu B, editor. Glucocorticoid Receptor Structure and Leukemic Cell Responses. R. G. Landes Co.; Austin, TX, USA: 1995. pp. 163–176. [Google Scholar]
- Greene GL, Sobel NB, King WJ, Jensen EV. Immunochemical studies of estrogen receptors. Journal of Steroid Biochemistry. 1984;20:51–56. doi: 10.1016/0022-4731(84)90188-2. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Lieberman ME. Estrogen-stimulated prolactin synthesis in vitro. Classification of agonist, partial agonist, and antagonist actions based on structure. Molecular Pharmacology. 1984;26:279–285. [PubMed] [Google Scholar]
- Kim YS, MacDonald PN, Dedhar S, Hruska KA. Association of 1 alpha, 25-dihydroxyvitamin D3-occupied vitamin D receptors with cellular membrane acceptance sites. Endocrinology. 1996;137:3649–3658. doi: 10.1210/endo.137.9.8756529. [DOI] [PubMed] [Google Scholar]
- King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Lackner C, Daufeldt S, Wildt L, Allera A. Glucocorticoid-recognizing and -effector sites in rat liver plasma membrane, kinetics of corticosterone uptake by isolated membrane vesicles -III - specificity and stereospecificity. Journal of Steroid Biochemistry and Molecular Biology. 1998;64:69–82. doi: 10.1016/s0960-0760(97)00141-6. [DOI] [PubMed] [Google Scholar]
- McLachlan JA. Functional toxicology: A new approach to detect biologically active xenobiotics. Environmental Health Perspectives. 1993;101:386–387. doi: 10.1289/ehp.93101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madar Z, MacLusky NJ, Naftolin F. Estrogen stimulation of 3-o-methyl-D-glucose uptake in isolated rat hepatocytes. Endocrinology. 1982;110:330–335. doi: 10.1210/endo-110-2-330. [DOI] [PubMed] [Google Scholar]
- Martin TF. Thyrotropin-releasing hormone rapidly activates the phosphodiester hydrolysis of polyphosphoinositides in GH3 pituitary cells. Evidence for the role of a polyphosphoinositide-specific phospholipase C in hormone action. Journal of Biological Chemistry. 1983;258:14816–14822. [PubMed] [Google Scholar]
- Nadal A, Rovira JM, Laribi O, Leonquinto T, Andreu E, Ripoll C, Soria B. Rapid insulinotropic effect of 17-β-estradiol via a plasma membrane receptor. FASEB Journal. 1998;12:1341–1348. doi: 10.1096/fasebj.12.13.1341. [DOI] [PubMed] [Google Scholar]
- Nakhla AM, Khan MS, Romas NP, Rosner W. Estradiol causes the rapid accumulation of cAMP in human prostate. Proceedings of the National Academy of Sciences of the USA. 1994;91:5402–5405. doi: 10.1073/pnas.91.12.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhla AM, Romas NA, Rosner W. Estradiol activates the prostate androgen receptor and prostate-specific antigen secretion through the intermediacy of sex hormone-binding globulin. Journal of Biological Chemistry. 1997;272:6838–6841. doi: 10.1074/jbc.272.11.6838. [DOI] [PubMed] [Google Scholar]
- Norfleet AM, Clarke C, Gametchu B, Watson CS. Antibodies to the estrogen receptor-α modulate prolactin release from rat pituitary tumor cells through plasma membrane estrogen receptors. FASEB Journal. 1999a doi: 10.1096/fasebj.14.1.157. in the Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norfleet AM, Thomas ML, Gametchu B, Watson CS. Estrogen receptor-alpha detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary tumor cells by enzyme-linked immunocytochemistry. Endocrinology. 1999b;140:3805–3814. doi: 10.1210/endo.140.8.6936. [DOI] [PubMed] [Google Scholar]
- Ohmichi M, Sawada T, Kanda Y, Koike K, Hirota K, Miyake A, Saltiel AR. Thyrotropin-releasing hormone stimulates MAP kinase activity in GH3 cells by divergent pathways. Evidence of a role for early tyrosine phosphorylation. Journal of Biological Chemistry. 1994;269:3783–3788. [PubMed] [Google Scholar]
- Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptor-enriched GH3/B6 cells have an enhanced non-genomic response to estrogen. Endocrine. 1995a;3:743–749. doi: 10.1007/BF03000207. [DOI] [PubMed] [Google Scholar]
- Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody and impeded ligand labeling. FASEB Journal. 1995b;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- Pappas TC, Gametchu B, Yannariello-Brown J, Collins TJ, Watson CS. Membrane estrogen receptors in GH3/B6 cells are associated with rapid estrogen-induced release of prolactin. Endocrine. 1994;2:813–822. [Google Scholar]
- Ramamoorthy K, Vyhlidal C, Wang F, Chen I, Safe S, McDonnell DP, Leonard LS, Gaido KW. Additive estrogenic activities of a binary mixture of 2′,4′,6′-trichloro- and 2′,3′,4′,5′-tetrachloro-4-biphenylol. Toxicology and Applied Pharmacology. 1997;147:93–100. doi: 10.1006/taap.1997.8281. [DOI] [PubMed] [Google Scholar]
- Ruehlmann DO, Steinert JR, Valverde MA, Jacob R, Mann GE. Environmental estrogenic pollutants induce acute vascular relaxation by inhibiting L-type Ca2+ channels in smooth muscle cells. FASEB Journal. 1998;12:613–619. doi: 10.1096/fasebj.12.7.613. [DOI] [PubMed] [Google Scholar]
- Sabeur K, Edwards DP, Meizel S. Human sperm plasma membrane progesterone receptor(s) and the acrosome reaction. Biology of Reproduction. 1996;54:993–1001. doi: 10.1095/biolreprod54.5.993. [DOI] [PubMed] [Google Scholar]
- Shorte SL, Stafford SJ, Collett VJ, Schofield JG. Simultaneous measurement of [Ca2+]i and secretion-coupled membrane turnover, by single cell fluorescence microscopy. Cell Calcium. 1995;18:440–454. doi: 10.1016/0143-4160(95)90059-4. [DOI] [PubMed] [Google Scholar]
- Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn RW. The serum transport of steroid hormones. Recent Progress in Hormone Research. 1982;38:457–510. doi: 10.1016/b978-0-12-571138-8.50016-0. [DOI] [PubMed] [Google Scholar]
- Sonnenschein C, Soto AM, Michaelson CL. Human serum albumin shares the properties of estrocolyone-I, the inhibitor of the proliferation of estrogen-target cells. Journal of Steroid Biochemistry and Molecular Biology. 1996;59:147–154. doi: 10.1016/s0960-0760(96)00112-4. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A, Perrot-Applanat M, Dinerstein H, Pallier A, Postel-Vinay M, Finidori J, Kelly PA. Distinct cytoplasmic regions of the growth hormone receptor are required for activation of JAK2, mitogen-activated protein kinase, and transcription. Endocrinology. 1994;135:1292–1298. doi: 10.1210/endo.135.4.7925092. [DOI] [PubMed] [Google Scholar]
- Tashjian AH, Jr, Yasumura Y, Levine L, Sato GH, Parker ML. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968;82:342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Tsai LW, Sapolsky RM. Rapid stimulatory effects of testosterone upon myotubule metabolism and sugar transport, as assessed by silicon microphysiometry. Aggressive Behavior. 1996;22:357–364. [Google Scholar]
- Tsien RY, Harootunian AT. Practical design criteria for a dynamic ratio imaging system. Cell Calcium. 1990;11:93–109. doi: 10.1016/0143-4160(90)90063-z. [DOI] [PubMed] [Google Scholar]
- Watson CS, Gametchu B. Membrane-initiated steroid actions and the proteins which mediate them. Proceedings of the Society of Experimental Biology and Medicine. 1999;220:9–19. doi: 10.1046/j.1525-1373.1999.d01-2.x. [DOI] [PubMed] [Google Scholar]
- Watson CS, Gametchu B, Norfleet AM, Campbell CH, Thomas ML. Rapid, nongenomic actions of estrogens. Women and Cancer. 1998;1:21–28. [Google Scholar]
- Watson CS, Pappas TC, Gametchu B. The other estrogen receptor in the plasma membrane: Implications for the actions of environmental estrogens. Environmental Health Perspectives. 1995;103 (suppl 7):41–50. doi: 10.1289/ehp.95103s741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling M, Eisen C, Aktas J, Christ M, Theisen K. Photoaffinity labeling of plasma membrane receptors for aldosterone from human mononuclear leukocytes. Biochemical and Biophysical Research Communications. 1992;189:1424–1428. doi: 10.1016/0006-291x(92)90233-b. [DOI] [PubMed] [Google Scholar]
- Wu J, GORENSTEIN DG. Structure and dynamics of cytochrome C in non-aqueous solvents by 2D NH-exchange NMR spectroscopy. Journal of the American Chemical Society. 1993;115:6843–6850. [Google Scholar]
- Zheng JB, Ramirez VD. Demonstration of membrane estrogen binding proteins in rat brain by ligand blotting using a 17-beta-estradiol-[i-125]bovine serum albumin conjugate. Journal of Steroid Biochemistry and Molecular Biology. 1997;62:327–336. doi: 10.1016/s0960-0760(97)00037-x. [DOI] [PubMed] [Google Scholar]


