Abstract
From the perspective of comparative morphology, the distribution of non-monoaminergic neurons in the common marmoset (Callithrix jacchus) was investigated using an immunohistochemical method with specific antibodies to tyrosine hydroxylase (TH) and aromatic-L-amino acid decarboxylase (AADC).
TH-immunoreactive (IR) neurons (but not AADC-IR) neurons were observed in the olfactory tubercle, preoptic suprachiasmatic nucleus, periventricular hypothalamic nucleus, arcuate nucleus, paraventricular nucleus, periaqueductal gray matter, medial longitudinal fasciculus, substantia nigra, and nucleus solitaris.
In contrast, AADC-IR (but not TH-IR), small, oval and spindle-shaped neurons were sparsely distributed in the following areas: the hypothalamus from the anterior nucleus to the lateral nucleus, the dorsomedial nucleus, the dorsomedial area of the medial mammillary nucleus and the arcuate nucleus; the midbrain, including the stria medullaris and substantia nigra; and the medulla oblongata, including the dorsal area of the nucleus solitaris and the medullary reticular nucleus. The distribution of AADC-IR neurons was not as extensive in the marmoset as it is in rats. However, these neurons were located in the marmoset, but not the rat substantia nigra. Furthermore, AADC-IR neurons that are present in the human striatum were absent in that of the marmoset.
The present results indicate that the distribution of non-monoaminergic neurons in the brain of the common marmoset is unique and different from that in humans and rodents.
Keywords: common marmoset, immunohistochemistry, non-monoaminergic neuron, tyrosine hydroxylase, aromatic L-amino acid decarboxylase
I. Introduction
Tyrosine hydroxylase (TH) is the rate-limiting enzyme for the biosynthesis of catecholamines (dopamine, noradrenaline, and adrenaline). TH is transiently expressed in some non-catecholaminergic (CA) neurons of the mammalian brain during prenatal and postnatal development of the cerebral cortex [2, 6, 50], anterior olfactory nucleus [42], medial geniculate nucleus [45], and inferior colliculus [11]. TH-immunoreactive (IR) non-CA neurons have also been observed in the brains of adult goldfish [15], rats [34], monkeys [30], and humans [7, 8]. Furthermore, the number of TH-IR neurons increases after treatment with parachlorophenylalanine [26] or colchicines [31, 52], and TH-IR neurons are present in non-CA regions of transgene in transgenic mice carrying human TH-chloramphenicol acetyltransferase fusion gene [43, 44].
TH has been reported to appear transiently in the neonatal stage as well as in response to drug administration as described above. However, the actual conditions of these phenomena have yet to be elucidated. Herein, we report the distribution of neurons containing TH in the brain of the marmoset, a primate species closely related to humans, from a phylogenetic perspective, and discuss the significance of the existence of these neurons.
Aromatic L-amino acid decarboxylase (AADC) catalyzes the conversion of L-3, 4-dihydroxyphenylalanine to dopamine and the conversion of 5-hydroxy-L-tryptophan (5-HTP) to 5-hydroxytryptamine [35]. In addition, AADC is also intimately involved in the synthesis of trace amines (tryptamine, phenylethylamine, tyramine, and octopamine) in the brain [14]. Jaeger et al. [12, 13] identified AADC in certain non-monoaminergic neurons and labeled them as belonging to the D neuron system located in the rat brain and spinal cord. D neurons have been demonstrated immunohistochemically in the brain of laboratory shrews [14, 19, 20], mice [35], rats [39, 41], cats [21], and humans [9, 27].
In the present study, we report and discuss the significance of the existence of AADC-IR neurons, which are widely distributed in the marmoset brain. Neurons containing both TH and AADC are thought to exert various physiological actions as dopaminergic neurons, and have already been reported to be widely distributed in the marmoset brain [25]. Furthermore, we closely investigated the existence of neurons containing only TH or only AADC using an immunohistochemical method with originally produced specific antibodies [40, 41].
II. Materials and Methods
Experimental animals
Two male common marmosets (Callithrix jacchus: C.j-153, body weight, 300 g; age, 2 years) were bred at the Primate Research Institute of Kyoto University. All animal experiments proceeded in accordance with the Guide for the Care and Use of Laboratory Animals established by the US National Institutes of Health (1985) and the Guide for the Care and Use of Laboratory Primates (2002) established by the Primate Research Institute of Kyoto University.
Immunohistochemistry
1) Tissue preparation
The animals were administered ketamine hydrochloride i.m. (1 mg/100 g) and then deeply anesthetized with pentobarbital sodium (Nembutal 2.5 mg/100 g) i.v. Heparin sodium (1,000 units/ml) was injected directly into the left ventricle (0.5 ml). The animals were then perfused through the ascending aorta with 0.15 M NaCl, followed by 4% formaldehyde (FA) and 0.2% glutaraldehyde in 0.1 M phosphate buffer (PB, pH 7.4). During perfusion, the heads of the animals were chilled in crushed ice. The brain was removed from the skull, postfixed in 2% FA for 10 hr and washed in 30% sucrose PB at 4°C for 2 days before slicing into 30 µm-thick sections using a DSK-3000W microslicer (Dosaka EM, Japan).
2) Antisera
Highly specific polyclonal antisera were raised from bovine adrenal TH and rabbit AADC. The specificity of each is described elsewhere [40, 41].
3) Avidin-biotin peroxidase complex (ABC) method
Floating sections were processed according to the ABC method as follows: pre-incubation in 0.1 M phosphate-buffered saline (PBS) containing 0.3% normal swine serum (NSS) for 1 day; subsequently, sections were washed 3 times with PBS at 10-min intervals and immersed in 10% NSS for 30 min. The sections were then sequentially incubated with anti-TH (1:10,000) or anti-AADC (1:5,000) antisera at 4°C for 2 days, biotinylated anti-rabbit IgG diluted 1:1,000, and avidin-biotin peroxidase complex (1:1,000) for 2 hr at room temperature. After immersion in 0.15 M Tris buffer (pH 7.6) containing 0.005% diaminobenzidine, 0.05% H2O2 and 0.8% nickel ammonium sulfate, sections were washed in water, mounted on slides, dehydrated, and coverslipped for light microscopy.
4) Double-labeling immunofluorescence method
Mouse antiserum against TH (MAB318, 1:1,000; Chemicon Co. Ltd.) [4, 55], and rabbit antiserum against AADC (1:5,000) [41] were the primary antisera. Secondary antibodies for TH and AADC staining included fluorescein-conjugated goat anti-mouse IgG (AP124F9, 1:200; Chemicon Co. Ltd.) and rhodamine-conjugated goat anti-rabbit IgG (AP156R, 1:200; Chemicon Co. Ltd.). Stained sections were observed using an Axioskop 2 plus microscope (Carl Zeiss, Germany).
III. Results
TH-immunoreactive (IR) neurons
1) Hypothalamus
Although a few moderately IR, small, oval and spindle-shaped neurons were diffusely distributed in the preoptic suprachiasmatic nucleus (Figs. 1A, 2A), AADC-IR neurons were undetectable (data not shown). Small, oval, green neurons were sparsely distributed from the lateral to the ventrolateral area of the arcuate nucleus (Figs. 1B, 2B). Furthermore, the paraventricular nucleus contained very few moderately IR, small, oval neurons (Figs. 1D, 2C), but no AADC-IR neurons (data not shown).
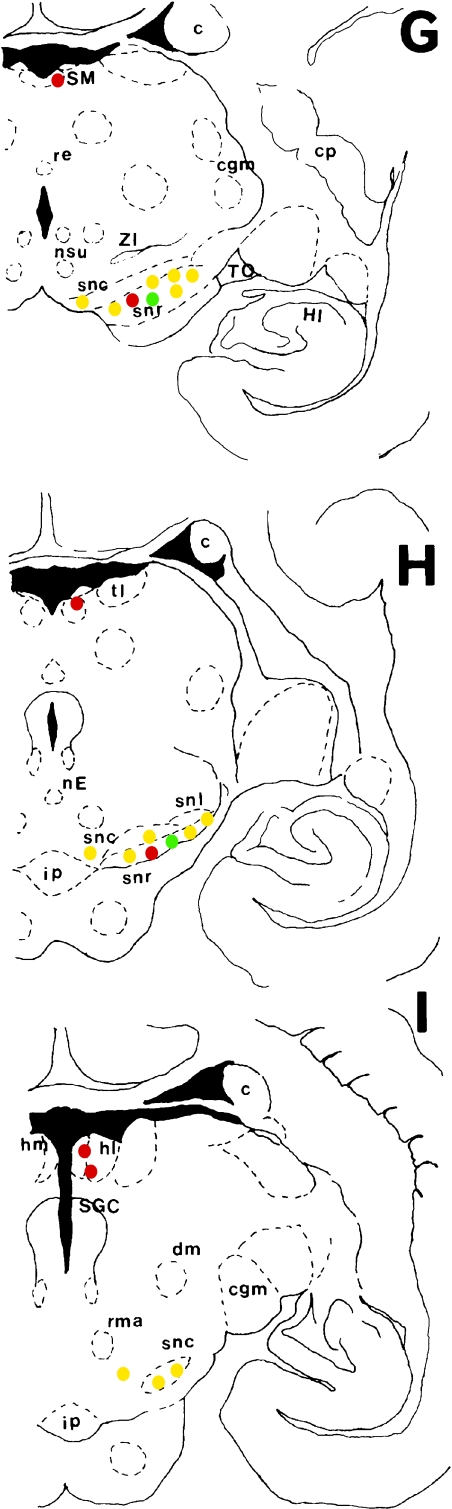
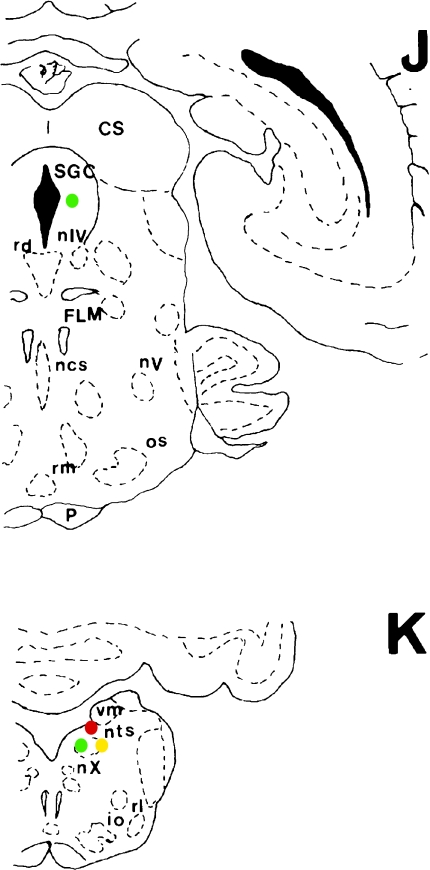
Fig. 1.
Schematic of frontal sections of brain showing distribution of TH-immunoreactive (IR) (green circles), AADC-IR (red circles) and TH with AADC double IR (yellow circles) neurons. Symbol indicating neuronal somata represents 5–10 neurons.
Abbreviations: ac, central amygdaloid nucleus; c, caudate nucleus head; CA, anterior commissure; cgm, medial geniculate body; CO, optic chiasm; cp, caudate putamen; CS, superior colliculus; dm, deep mesencephalic nucleus; FLM, medial longitudinal fasciculus; gp, globus pallidus; HI, hippocampus; hl, lateral habenular nucleus; hm, medial habenular nucleus; io, inferior olive; ip, interpeduncular nucleus; ma, ventromedial nucleus, anteromedial part; me, median eminence; na, arcuate nucleus; ncs, median raphe nucleu; ndm, dorsomedial nucleus; nE, Edinger-Westphal nucleus; nha, anterior hypothalamic nucleus; nme, medial mammillary nucleus; nmm, medial mammillary nucleus, medial part; npe, periventricular hypothalamic nucleus; npv, paraventricular nucleus; nso, supraoptic nucleus; nsu, supramammillary nucleus; nts, nucleus of the solitary tract; nIV, trochlear nucleus; nV, trigeminal motor nucleus; nX, dorsal vagal nucleus; os, superior olive; P, pyramidal tract; pos, preoptic suprachiasmatic nucleus; rd, dorsal raphe nucleus; re, reunions nucleus; rl, lateral reticular nucleus; rm, nucleus raphe magnus; rma, red nucleus, magnocellular part; SGC, periaqueductal gray matter; sin, substantia innominata; sm, medial septal nucleus; SM, stria medullaris; snc, substantia nigra, compact zone; snl, substantia nigra, lateral part; snr, substantia nigra, reticular zone; tad, anterior dorsal thalamic nucleus; tl, lateral thalamic nucleus; tmd, mediodorsal thalamic nucleus; TO, optic tract; tpl, ventroposterior lateral thalamic nucleus; tpm, ventroposterior medial thalamic nucleus; vm, medial vestibular nucleus; ZI, zona incerta.
Fig. 2.
Immunohistochemical microphotograph (A, C, E) and immunofluorescence double immunostaining (B, D, F). A) Small, oval and spindle-shaped TH-IR neurons are diffusely distributed in the preoptic suprachiasmatic nucleus (thin arrows). ×350. B) TH-IR neurons are distributed in the lateral to ventrolateral area (green, open arrows), and AADC-IR neurons are distributed in the ventromedial area (red, thin arrows) of the arcuate nucleus. ×250. C) A few moderately stained TH-IR neurons are distributed in the paraventricular nucleus (open arrow). III: third ventricle. ×350. D) Co-expression of TH- and AADC-IR is observed in the neurons of substantia nigra as shown in yellow. Green (open arrows) and red (thin arrows) cells are TH-IR and AADC-IR neurons, respectively. ×150. E) Intensely TH-IR small neurons are distributed in periaqueductal gray matter (open arrow). White star: cerebral aqueduct. ×350. F) Green oval (open arrows) and yellow (thin arrows) cells in solitary tract nucleus are TH-IR, and TH-AADC double IR neurons, respectively. White star: capillary. ×600.
2) Midbrain
Medium TH-IR green neurons were sparsely distributed and intermixed with TH- and AADC-IR yellow dopaminergic neurons (Figs. 1G, 1H, 2D) in the substantia nigra. A few intensely IR, small, round neurons were distributed in the periaqueductal gray matter (Figs. 1J, 2E).
3) Medulla oblongata
A few oval, green neurons were distributed around the blood vessels in the ventral region of the solitary tract nucleus (Figs. 1K, 2F).
AADC-IR neurons
1) Hypothalamus
A group of moderately IR, small, oval neurons ranged from the anterior nucleus to the lateral nucleus (Figs. 1A, 3A), but TH-IR neurons were undetectable in this region (data not shown). These AADC-IR neurons are considered to correspond to the rat group D14 neurons reported by Jaeger et al. [12, 13]. The dorsomedial nucleus contained a group of moderately IR, small, circular or oval, red neurons (Figs. 1C, 1D, 3B), which comprised the largest group of AADC-IR neurons in the brain of the common marmoset. These neurons were distributed over a broad area from the rostral to the caudal regions of the hypothalamus and were considered to correspond to the rat group D12 neurons reported by Jaeger et al. [12, 13]. The premammillary nucleus ventral to the medial mammillary nucleus contained a large group of D8 neurons in rat and other animals, but AADC-IR red neurons were only sparsely distributed in this region of the common marmoset (Figs. 1E, 3C). Small, oval, red AADC-IR neurons were diffusely distributed in the ventromedial area of the arcuate nucleus (Figs. 1B, 1C, 2B).
Fig. 3.
Immunohistochemical microphotographs (A, D, E, F) and immunofluorescence double immunostaining (B, C). A) Small, oval and spindle-shaped AADC-IR neurons are diffusely distributed in the anterior nucleus of the hypothalamic area. ×350. B) Small, oval-shaped AADC-IR neurons (red cells, thin arrows) are diffusely distributed in the dorsomedial nucleus of the hypothalamic area. ×500. C) Round AADC-IR neurons (red cells, thin arrows) are scattered in the dorsomedial area of the medial mammillary nucleus. ×400. D) Moderately stained, very small AADC-IR cells are located in the stria medullaris. ×350. E) Moderately AADC-IR, oval neurons are distributed in the solitary tract nucleus (thin arrows). ×350. F) TH-IR varicose fibers are located in the solitary tract nucleus (thin arrows). ×350.
2) Midbrain
A group of moderately IR, very small, round cells was identified in the stria medullaris (Figs. 1F, 1I, 3D), but no TH-IR cells were identified in this area (data not shown). These AADC-IR neurons were considered to correspond to the group D6 neurons described by Jaeger et al. [12, 13]. In marmosets, the AADC-IR neurons in this area are likely to be glia cells based on their morphological characteristics, including small neurons, and ambiguous nerve fiber projections extending from neurons. Medium to relatively large spindle-shaped red neurons were sparsely distributed from the deep compacta region to the reticular region of the substantia nigra (Figs. 1G, 1H, 2D), and the nerve fibers extended from the inside to the outside of the substantia nigra.
3) Medulla oblongata
Moderately IR, small, oval neurons were diffusely distributed from the dorsal to the ventral regions of the nucleus of the solitary tract (Figs. 1K, 3E). On the other hand, although this region contained relatively large number of TH-IR fibers (Fig. 3F), TH-IR neurons were not evident (Fig. 3F). The neurons in this region were considered to correspond to the group D2 neurons described by Jaeger et al. [12, 13].
Distribution map of non-monoaminergic neurons
An atlas of the distribution of non-monoaminergic neurons was constructed based on The Brain of the Common Marmoset by Stephan et al. [25, 31, 51].
IV. Discussion
TH is the rate-limiting enzyme in catecholamine (CA) neuron synthesis. AADC, dopamine-β-hydroxylase (DBH), and phenylethanolamine-N-methyltransferase (PNMT) are essential enzymes in the syntheses of dopamine, noradrenaline, and adrenaline, respectively. The existence of non-monoaminergic neurons containing only TH [2, 6–8, 11, 15, 30, 34, 42–45, 50] or only AADC [9, 12, 13, 27, 29, 39, 41, 47, 53] has been reported in the brain of many mammals, including humans. We previously reported the distribution of monoaminergic neurons in the brain of vertebrates with respect to their comparative anatomy [15, 16, 25]. In the present study, as part of such comparative anatomical investigation, we clarified the distribution of TH-IR (AADC-negative) and TH-negative (AADC-IR) neurons.
The existence of TH-IR neurons in the arcuate nucleus has been reported in mammals other than the shrew [5, 18, 37]. We previously generated an anti-L-DOPA antibody [29] and reported the high probability that L-DOPA is the end product of TH-IR neurons observed in the arcuate nucleus of the laboratory shrew [18].
Although is also possible that L-DOPA is the end product of TH-positive neurons observed in the arcuate nucleus of marmosets, this possibility was not confirmed by double immunostaining using anti-TH and anti-L-DOPA antibodies. Therefore, it is not currently possible to draw specific conclusions. A unique observation in the present study pertains to the unique distribution of TH-IR neurons in marmosets in comparison to other mammals. TH-IR neurons were occasionally observed in the substantia nigra, which is the main nucleus containing the largest number of dopamine-IR neurons. However, no TH-IR neurons were observed in the locus ceruleus, which contains the largest number of noradrenaline-IR neurons, or in the dorsal raphe nucleus, which contains the largest number of serotonin (5-HT)-IR neurons. The absence of TH-IR neurons in the cerebral cortex [2, 8, 34, 50] and vagal motor nucleus [23, 26, 36], in which TH-IR neurons were previously observed in other mammals, is also a characteristic feature of the distribution of TH-IR neurons in the marmoset.
Many researchers have reported the possibility that L-DOPA is the end product of TH-IR neurons [17, 18, 28, 32, 37, 38, 46, 54]. We previously confirmed this possibility on the basis of our study of the arcuate and the lateral habenular nuclei in the laboratory shrew. Researchers have also reported that TH-IR neurons colocalize with other neurotransmitters, such as choline acetyltransferase (ChAT) [1, 23, 36], γ-aminobutyric acid (GABA) [5, 24, 33], glutamic acid decarboxylase (GAD) [3], which is a GABA-synthesizing enzyme, calretinin [10, 49], and 5-HT [22]. Many of these studies have indicated that TH appears transiently in the neonatal stage and colocalizes with other neurotransmitters. We have previously reported on the colocalization of TH-IR neurons with ChAT in the vagal motor nucleus and with 5-HT in the raphe nucleus in the laboratory shrew during the neonatal period. This suggests the possibility that TH, in the presence of other neurotransmitters, has a neurohormonal action during postnatal development. TH-IR neurons were observed in more parts of the marmoset brain than in the brains of other mammals. Our future experiments will focus on whether L-DOPA is the end product of these TH-IR neurons and whether these neurons exert a neurohormonal physiologic action in the presence of other neurotransmitters.
On the other hand, AADC is a nonspecific enzyme involved in the synthesis of CA, 5-HT, and trace amines, such as tyramine, tryptamine, and phenylethylamine [14], in the brain. The existence of a group of neurons containing only AADC in mammals was first reported by Jaeger et al. [12, 13]. Since then, the existence of such a group has been reported in many mammals including humans [9, 27, 29, 39, 41, 47, 53]. In the present study of the marmoset, the distribution of neurons containing only AADC was observed in a broad range of brain structures, from the rostral to the caudal parts. As characteristic findings in the marmoset, we observed such neurons in the arcuate nucleus and substantia nigra, areas where such neurons were not observed in rats.
AADC-IR neurons can synthesize amines from a precursor of amines [48]. As we have previously shown using an immunocytochemical method, AADC-IR neurons in the laboratory shrew produce dopamine when L-DOPA is administered; however, they produce 5-HT when 5-HTP is administered [19–21]. It is possible that AADC-IR neurons in the marmoset brain have similar functions to those in the laboratory shrew. We plan to examine this in our future experiments. AADC-IR neurons have been observed in the corpus striatum [9] and hypothalamus [29] of humans, although no such neurons were observed in these regions of the marmoset brain. Therefore, there is a high possibility that dopamine is produced in brain regions other than the substantia nigra, such as the corpus striatum and hypothalamus. This possibility should be considered when using L-DOPA treatment for Parkinson’s disease.
Furthermore, AADC-IR neurons may form a system containing trace amines, such as tyramine, tryptamine, and phenylethylamine. The existence of this system has not been demonstrated by immunohistochemistry because specific enzymes, including the synthesis reaction for these amines, have yet to be elucidated. As the synthesis of these trace amines is enhanced by the administration of a monoamine oxidase inhibitor, we are planning to develop an appropriate method to further clarify the function of AADC-IR neurons, as well as a technique for verifying their morphologies.
V. Acknowledgment
The authors are grateful to the members of the Functional Anatomy Club of Seijoh University for their technical assistance.
VI. References
- 1.Armstrong M. D., Manley L., Haycock W. J., Hersh B. L. Co-localization of choline acetyltransferase and tyrosine hydroxylase within neurons of the dorsal motor nucleus of the vagus. J. Chem. Neuroanat. 1990;3:133–140. [PubMed] [Google Scholar]
- 2.Berger B., Verney C., Gaspar P., Febvret A. Transient expression of tyrosine hydroxylase immunoreactivity in some neurons of the rat neocortex during postnatal development. Dev. Brain Res. 1985;23:141–144. doi: 10.1016/0165-3806(85)90013-6. [DOI] [PubMed] [Google Scholar]
- 3.Campbell K. J., Takada M., Hattori T. Co-localization of tyrosine hydroxylase and glutamate decarboxylase in a subpopulation of single nigrotectal projection neurons. Brain Res. 1991;558:239–244. doi: 10.1016/0006-8993(91)90774-p. [DOI] [PubMed] [Google Scholar]
- 4.Chu J., Wilczynski M. Androgen effects on tyrosine hydroxylase cell in the northern leopard frog, Rana pipiens. Neuroendocrinology. 2002;76:18–27. doi: 10.1159/000063680. [DOI] [PubMed] [Google Scholar]
- 5.Everitt B. J., Wu J-Y., Goldstein M. Coexistence of tyrosine hydroxylase-like and gamma-aminobutyric acid-like immunoreactivities in neurons of the arcuate nucleus. Neuroendocrinology. 1984;39:189–191. doi: 10.1159/000123977. [DOI] [PubMed] [Google Scholar]
- 6.Fujii T., Komori K., Sakai M., Yamada K., Karasawa N., Miura K., Nagatsu I. Immunocytochemical study on transient expression of tyrosine hydroxylase-immunoreactive neurons in the mouse telencephalon during postnatal development. Biog. Amines. 1992;9:115–122. [Google Scholar]
- 7.Gasper P., Berger B., Alvarez C., Vigny A., Henry J. P. Catecholaminergic innervation of the septal area in man: immunocytochemical study using TH and DBH antibodies. J. Comp. Neurol. 1985;241:12–33. doi: 10.1002/cne.902410103. [DOI] [PubMed] [Google Scholar]
- 8.Hornung P. J., Tork I., De Tribolet N. Morphology of tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex. Exp. Brain Res. 1989;76:12–20. doi: 10.1007/BF00253618. [DOI] [PubMed] [Google Scholar]
- 9.Ikemoto K., Kitahama K., Jouvet A., Arai R., Nishimura A., Nishi K., Nagatsu I. Demonstration of L-dopa decarboxylating neurons specific to human striatum. Neurosci. Lett. 1997;232:111–114. doi: 10.1016/s0304-3940(97)00587-9. [DOI] [PubMed] [Google Scholar]
- 10.Isaacs K. R., Jacobowitz D. M. Mapping of the colocalization of calretinin and tyrosine hydroxylase in the rat substantia nigra and ventral tegmental area. Exp. Brain Res. 1994;99:34–42. doi: 10.1007/BF00241410. [DOI] [PubMed] [Google Scholar]
- 11.Jaeger C. B., Joh T. J. Transient expression of tyrosine hydroxylase in some neurons of the developing inferior colliculus of the rat. Dev. Brain Res. 1983;11:128–132. doi: 10.1016/0165-3806(83)90208-0. [DOI] [PubMed] [Google Scholar]
- 12.Jaeger C. B., Teitelman G., Joh T. H., Albert V. R., Park D. H., Reis D. J. Some neurons of tha rat central nervous system contain aromatic-L-amino-acid decarboxylase but not monoamines. Science. 1983;219:1233–1235. doi: 10.1126/science.6131537. [DOI] [PubMed] [Google Scholar]
- 13.Jaeger C. B., Ruggiero D. A., Albert V. R., Joh T. H., Reis D. J. In “Handbook of Chemical Neuroanatomy”, ed. by A. Björklund and T. Hökfelt, Vol. 2. Elsevier; Amsterdam: 1984. Immunocytochemical localization of aromatic-L-amino acid decarboxylase; pp. 387–408. [Google Scholar]
- 14.Jones R. S. G. Trace biogenic amines: a possible functional role in the CNS. Trends Pharmacol. Sci. 1983;4:426–429. [Google Scholar]
- 15.Karasawa N., Yoshida M., Kawakami-Kondo Y., Okumura A., Sato T., Nagatsu I. Immunohistochemical demonstration of big monoaminergic neurons of the goldfish hypothalamus. Biog. Amines. 1984;1:133–141. [Google Scholar]
- 16.Karasawa N., Isomura G., Yamada K., Nagatsu I. Immunocytochemical localization of monoaminergic and non-aminergic neurons in the house-srew (Suncus murinus) brain. Acta Histochem. Cytochem. 1991;24:465–475. [Google Scholar]
- 17.Karasawa N., Isomura G., Nagatsu I. Production of specific antibody against L-DOPA and its ultrastructural localization of immunoreactivity in the house-shrew (Suncus murinus) lateral habenular nucleus. Neurosci. Lett. 1992;143:267–270. doi: 10.1016/0304-3940(92)90280-k. [DOI] [PubMed] [Google Scholar]
- 18.Karasawa N., Isomura G., Yamada K., Sakai K., Nagatsu I. L-DOPA immunoreactive neurons in the ventrolateral area of arcuate nucleus of the house-shrew (Suncus murinus) Biog. Amines. 1994;10:287–293. [Google Scholar]
- 19.Karasawa N., Aria R., Isomura G., Yamada K., Sakai M., Nagatsu T., Nagatsu I. D-neurons (TH-negative, AADC-positive neurons) may belong to APUD system in the laboratory shrew (Suncus murinus) brain. Biog. Amines. 1994;10:311–318. [Google Scholar]
- 20.Karasawa N., Arai R., Isomura G., Yamada K., Sakai K., Sakai M., Nagatsu T., Nagatsu I. Phenotypic changes of AADC-only immunopositive premammillary neurons in the brain of laboratory shrew Suncus murinus by systemic administration of monoamine precursors. Neurosci. Lett. 1994;179:65–70. doi: 10.1016/0304-3940(94)90936-9. [DOI] [PubMed] [Google Scholar]
- 21.Karasawa N., Arai R., Isomura G., Nagatsu T., Nagatsu I. Chemical features of monoaminergic and non-monoaminergic neurons in the brain of laboratory shrew (Suncus murinus) are changed by systemic administration of monoamine precursors. Neurosci. Res. 1995;24:67–74. doi: 10.1016/0168-0102(95)00976-0. [DOI] [PubMed] [Google Scholar]
- 22.Karasawa N., Arai R., Isomura G., Nagatsu T., Nagatsu I. Coexistence of tyrosine hydroxylase and serotonin in the raphe nucleus of the laboratory shrew (Suncus murinus) during postnatal life. Dev. Brain Res. 1997;99:121–125. doi: 10.1016/s0165-3806(96)00203-9. [DOI] [PubMed] [Google Scholar]
- 23.Karasawa N., Arai R., Isomura G., Sakai K., Takeuchi T., Nagatsu T., Nagatsu I. Postnatal colocalization of tyrosine hydroxylase and choline acetyltransferase in neurons of the dorsal motor nucleus of the vagus of the laboratory shrew (Suncus murinus) Biog. Amines. 1997;13:171–179. [Google Scholar]
- 24.Karasawa N., Arai R., Yamawaki Y., Shino M., Watanabe K., Onozuka M., Kawase T., Jacobowitz D. M., Nagatsu I. Transient coexistence of tyrosine hydroxylase and γ-aminobutyric acid immunoreactivities in the developing anterior olfactory nucleus of the mouse. Acta Histochem. Cytochem. 1999;32:333–339. [Google Scholar]
- 25.Karasawa N., Hayashi M., Katayama K., Mori T., Shimizu K., Yamada K., Nagatsu I., Iwasa M., Takeuchi T., Onozuka M. Immunohistochemical analysis of monoaminergic neurons in the brain of the common marmoset, Callithrix jacchus. Acta Histochem. Cytochem. 2005;38:353–366. doi: 10.1267/ahc.06019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitahama K., Berod A., Denoyer M., Jouvet M. Visualization of tyrosine hydroxylase-immunoreactive neurons in the cat dorsal motor vagal cells after treatment with parachlorophenylalanine. Neurosci. Lett. 1987;77:155–160. doi: 10.1016/0304-3940(87)90578-7. [DOI] [PubMed] [Google Scholar]
- 27.Kitahama K., Denoyer M., Raynaud B., Borri-Voltattorni C., Weber M., Jouvet M. Immunohistochemistry of aromatic L-amino acid decarboxylase in the cat forebrain. J. Comp. Neurol. 1988;270:337–353. doi: 10.1002/cne.902700304. [DOI] [PubMed] [Google Scholar]
- 28.Kitahama K., Mons N., Okamura H., Jouvet M., Geffard M. Endogeneous L-DOPA, its immunoreactivity in neurons of midbrain and its projection fields in the cat. Neurosci. Lett. 1988;95:47–52. doi: 10.1016/0304-3940(88)90630-1. [DOI] [PubMed] [Google Scholar]
- 29.Kitahama K., Ikemoto K., Nagatsu I., Geffard M., Okamura H., Pearson J. Aromatic L-amino acid decarboxylase and tyrosine hydroxylase immunohistochemistry in the adult human hypothalamus. J. Chem. Neuroanat. 1998;16:43–55. doi: 10.1016/s0891-0618(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 30.Kohler C., Everitt J. B., Pearson J., Goldstein M. Immunohistochemical evidence for a new group of catecholamine-containing neurons in the basal forebrain of the monkey. Neurosci. Lett. 1983;37:161–166. doi: 10.1016/0304-3940(83)90147-7. [DOI] [PubMed] [Google Scholar]
- 31.Komori K., Sakai M., Karasawa N., Yamada K., Nagatsu I. Evidence for transient expression of tyrosine hydroxylase immunoreactivity in the mouse striatum and the effect of colchicines. Acta Histochem. Cytochem. 1991;24:223–231. [Google Scholar]
- 32.Komori K., Uesaka S., Yamaoka H., Fujita K., Yamaoka K., Naitoh H., Kuroda M., Karasawa N., Ito T., Kasahara Y., Nagatsu I. Identification of L-DOPA immunoreactivity in some neurons in the human mesencephalic region: a novel DOPA neuron group? Neurosci. Lett. 1993;157:13–16. doi: 10.1016/0304-3940(93)90631-t. [DOI] [PubMed] [Google Scholar]
- 33.Kosaka T., Hataguchi Y., Hama K., Nagatsu I., Wu J.-Y. Coexistence of immunoreactivities for glutamate decarboxylase and tyrosine hydroxylase in some neurons in the periglomerular region of the rat main olfactory bulb: possible coexistence of gammaaminobutyric acid (GABA) and dopamine. Brain Res. 1985;343:166–171. doi: 10.1016/0006-8993(85)91172-2. [DOI] [PubMed] [Google Scholar]
- 34.Kosaka T., Hama K., Nagatsu I. Tyrosine hydroxylase-immunoreactive intrinsic neurons in the rat cerebral cortex. Exp. Brain Res. 1987;68:393–405. doi: 10.1007/BF00248804. [DOI] [PubMed] [Google Scholar]
- 35.Lovenberg W., Weissbach H., Udenfriend S. Aromatic L-amino acid decarboxylase. J. Biol. Chem. 1962;237:89–93. [PubMed] [Google Scholar]
- 36.Manier M., Mouchet P., Feuerstein C. Immunohistochemical evidence for the coexistence of cholinergic and catecholaminergic phenotypes in neurons of the vagal motor nucleus in the adult rat. Neurosci. Lett. 1987;80:141–146. doi: 10.1016/0304-3940(87)90643-4. [DOI] [PubMed] [Google Scholar]
- 37.Meister B., Hökfelt T., Steinbusch H. W. M., Skagerberg G., Lindvall O., Geffard M., Joh T. H., Cuello A. C., Goldstein M. Do tyrosine hydroxylase-immunoreactive neurons in the ventrolateral arcuate nucleus produce dopamine or only L-DOPA? J. Chem. Neuroanat. 1988;1:59–64. [PubMed] [Google Scholar]
- 38.Mons N., Tison F., Geffard M. Existence of L-DOPA immunoreactive neurons in the rat preoptic area and anterior hypothalamus. Neuroendocrinology. 1990;51:425–428. doi: 10.1159/000125369. [DOI] [PubMed] [Google Scholar]
- 39.Mura A., Linder J. C., Young S. J., Groves P. M. Striatal cells containing aromatic L-amino acid decarboxylase: an immunohistochemical comparision with other classes of striatal neurons. Neuroscience. 2000;98:501–511. doi: 10.1016/s0306-4522(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 40.Nagatsu I., Inagaki S., Kondo Y., Karasawa N., Nagatsu T. Immunofluorescent studies on the localization of tyrosine hydroxylase and dopamine-β-hydroxylase in the mes-, di-, and telencephalon of the rat using unperfused fresh frozen sections. Acta Histochem. Cytochem. 1979;12:20–37. [Google Scholar]
- 41.Nagatsu I., Sakai M., Yoshida M., Nagatsu T. Aromatic L-amino acid decarboxylase-immunoreactive neurons in and around the mouse and rat spinal cord. Brain Res. 1988;475:91–102. doi: 10.1016/0006-8993(88)90202-8. [DOI] [PubMed] [Google Scholar]
- 42.Nagatsu I., Komori K., Takeuchi T., Sakai M., Yamada K., Karasawa N. Transient tyrosine hydroxylase-immunoreactive neurons in the region of the anterior olfactory nucleus of pre- and postnatal mice do not contain dopamine. Brain Res. 1990;511:55–62. doi: 10.1016/0006-8993(90)90224-y. [DOI] [PubMed] [Google Scholar]
- 43.Nagatsu I., Yamada K., Karasawa N., Sakai M., Takeuchi T., Kanada N., Sasaoka T., Kobayashi K., Yokoyama M., Nomura T., Katsuki M., Fujita K., Nagatsu T. Expression in brain sensory neurons of the transgene in transgenic mice carrying human tyrosine hydroxylase gene. Neurosci. Lett. 1991;127:91–95. doi: 10.1016/0304-3940(91)90902-6. [DOI] [PubMed] [Google Scholar]
- 44.Nagatsu I., Karasawa N., Yamada K., Sakai M., Fujii T., Takeuchi T., Arai R., Kobayashi K., Nagatsu T. Expression of human tyrosine hydroxylase-chloramphenicol acetyltransferase (CAT) fusion gene in the brains of transgenic mice as examined by CAT immunocytochemistry. J. Neural Transm. 1994;96:85–104. doi: 10.1007/BF01277931. [DOI] [PubMed] [Google Scholar]
- 45.Nagatsu I., Takeuchi T., Sakai M., Karasawa N., Yamawaki Y., Arai R., Nagatsu T. Transient appearance of tyrosine hydroxylase-immunoreactive non-catecholaminergic neurons in the medial geniculate nucleus of postnatal mice. Neurosci. Lett. 1996;211:183–186. doi: 10.1016/0304-3940(96)12753-1. [DOI] [PubMed] [Google Scholar]
- 46.Okamura H., Kitahama K., Mons N., Ibata Y., Jouvet M., Geffard M. L-DOPA-immunoreactive neurons in the rat hypothalamic tuberal region. Neurosci. Lett. 1988;95:42–46. doi: 10.1016/0304-3940(88)90629-5. [DOI] [PubMed] [Google Scholar]
- 47.Okamura H., Kitahama K., Raynaud B., Nagatsu I., Borri-Voltattorni C., Weber M. Aromatic L-amino acid decarboxylase (AADC)-immunoreactive cells in the tuberal region of the rat hypothalamus. Biomed. Res. 1988;9:261–267. [Google Scholar]
- 48.Pearse A. G. E., Takor T. T. Neuroendocrine embryology and the APUD concept. Clin. Endocrinol. 1976;5:229–244. doi: 10.1111/j.1365-2265.1976.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 49.Rogers J. H. Immunohistochemical markers in rat brain: colocalization of calretinin and calbindin-D28k with tyrosine hydroxylase. Brain Res. 1992;587:203–210. doi: 10.1016/0006-8993(92)90998-o. [DOI] [PubMed] [Google Scholar]
- 50.Satoh J., Suzuki K. Tyrosine hydroxylase-immunoreactive neurons in the mouse cerebral cortex during the postnatal period. Dev. Brain Res. 1990;53:1–5. doi: 10.1016/0165-3806(90)90119-j. [DOI] [PubMed] [Google Scholar]
- 51.Stephan H., Baron G., Schwerdtfeger W. K. The Brain of the Common Marmoset (Callithrix jacchus): A Stereotaxic Atlas. Springer-Verlag; Berlin, Heidelberg: 1980. [Google Scholar]
- 52.Takada M., Sugimoto T., Hattori T. Tyrosine hydroxylase immunoreactivity in cerebellar Purkinje cells of the rat. Neurosci. Lett. 1993;150:61–64. doi: 10.1016/0304-3940(93)90108-w. [DOI] [PubMed] [Google Scholar]
- 53.Kaneko T., Tashiro Y., Sugimoto T., Nagatsu I., Kikuchi H., Mizuno N. Striatal neurons with aromatic L-amino acid decarboxylase-like immunoreactivity in the rat. Neurosci. Lett. 1989;100:29–34. doi: 10.1016/0304-3940(89)90655-1. [DOI] [PubMed] [Google Scholar]
- 54.Tison F., Mons N., Rouet-Karama S., Geffard M., Henry P. Endogeneous L-DOPA in the rat dorsal vagal complex: an immunocytochemical study by light and electron microscopy. Brain Res. 1989;497:260–270. doi: 10.1016/0006-8993(89)90271-0. [DOI] [PubMed] [Google Scholar]
- 55.Wolf M., Zigmond M. J., Kapatos G. Tyrosine hydroxylase content of residual striatal dopamine nerve terminals following 6-hydroxydopamine administration: A flow cytometric study. J. Neurochem. 1989;53:879–885. doi: 10.1111/j.1471-4159.1989.tb11786.x. [DOI] [PubMed] [Google Scholar]