Abstract
Objective
To determine whether all boys with Duchenne muscular dystrophy (DMD) have a similar verbal and memory profile of skills, or whether only a subset is affected, and to determine whether the weak areas in their profile are substantially different from a control group.
Methods
Performance of patients with DMD on neuropsychological tests of verbal and memory skills was examined in two ways. Standardized test scores for 80 boys with DMD (estimated IQ range, 70 to 160) were ranked individually from worst to best, and the individual rankings were compared across the group using Friedman rank analysis. Additionally, performance of 41 boys with DMD was compared with that of their sibling control subjects of similar age and estimated IQ using multivariate analysis of variance.
Results
Individual cognitive profiles were significantly similar among the subjects with DMD, such that for most subjects digit span, story recall, and comprehension were the tests on which each performed most poorly. This finding remained true regardless of whether they were of high or low intellectual function. In contrast, no significant cognitive profile was found among their sibling control subjects, and when compared with their siblings, the DMD group scored significantly more poorly on digit span, comprehension, and story recall, but not on other verbal and memory measures.
Conclusions
Boys with DMD have a specific cognitive profile, regardless of their general level of cognitive function. Specifically, boys with DMD performed more poorly on tests requiring attention to complex verbal information than they did on other verbal or memory measures. The possibility that the missing dystrophin brain products may contribute to selective cognitive processing is considered.
Keywords: Duchenne muscular dystrophy, Cognitive profile, Dystrophin, Brain development, Developmental disability
Duchenne muscular dystrophy (DMD) has long been recognized as a cause of mental retardation, yet most individuals with DMD are not mentally retarded. Mean IQ scores are shifted down approximately 1 SD from the normal population, with approximately 19% of boys scoring in the mentally retarded range.1 It has been proposed that all affected individuals with DMD have some cognitive impairment, and that this overall shift in scores would necessarily increase the number that fall in the mentally retarded range.2 Although IQ scores in DMD appear to be distributed normally, specific analyses have not addressed whether all or only a subpopulation of DMD individuals are compromised cognitively. The possibility exists that only boys with lower intellectual function have cognitive involvement related to the disorder, whereas the other boys are spared. We studied this question by examining cognitive skills across a large sample of children with DMD of varying intellectual level. Secondary analyses then compared skills between a subgroup of boys with DMD and their unaffected siblings.
The cause of cognitive impairment in DMD has not been established. It has been proposed that physical impairment may contribute to the detection of mental retardation; however, severity of cognitive impairment is not correlated with severity of physical decline. Also, comparison of patients with DMD with comparably physically impaired patients with spinal muscular atrophy (SMA) demonstrates DMD patients have lower intellectual levels than SMA patients,3–6 and verbal and memory skills appear to be selectively compromised. Other studies have also demonstrated specific verbal and memory deficits in DMD, whereas nonverbal skills (which do not require a heavy motor component) are generally intact.7–13 The majority of studies have demonstrated broad verbal versus performance IQ differences, and have not examined verbal and memory functions in detail or among high-functioning boys with DMD.
The discovery that the mutated gene in DMD codes for multiple protein products that localize to separate tissue types, including muscle and brain, offers a potential explanation for the cognitive manifestation of the DMD phenotype. In the brain, dystrophin isoforms normally localize to circumscribed cerebral and cerebellar cortical regions1–6,14–16 and are assumed to be absent in children with DMD. Although the contribution of the dystrophin brain products to function is unknown, their specific anatomic distribution suggests that they may well be involved in selective cognitive processes. Furthermore, because different mutations could potentially compromise some brain isoforms selectively over others, the possibility exists that there may be multiple cognitive phenotypes within the group.
The current study addressed three questions about cognition in boys with DMD: 1) Do all testable boys with DMD have some specific cognitive involvement or is just a subset affected? 2) Is general intellectual function associated with the specificity of the profile? 3) Is the profile of verbal and memory performance associated with DMD substantially different from a control comparison group? During part 1 of the current study we examined cognitive function in a large sample of boys with DMD who had a wide range of intellectual function. During part 2 we compared a smaller sample of DMD patients with their unaffected siblings.
Methods
Subjects
Part 1: probands’ performance
A total of 92 boys with DMD were enrolled. Subjects were recruited from 1) the cohort ascertained in the Duchenne Muscular Dystrophy Clinical Trials Study (n = 21); 2) private physicians associated with the Muscular Dystrophy Association clinics at Columbia Presbyterian Medical Center (CPMC; n = 11), and the Albert Einstein Medical Center (n = 3) in New York, NY, the Scottish Rite Children’s Medical Center (SRCMC) in Atlanta, GA (n = 15), and at Newington Children’s Hospital in Hartford, CT (n = 6); and 3) announcements and mailings through the Muscular Dystrophy Association and the Duchenne Parent Project (n = 36). All were boys between 6 and 16 years of age, in otherwise good general health. They all spoke English as their primary language and were willing to participate. Diagnosis of DMD was based on clinical onset of progressive weakness before 5 years of age, elevated serum creatine kinase levels, and either molecular assessment of mutation in the DMD gene or muscle biopsy that was deficient in dystrophin and compatible with DMD. For those families in which more than one boy met criteria for inclusion (six families had two eligible sons), only one affected boy was included. The selected proband was chosen randomly; preference for the elder and then the younger boy alternated between families. A total of 88% of the sample were white, 5% were black, 3.5% were Hispanic, and the remainder were Asian.
Part 2: probands versus siblings
When possible, one healthy sibling without DMD was also recruited from each family. Selection criteria included the following: 6 to 16 years old, age within 5 years of the proband’s age, in good general health, English as the primary language, and willingness to participate. When more than one control subject was available, preference was given first to boys and then to closeness of age. A total of 41 siblings met these criteria and participated. A total of 93% of the subjects were white, 5% were Hispanic, and 2% were black.
Procedures
All subjects received a battery of neuropsychological tests. Measures were chosen that emphasized verbal and memory skills, and that made minimal motor demands to minimize the potential confounding effects of impaired physical agility. The tests measured a broad range of general intellectual function, and included selected subtests from two composite neuropsychological measures. Data were collected either at CPMC (n = 31), SRCMC (n = 6), or in the subjects’ homes (n = 55). All subjects were assessed individually in a quiet room, and each assessment took approximately 3 hours. Subjects were given breaks as needed. Testing was conducted in English. All tests were scored twice, once by the person who administered them and once by a research assistant who had not had direct contact with the subject to ensure accuracy of the scored data. Discrepancies were resolved by consensus.
Measures
Because of the anticipated wide variability of IQ in DMD, the Peabody Picture Vocabulary Test–Revised (PPVT-R)17 was included as a screening measure of general intellectual function. This test requires the subject to listen to a word and point to one of four pictures shown to describe the word best. The test has very simple starting items and measures the higher limits of each subject’s functioning, making it a reliable measure of single-word comprehension in both low- and high-functioning individuals. Furthermore, the PPVT-R has clear and direct instructions and is correlated strongly with more detailed measures of verbal IQ. A standard score with a mean of 100 and an SD of 15 was computed for the PPVT-R and was used as an estimate of each subject’s overall verbal intellectual function.
For the purposes of the current study’s data analysis, only well-standardized measures with equivalent normative information for each subtest were chosen from a battery of tests administered. These included selected verbal subtests from the Wechsler Intelligence Scale for Children–III (WISC-III)—information, similarities, comprehension, and digit span18—and selected subtests from the Wide Range Assessment of Memory and Learning (WRAML)—verbal learning, visual learning, story memory, and picture memory.19
Data analysis
Part 1: probands’ performance
The range of standard scores on the PPVT-R was examined to determine the distribution of patients’ scores. Performance on the PPVT-R was used as a way to segregate the patients scoring in the “mentally retarded” range from the rest of the sample. For the data analysis of the current study, only patients with standard scores more than 70 on the PPVT-R were included.
On the composite neuropsychological measures, non-parametric rank order analyses were used to determine whether patients had similar cognitive strengths and weaknesses irrespective of general intellectual level. Because each test included in these analyses was standardized on the same population, similar performance across the tests would be expected for each individual. Furthermore, within a group of subjects, individual variation of scores across tests should be random. Thus, a significant finding on the rank order analyses indicates across-subject consistency in the relative performance on the subtests. For each subject, the scaled scores (SS) obtained on the individual subtests of the WISC-III and WRAML were rank ordered: Each subject’s subtest with the lowest SS was assigned a “one,” the next lowest score a “two,” and so on, across the four subtests. Then, each individual’s rankings were combined with those of the other patients in the sample, and a Friedman analysis of related samples determined the similarity of rankings across patients. During follow-up analyses, the patients were segregated into two groups: those whose PPVT-R SS was more than the median of the distribution and those who SS was less than the median. The nonparametric rank order analyses were then repeated separately in those two groups to determine whether patterns of strengths and weaknesses differed as a function of general level of intelligence. Standard scores were also plotted against IQ levels to look for tendencies in the data as a function of IQ.
Part 2: probands versus siblings
To investigate further the cognitive profile associated with boys with DMD and normal IQ, probands’ performance on the tests was compared with that of their unaffected siblings. Individual Student’s t-tests were calculated for age, grade, and PPVT-R standard scores to ensure similarity of the groups. Socioeconomic and background variables did not require statistical control because subjects were from the same family and household. To determine whether the subgroup of probands with siblings accurately reflected characteristics of the larger sample, rank order analyses were calculated using the WISC-III and WRAML subtests. Rank order analyses were also conducted to determine whether there was a profile of cognitive strengths and weaknesses among the siblings in the comparison group.
To determine whether the cognitive profiles were substantially different, scores on each of the standardized test measures were then compared across the DMD and sibling control groups using multivariate analyses of variance (MANOVAs) with gender included as a covariate.
Results
Part 1: probands’ performance
All 92 patients took the PPVT-R. As expected, the range of scores was great, from less than 40 (the minimum PPVT-R standardized score) to 160 (the maximum PPVT-R standardized score; figure 1). Ten patients (approximately 11%) had a standard score of ≤70, falling within the “mentally retarded” range. To ensure that results from other tests were analyzed only from DMD patients with “normal” verbal IQs, the PPVT-R was used to segregate the patients for further analyses.
Figure 1.
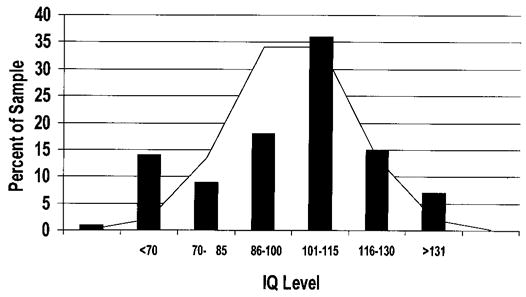
Histogram of Duchenne muscular dystrophy subjects’ estimated verbal IQ scores as derived from performance on the Peabody Picture Vocabulary Test–Revised, superimposed on the distribution of expected values for the general population.
For the remaining 82 patients with an estimated verbal IQ of more than 70, testing was attempted on the WISC-III and WRAML subtests. Two patients were unable to complete the tests due to severe behavior problems; both exhibited autistic-like behavior and were unable to comply with the structure necessary in a standardized testing environment. As such, data for the WISC-III subtests are presented on 80 patients. Of those 80 patients, one refused to complete the WRAML subtests.
Examination of the data collected on the probands with “normal” intellectual function (estimated verbal IQ range, 71 to 160) indicated that a specific neuropsychological profile existed, irrespective of general intellectual level. Friedman rank order analysis on the four WISC-III verbal subtests was highly significant (χ2 = 67.87, p < 0.001), indicating that there was a consistent similarity in the rank ordering of test performance across patients. Performance was ranked from worst to best in the following order: digit span, comprehension, similarities, and information. Friedman rank order analysis on the four WRAML subtests was also highly significant (χ2 = 27.00, p < 0.001). Tests were ranked from worst to best in the following order: story memory, picture memory, verbal learning, and visual learning.
To determine whether the WISC-III profile existed independently of intellectual function, the group was split into those whose PPVT-R score was either more or less than the median of the probands’ distribution of scores, and Friedman analyses were repeated on the two subgroups. Ranks were consistent regardless of estimated verbal IQ level (patients with an estimated verbal IQ > 108: χ2 = 50.56, p < 0.001; patients with an estimated IQ < 108: χ2 = 22.03, p < 0.001). Figures 2 and 3 represent the patients’ profile on the WISC-III and WRAML subtests across their estimated IQ range. Note that the SS for digit span and comprehension were consistently lower than the SS of the other WISC-III verbal subtests across the wide rage of intellectual function. Similarly, note that the story memory SS were consistently lower than those of the other WRAML subtests, across the IQ range.
Figure 2.
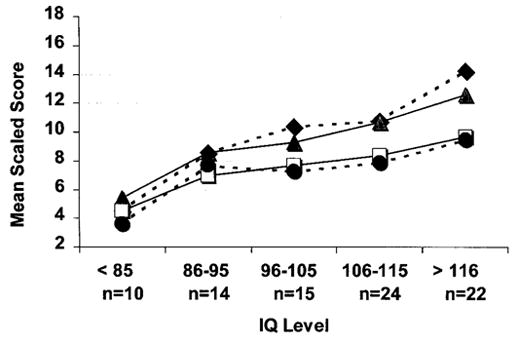
Plot of the Duchenne muscular dystrophy group’s mean scaled scores for the four Wechsler Intelligence Scale for Children–III verbal subtests across IQ levels (◆, information; □, comprehension; ▲, similarities; ●, digit span). Note that the digit span and comprehension scores are lower than the scores for information and similarities across the IQ range.
Figure 3.
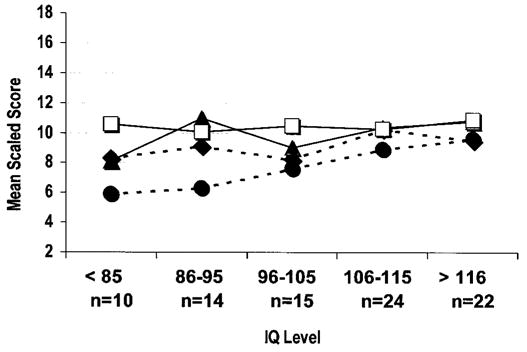
Plot of the Duchenne muscular dystrophy group’s mean scaled scores for the four Wide Range Assessment of Memory and Learning subtests across IQ levels (◆, picture memory; □, visual learning; ▲, verbal learning; ●, story memory). Note that the story memory scores are lower than the scores of the other memory tests across the IQ range.
Part 2: probands versus siblings
Probands’ performance was then compared with that of unaffected siblings. Among the previously described 80 patients, 41 had a sibling control. Twenty-four control subjects were boys and 17 control subjects were girls. Twenty-two siblings were older than the proband (13 boys and 9 girls) and 19 subjects were younger (11 boys and 8 girls). All 41 siblings took the PPVT-R, and WISC-III and WRAML subtests. The pro-band who refused to finish the WRAML subtests was in the matched group, so analysis of the WRAML is presented on only 40 subject pairs.
Comparison of the proband and matched sibling groups using Student’s t-test confirmed that the groups did not differ with respect to age, grade, or estimated verbal IQ (t = 1.02, 1.44, and 1.22 respectively; not significant).
To determine whether the proband group selected on the basis of sibling participation was similar to the larger proband sample, repeat rank order analyses were conducted. Rank order analysis of the subset of the proband group was similar to that of the larger sample for both the WISC III (χ2 = 35.34, p < 0.001) and WRAML subtests (probands, χ2 = 17.80, p < 0.001).
Rank order analyses were calculated to investigate whether the unaffected sibling comparison group had a cognitive profile similar to that of the probands. No consistent profile was observed across the WISC-III subtests (χ2 = 5.49, not significant) or WRAML subtests (χ2 = 1.23, not significant). This confirms that within the control group, individual cognitive strengths and weaknesses varied.
MANOVAs were used to examine whether the probands performed differently from their siblings on the neuropsychological tests. The groups did not differ in age, grade, or estimated verbal IQ, but the sibling group included girls as well as boys, so gender was used as a covariate. The omnibus comparison of the two groups’ performance on the WISC-III subtests was significant (F = 5.18, p < 0.01). Examining the univariate ANOVAs, significant intergroup effects were found for digit span and comprehension (F = 13.50, 6.78; p < 0.01), with the probands having lower scores on these tests than their siblings. No significant intergroup differences were observed for the similarities and information subtests.
The omnibus test for intergroup performance on the WRAML subtests was also significant (F = 2.61, p < 0.05). Examining the univariate ANOVAs, significant intergroup effects were found for story memory (F = 6.31, p < 0.05), with the probands performing more poorly than their siblings. Additionally, there was a trend toward poorer performance for the probands on the picture memory subtest. No significant intergroup differences were observed on the verbal and visual learning subtests.
Discussion
These data present clear evidence of a specific cognitive profile associated with DMD. Boys with DMD performed more poorly on tests of digit span, verbal comprehension, and story memory relative to their other verbal and memory skills. Furthermore, this finding was consistent across all levels of general intellectual function, suggesting that the pattern of cognitive strengths and weaknesses is found among all individuals with DMD, irrespective of their general intelligence. This was demonstrated by analyzing the data in two separate ways. Part 1 examined the probands’ performance by rank ordering nonretarded individuals’ scores and examining the nature of those rankings across the subject group and by plotting performance on individual subtests across intellectual level. Part 2 compared test performance of nonretarded affected boys with performance of their unaffected siblings, controlling for the effects of overall IQ.
Therefore, the answer to the first question—Do all boys affected by DMD have some cognitive involvement?—is yes. However, this clearly does not mean that all boys with DMD have cognitive deficits or intellectual impairment. Although the overall profile of cognitive strengths and weaknesses was similar across boys with DMD, the degree of cognitive involvement was variable. Notably, for many of the boys in the sample, being relatively weaker in their digit span, verbal comprehension, and story memory skills was still consistent with overall good cognitive ability. Within this sample of boys chosen on the basis of the physical characteristics of the disorder, the estimated verbal IQ ranged from the lowest measurable score (40) to the highest measurable score (160) on the test used (the PPVT-R). This wide range is consistent with previous reports in the literature.8,12,20–24 Furthermore, and also in agreement with the literature, the intellectual functioning of the majority of boys in the sample was well within normal limits, and only those boys whose IQs were more than 70 were chosen for the current analyses. Additionally, even when they were split by their median estimated IQ score, even those boys in the high-functioning group (PPVT-R SS, >108) had evidence of a significant cognitive profile. And when plotted across the IQ range, the ranking of the subtests remained consistent. Thus, the answer to the second question—Is general intellectual function associated with the specificity of the profile?—is no. Even though these data indicate that all the boys with DMD are characteristically weak in certain cognitive areas, for many, their general cognitive abilities are strong enough to preclude them from having learning difficulties.
The cognitive involvement in the DMD group did not generalize across all memory or verbal tasks. The children in the DMD group did relatively well and performed no differently than their siblings on rote memory tests that did not involve any “mental manipulations.” When asked to learn a list of 16 words or the positions of 14 visual designs hidden on a board, the boys with DMD did well. For both of these tests, the items are presented over four trials, and the total number of recalled items is computed. The boys with DMD were able to sustain their concentration and demonstrated increased recall with each successive trial, indicating intact attention, learning, and rote memory skills. Additionally, they did well on some verbal tasks. The boys with DMD had no trouble answering questions about general factual information or explaining the similarity between two given items. As such, they demonstrated intact general verbal understanding and verbal abstract thinking skills, as well as the ability to articulate well-formulated responses.
In contrast, the DMD group performed less well, relative to themselves and compared with their siblings, on tests of digit span, comprehension, and story memory than they did on the other tests administered. This was true regardless of degree of cognitive involvement in the DMD subjects. Each of the measures on which they did relatively more poorly requires attention and ability to “immediately hold” and “work with” aurally presented information. The subject must remember, manipulate, and repeat back a string of digits; comprehend, consider, and respond to a linguistically complex question; and listen to, process, and reconstruct a short story. Each task taxes the subject’s verbal working memory.
Thus, the cognitive profile associated with DMD has areas of cognitive deficit that appear to be related to deficient verbal working memory and auditory comprehension skills. According to a model by Baddeley et al.,25 these functions are integrally related. The current results suggest DMD may affect the proposed “phonologic loop” necessary for optimal development of verbal working memory.
Numerous studies, including an analysis of select subjects from the current sample on a more comprehensive battery of neuropsychological tests (described in a paper in press26) have shown visuospatial skills to be relatively intact in boys with DMD. In general, earlier work examining cognitive skills in DMD supports the theory that verbal working memory skills are compromised, but no previous study has examined it across such a wide range of intellectual function and found the profile to be consistent regardless of level of overall intellectual ability. Furthermore, the current results are more specific than those reported previously. Work comparing DMD patients with SMA patients noted deficient digit span in the DMD group.12,20,27 Other previously reported findings among boys with DMD include decreased visual memory as well as verbal memory,20 more generalized verbal involvement,27 and poor serial learning,7 which clearly were not replicated here. Rather, the current data indicate that rote learning and long-term memory are relative strengths for the DMD group. Although there have been many similarities in the findings across the studies, there have also been discrepancies, likely due to methodological issues. Some studies only evaluated children with learning difficulties9 and others lacked an appropriate comparison group.8,11,13,28 The design of the current study, ranking individual performance profiles across IQ levels and examining sibling pairs of similar general verbal IQ, likely controls for more potentially confounding variables than the previously reported studies. The finding that boys with DMD have relatively poor verbal working memory is both robust and reliable.
We hypothesize that dystrophin products contribute to the optimal brain function underlying working memory skills. Because the brains of children with DMD have developed without specific dystrophin isoforms, they likely function slightly differently from brains that developed with the dystrophin isoforms (like those of their siblings). Overt level of cognitive dysfunction in DMD is extremely variable. Other as yet unknown genetic and environmental factors likely contribute to the wide range of cognitive involvement. All boys with DMD are put at increased risk for learning difficulties because of their relatively weaker verbal working memory skills, yet the majority compensate well. For children with DMD, emphasizing learning strategies that rely more heavily on rote memorization and nonverbal methods, rather than verbal working memory skills, may enhance their quality of life substantially.
Footnotes
Supported by grant R29 HD34155 from the National Institute of Child Health and Development and the Muscular Dystrophy Association (V.J.H.).
References
- 1.Kim TW, Wu K, Xu JL, Black IB. Detection of dystrophin in the postsynaptic density of rat brain and deficiency in a mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 1992;89:11642–11644. doi: 10.1073/pnas.89.23.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchino M, Teramoto H, Naoe H, Yoshioka K, Miike T, Ando M. Localisation and characterisation of dystrophin in the central nervous system of control subjects and patients with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. 1994;57:426–429. doi: 10.1136/jnnp.57.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura S, Abe K, Suzuki M, et al. 2.1 Kb 5′-flanking region of the brain type dystrophin gene directs the expression of lacZ in the cerebral cortex, but not in the hippocampus. J Neurol Sci. 1997;147:13–20. doi: 10.1016/s0022-510x(96)05317-8. [DOI] [PubMed] [Google Scholar]
- 4.Lidov HG, Byers TJ, Watkins SC, Kunkel LM. Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature. 1990;348:725–728. doi: 10.1038/348725a0. [DOI] [PubMed] [Google Scholar]
- 5.Tian M, Jacobson C, Gee SH, Campbell KP, Carbonetto S, Jucker M. Dystroglycan in the cerebellum is a laminin alpha 2-chain binding protein at the glial–vascular interface and is expressed in Purkinje cells. Eur J Neurosci. 1996;8:2739–2747. doi: 10.1111/j.1460-9568.1996.tb01568.x. [DOI] [PubMed] [Google Scholar]
- 6.Uchino M, Teramoto H, Naoe H, Miike T, Yoshioka K, Ando M. Dystrophin and dystrophin-related protein in the central nervous system of normal control subjects and Duchenne muscular dystrophy. Acta Neuropathol. 1994;87:129–134. doi: 10.1007/BF00296181. [DOI] [PubMed] [Google Scholar]
- 7.Anderson SW, Routh DK, Ionasescu VV. Serial position memory of boys with Duchenne muscular dystrophy. Dev Med Child Neurol. 1988;30:328–333. doi: 10.1111/j.1469-8749.1988.tb14557.x. [DOI] [PubMed] [Google Scholar]
- 8.Karagan NJ, Zellweger HU. Early verbal disability in children with Duchenne muscular dystrophy. Dev Med Child Neurol. 1978;20:435–441. doi: 10.1111/j.1469-8749.1978.tb15244.x. [DOI] [PubMed] [Google Scholar]
- 9.Karagan NJ, Richman LC, Sorensen JP. Analysis of verbal disability in Duchenne muscular dystrophy. J Nerv Mental Dis. 1980;168:419–423. doi: 10.1097/00005053-198007000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Leibowitz D, Dubowitz V. Intellect and behaviour in Duchenne muscular dystrophy. Dev Med Child Neurol. 1981;23:577–590. doi: 10.1111/j.1469-8749.1981.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 11.Marsh GG, Munsat TL. Evidence of early impairment of verbal intelligence in Duchenne muscular dystrophy. Arch Dis Child. 1974;49:118–122. doi: 10.1136/adc.49.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogasawara A. Downward shift in IQ in persons with Duchenne muscular dystrophy compared with those with spinal muscular atrophy. Am J Ment Retard. 1989;93:544–547. [PubMed] [Google Scholar]
- 13.Cotton S, Crowe SF, Voudouris N. Neuropsychological profile of Duchenne muscular dystrophy. Child Neuropsychol. 1998;4:110–117. [Google Scholar]
- 14.Gorecki DC, Lukasiuk K, Szklarczyk A, Kaczmarek L. Kainate-evoked changes in dystrophin messenger RNA levels in the rat hippocampus. Neuroscience. 1998;84:467–477. doi: 10.1016/s0306-4522(97)00562-9. [DOI] [PubMed] [Google Scholar]
- 15.Jung D, Pons F, Leger JJ, Aunis D, Rendon A. Dystrophin in central nervous system: a developmental, regional distribution and subcellular localization study. Neurosci Lett. 1991;124:87–91. doi: 10.1016/0304-3940(91)90828-h. [DOI] [PubMed] [Google Scholar]
- 16.Jancsik V, Hajos F. Differential distribution of dystrophin in postsynaptic densities of spine synapses. Neuroreport. 1998;9:2249–2251. doi: 10.1097/00001756-199807130-00018. [DOI] [PubMed] [Google Scholar]
- 17.Dunn LF, Dunn LM. Manual, PPVT-R. Circle Pines: American Guidance Center; 1981. [Google Scholar]
- 18.Wechsler D. WISC-III manual. San Antonio, TX: The Psychological Corporation, Harcourt Brace; 1991. [Google Scholar]
- 19.Sheslow D, Adams W. WRAML: wide range assessment of memory and learning. Wilmington, DE: Wide Range Inc; 1990. [Google Scholar]
- 20.Billard C, Gillet P, Signoret JL, et al. Cognitive functions in Duchenne muscular dystrophy: a reappraisal and comparison with spinal muscular atrophy. Neuromuscul Disord. 1992;2:371–378. doi: 10.1016/s0960-8966(06)80008-8. [DOI] [PubMed] [Google Scholar]
- 21.Bushby KM, Appleton R, Anderson LV, Welch JL, Kelly P, Gardner–Medwin D. Deletion status and intellectual impairment in Duchenne muscular dystrophy. Dev Med Child Neurol. 1995;37:260–269. doi: 10.1111/j.1469-8749.1995.tb12000.x. [DOI] [PubMed] [Google Scholar]
- 22.Dorman C, Hurley AD, D’Avignon J. Language and learning disorders of older boys with Duchenne muscular dystrophy. Dev Med Child Neurol. 1988;30:316–327. doi: 10.1111/j.1469-8749.1988.tb14556.x. [DOI] [PubMed] [Google Scholar]
- 23.Rapaport D, Passos–Bueno MR, Brandao L, Love D, Vainzof M, Zatz M. Apparent association of mental retardation and specific patterns of deletions screened with probes cf 56a and cf 23a in Duchenne muscular dystrophy. Am J Med Genet. 1991;39:437–441. doi: 10.1002/ajmg.1320390414. [DOI] [PubMed] [Google Scholar]
- 24.Moizard MP, Billard C, Toutain A, Berret F, Marmin N, Moraine C. Are dp71 and dp140 brain dystrophin isoforms related to cognitive impairment in Duchenne muscular dystrophy? Am J Med Genet. 1998;80:32–41. doi: 10.1002/(sici)1096-8628(19981102)80:1<32::aid-ajmg6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Baddeley AD, Gathercole S, Papagno C. The phonological loop as a language learning device. Psychol Rev. 1998;105:158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- 26.Hinton VJ, DeVivo DC, Nereo NE, Goldstein E, Stern Y. Selective deficits in verbal working memory associated with a known genetic etiology: the neuropsychological profile of Duchenne muscular dystrophy. J Int Neurophychol Soc. 2000 doi: 10.1017/s1355617701711058. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whelan TB. Neuropsychological performance of children with Duchenne muscular dystrophy and spinal muscle atrophy. Dev Med Child Neurol. 1987;29:212–220. doi: 10.1111/j.1469-8749.1987.tb02138.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith RA, Sibert JR, Harper PS. Early development of boys with Duchenne muscular dystrophy. Dev Med Child Neurol. 1990;32:519–527. doi: 10.1111/j.1469-8749.1990.tb16978.x. [DOI] [PubMed] [Google Scholar]


