Abstract
Francisella tularensis is a gram-negative intracellular bacterium, and the causative agent of tularemia. The infection can be initiated by various routes and can manifest itself in several clinical forms with the disseminated typhoidal form initiated by inhalation being most fatal. The attenuated live vaccine strain (LVS), developed almost 50 years ago, remains the sole effective tularemia vaccine, which is still only available as an Investigational New Drug for at-risk individuals. This vaccine, when given by scarification, appears to provide solid protection against subsequent systemic infection with clinical strains of F. tularensis, but its efficacy against respiratory infection is less satisfactory. In this study, we evaluated the potential of oral immunization with LVS for eliciting protection against systemic and respiratory infection with virulent F. tularensis strains in a mouse model of tularemia. Oral LVS immunization was highly effective at protecting Balb/c mice against lethal systemic or respiratory challenges with type A and type B F. tularensis. Compared to sham-immunized mice, oral LVS-immunized mice showed significant reductions in burdens of virulent F. tularensis in the lung and spleen and milder tissue damage and inflammation in the liver. The immunization induced F. tularensis -specific antibody responses in the serum and bronchoalveolar lavage fluids, as well as antigen-specific splenocyte proliferation and IFN-γ and IL-2 production. The protective efficacy was related to the size of the immunizing dose but not the number of doses administered. Like other routes of LVS immunization in mice, the protective immunity induced by oral immunization was relatively short-lived. These results suggest that oral immunization should be explored further as an alternative vaccination strategy to combat tularemia.
Keywords: Francisella tularensis, oral immunization, mucosal infection, mice
1. Introduction
Francisella tularensis is a Gram-negative facultative intracellular bacterium and the causative agent of tularemia, a highly debilitating and potentially fatal disease in humans and other mammals [1, 2]. Two subspecies of F. tularensis, subspecies tularensis (type A) and subspecies holarctica (type B), exist in nature and both are highly infectious for humans [3]. However, only type A F. tularensis routinely cause lethal infection in people especially following exposure to small particle aerosols of the pathogen; inhalation of as few as 10 virulent type A bacilli is sufficient to initiate severe disease including typhoidal tularemia with a mortality rate of 30 - 60%, if left untreated [4-6]. Because of its high infectivity, ease of dissemination by aerosol, and capacity to cause severe morbidity and mortality, type A F. tularensis has long been considered a potential biothreat agent [6].
Despite the clinical and biodefense significance of F. tularensis, currently there is no licensed tularemia vaccine or facile immunization strategy available. The attenuated live vaccine strain (LVS) of F. tularensis, developed almost 50 years ago from a clinical type B isolate [7], remains the sole vaccine available to combat tularemia. Historically, it was extensively tested for efficacy in human volunteers and was used as an investigational new drug (IND) to vaccinate at-risk individuals, primarily tularemia researchers. This vaccine, when given by scarification, protects well against subsequent exposure to large dose systemic and small dose aerosol challenge with type A F. tularensis, but appears to be less protective against large dose aerosol challenge [4, 5, 8, 9]. These findings were mimicked in animal models of tularemia using monkeys, guinea pigs and mice [10-14]. Thus, continued efforts to explore improved tularemia vaccine formulations and/or alternative immunization strategies for effective control of respiratory infection with the highly virulent type A F. tularensis are warranted. In this regard, it has been shown that, compared to systemic immunization, immunization of mice, monkeys, guinea pigs, and humans with LVS by aerosol or intranasal inoculation afforded better protection against subsequent respiratory challenge with type A F. tularensis [8, 11, 14, 15]. However, LVS is much more virulent for humans and experimental animals when administered by the respiratory route than the dermal route [8, 11, 16].
Oral immunization is a promising, and potentially safer alternative route of vaccine administration [17] that could provide better protection than scarification by stimulating the common mucosal immune system as well as inducing systemic immunity [17]. The Sabin polio vaccine, which has been instrumental in achieving the World Health Organization′s polio eradication goal, is one of the most well-known oral vaccines. Other oral vaccines that are currently licensed in the United States are the Ty21a typhoid vaccine, which is administered to travelers, and the relatively new rotavirus vaccine [18]. In this study we evaluated the feasibility and the relative efficacy of oral LVS immunization against subsequent systemic and respiratory challenge with virulent strains of F. tularensis. The results showed that oral LVS immunization protects Balb/c mice against tularemia initiated by either route with type A and type B strains of F. tularensis. These results suggest that oral route of immunization should be further explored as a potential alternative route for administration of tularemia vaccines.
2. Material and methods
2.1. Mice and F. tularensis
Six to eight-week-old female Balb/c and C57BL/6 mice were purchased from Charles Rivers Laboratories (St Constant, Quebec). The animals were housed under specific-pathogen-free conditions in a federally-licensed small animal containment level 3 facility and given free access to sterile water and certified mouse chow. The animals were maintained and used in accordance with the recommendations of the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals, and the experimental procedures were approved by the institutional animal care committee.
F. tularensis LVS (ATCC 29684) was obtained from the American Type Culture Collection (Manassas, VA). Type A F. tularensis strain FSC33/snMF (strain FSC033) was originally isolated from a squirrel in Georgia USA [19]. Type B strain FSC108/SBL R45/81 (strain FSC108) was isolated in Sweden from an ulcer of a tularemia patient [19].
2.2. Immunization of mice and challenge with F. tularensis
For oral immunization, approximately 2 × 108 cfu LVS (a dose established by pilot studies) in 0.5 ml PBS were administered by gavage via a 18-gauge feeding needle. Unless otherwise stated, the mice were immunized twice at a 2-wk interval based on the immunization schedule described by Wahid et al.[20]. For intradermal (i.d.) immunization, 2 × 105 cfu in 50 μl PBS were injected into a fold of skin in the shaved right-belly. In some experiments, sera, fecal pellets, and bronchoalveolar lavage (BAL) fluid were collected seven days after the last immunization, and used for the determination of Francisella-specific antibodies.
At various time after the immunization, some age- and sex-matched LVS- or sham-immunized mice were challenged by intranasal (i.n.) or i.d route with various strains of F. tularensis in 50 μl PBS. Actual concentrations of the inoculum in each experiment were determined by plating 10-fold serial dilutions on cystine heart agar supplemented with 1% (w/v) hemoglobin [21]. In some experiments, mice were challenged by aerosol with type A F. tularensis, as described previously [21]. Briefly, low-dose aerosols of F. tularensis were generated with a Lovelace nebulizer and a customized commercial nose-only exposure apparatus (In-tox Products, Albuquerque, NM), which delivered an initial retained dose of approximately 20 cfu F. tularensis into the lungs [21].
2.3. Quantitative bacteriology and histopathology
At various times after intranasal challenge with type A F. tularensis, groups of 5 oral LVS- or sham-immunized mice were killed by CO2 asphyxiation and their lungs and spleens were removed aseptically for quantitative bacteriology. The tissues were cut into small pieces and homogenized using an aerosol-proof homogenizer. Ten-fold serial dilutions of the tissue homogenates were plated on cystine heart agar supplemented with 1% (w/v) hemoglobin and sulfamethoxazole and trimethoprim [21]. Colonies were counted after 72 hours of incubation at 37°C. The livers were removed, fixed immediately by immersion in 10% neutral buffered formalin, and then processed by standard paraffin embedding methods for histopathology. Sections were cut 4-μm thick, stained with haematoxylin-eosin (HE) and examined by light microscopy.
2.4. Detection of F. tularensis-specific antibodies by ELISA
Levels of F. tularensis-specific antibodies in sera, fecal extracts and BAL fluid were measured by an enzyme-linked immunosorbent assay (ELISA) modified from a previously described procedure [12]. Briefly, 96-well Immulon 2 plates (Thermo Labsystems, Franklin, MA) were coated with sonicated whole-cell extracts (10 μg/ml protein; 100 μl/well) or purified LPS (5 μg/ml; 100 μl/well) of F. tularensis LVS in 0.1M sodium carbonate buffer (pH 9.5) at 4°C overnight. The plates were blocked by incubation with 3% calf serum in phosphate-buffered saline at room temperature for 1 h, and then rinsed three times with PBS with 0.05% Tween 20. Duplicates of 100 μl prediluted samples (1:100 for serum, 1:2 for fecal extract and 1:1 for BAL fluid) were added to the wells, and the plates were incubated at room temperature for 3 h. Thereafter, alkaline phosphatase-conjugated goat antibodies specific for mouse IgA, IgG, IgG1, and IgG2a (all from Caltag Laboratories, Burlingame, CA) were added for 1 h at room temperature. Color reactions were developed by the addition of ρ-nitrophenyl phosphate (pNPP) substrates (KPL, Inc., Gaithersburg, MD), and optical density was measured at 405 nm with an automated ELISA plate reader (Multiskan Ascent, Thermo Labsystems, Vantaa, Finland). Positive and negative controls for the assay included samples from mice intranasally immunized with F. tularensis antigens with cholera toxin as an adjuvant, and serum samples from naïve mice, respectively. OD values for positive controls were 1.061 for fecal IgA, 1.331 for serum IgA, 1.880 for serum IgG, 1.283 for serum IgG1, and 1.892 for serum IgG2a. OD values for negative controls were all below 0.050.
2.5. Determination of splenocyte cytokine production
In selected experiments, oral LVS- or sham-immunized mice were sacrificed and their spleens aseptically removed and used to prepare single cell suspensions. Spleen cells were suspended at a concentration of 2.5 × 106 / ml in DMEM containing 2 mM L-glutamine, 25 mM HEPES, 10% fetal bovine serum, 5 × 10-5 M 2-mercaptoethanol, 100 U of penicillin / ml, and 100 μg of streptomycin /ml in the presence of formalin-inactivated F. tularensis LVS (2 × 106 bacterial cells/ml), Con A (5 μg/ml) or medium only. The cells were cultured in duplicates in 24-well (for culture supernatant) or 96-well flat-bottom (for proliferation assay) tissue culture plates at 37°C and 5% CO2. Spleen cell proliferation was assessed according to the procedures of the CellTiter 96 AQueous One Solution cell proliferation assay kit (Promega, Madison, WI). The absorbance in the presence of culture medium only (no cells) is subtracted as background. Cell culture supernatants were collected at 48 h, centrifuged, and stored at -80°C. The levels of interleukin-2 (IL-2) and gamma interferon (IFN-γ) in the culture supernatants were measured by the Beadlyte® Mouse Multi-Cytokine Flex Kit (Upstate, Charlottesville, VA) on a Luminex® 100IS system (Luminex Corp., Austin, TX).
2.7. Statistical analysis
Data are presented as mean ± standard deviation (SD) for parametric data, and median with ranges for non-parametric data. Differences in the antibody titers, cytokine levels and the number of bacteria between groups of animals were determined by Mann-Whitney U test or one-way ANOVA followed by Bonferroni multiple pairwise comparison test, when appropriate. Survival rates between groups were compared using the Mantel–Haenszel log rank test. Differences were considered significant at P < 0.05. All statistical analyses were conducted using GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA).
3. Results
3.1. Oral immunization of mice with F. tularensis LVS
As a first step to evaluate the potential of oral LVS immunization, we examined the relative susceptibility of Balb/c and C57BL/6 mice to gavage with varying doses of LVS, and their ability to resist a subsequent systemic (i.p.) or respiratory (i.n.) challenge with lethal doses of LVS. Both Balb/c and C57BL/6 mice were relatively resistant to oral inoculation of 106 - 109 cfu LVS. An oral dose of 106 cfu failed to establish infection and only about 40% of mice succumbed to oral inoculation with 109 cfu LVS (data not shown). Moreover, oral immunization of mice with 106 cfu LVS generated no protection at all against i.p challenge and only limited protection against i.n. challenge with 2.2 × 104 cfu LVS whereas immunization with 107 cfu LVS provided full protection against i.p challenge but failed to protect all animals against i.n. challenge (Fig. 1A). On the other hand, oral immunization of mice with 108 cfu LVS caused only incidental (5%) death, which was in the same range as i.d. immunization with 2 × 105 cfu LVS in our hands, and protected all immunized animals against high dose i.p. (106 cfu, ~105 LD50) or i.n.(2 × 105 cfu, ~100 LD50) LVS challenge (Fig. 1 A and B). Similar protection efficacy was observed in C57BL/6 mice orally immunized with 108 cfu LVS (data not shown). These results indicate that oral immunization with > 107 cfu LVS elicits effective protection against normally lethal systemic and respiratory challenges with LVS.
Fig. 1.
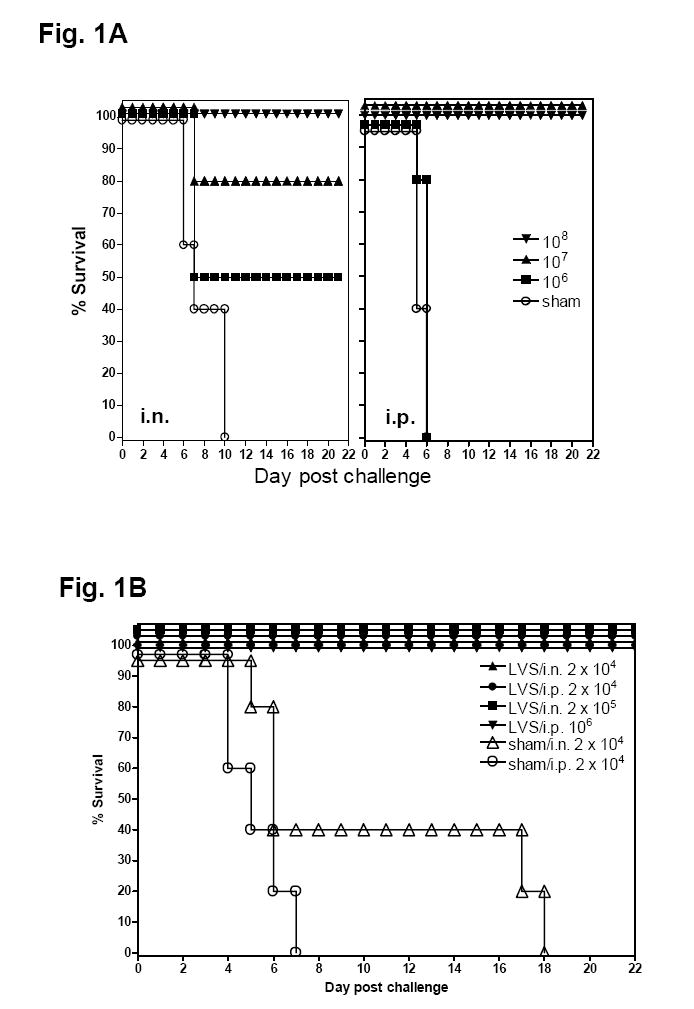
Effect of oral LVS immunization on the protection against lethal systemic or respiratory challenge with LVS. (A) Effect of the immunization doses. Groups of Balb/c mice (5-9 animals per group) were immunized by gavage with varying numbers (106 to 108 cfu) of F. tularensis LVS as indicated at day 0 and 14. The mice were challenged by either i.n. (left panel) or i.p. (right panel) route with 2.2 × 104 cfu LVS 2 weeks after the last immunization and their survival monitored. (B) Resistance to large dose LVS challenge in Balb/c mice orally immunized with LVS. Groups of Balb/c mice (5-9 animals per group) were immunized by gavage with 108 cfu F. tularensis LVS at day 0 and 14. The mice were challenged by either i.n. or i.p. route 2 weeks after the last immunization with the indicated numbers of LVS and their survival monitored.
3.2. Oral LVS immunization protects Balb/c mice against type A and type B F. tularensis infection
To explore the potential of oral LVS administration as an alternative vaccination strategy against clinical tularemia, we next determined whether it elicits effective protection against systemic and respiratory infection with virulent F. tularensis strains. Based on the dosing studies performed in the preceding section (Fig. 1A), ~108 cfu LVS was chosen as a standard oral immunization dosage in all subsequent experiments to ensure that all immunized mice received an adequate number of organisms. This dose is also in keeping with previous limited studies on oral F. tularensis infection and immunization in monkeys and humans [22, 23]. In the first series of challenge studies using virulent F. tularensis strains, orally LVS- and sham-immunized mice were challenged with about 50 cfu type A or type B F. tularensis (LD100 <10 cfu) by either the i.d. or i.n. route. As expected from our previous work [13, 21], all sham-immunized mice died after challenge with the highly virulent type A strain by day 6 and the less virulent type B strain between days 7 and 8. In contrast, all LVS-immunized mice survived the challenge except one mouse that died on day 6 or 10, respectively, after i.d. or i.n. challenge with the type A F. tularensis (Fig. 2A). Moreover, oral LVS immunization also protected most (5/8) mice against aerosol challenge with the highly virulent type A F. tularensis strain, FSC033, whereas all sham-immunized mice died by day 5 (Fig. 2B). Mice orally immunized with LVS also showed a substantial survival advantage (MTD of 10 days) over sham-immunized mice after an intranasal challenge with large doses of type A F. tularensis (up to 500 cfu), but only a minor survival advantage when challenged with 1000 cfu (MTD of 7 days)(Fig. 2C). In contrast, as previously reported by us and others [13-15], i.d. LVS immunized Balb/c mice remained highly susceptible to respiratory challenge with ~50 cfu type A F. tularensis (Fig. 2D). Similarly, oral immunization with 5 × 108 cfu LVS provided little protection to C57BL/6 mice against respiratory (i.n. and aerosol) challenge with ~20 cfu type A F. tularensis, although those mice were resistant to aerosol challenge with a high dose (~700 cfu initial retained dose in the lung) of LVS (Fig. 2E). Thus, oral immunization of Balb/c mice, but not B6 mice, with LVS led to good protection against both systemic and respiratory challenge with the highly virulent type A F. tularensis strain FSC033.
Fig. 2.
Protection against lethal challenge with virulent type A and B strains of F. tularensis in Balb/c mice orally immunized with LVS. Survival rates of Balb/c mice immunized by gavage with 108 cfu LVS and challenged. Balb/c mice were immunized by gavage with 108 cfu F. tularensis LVS (LVS-immunized) or PBS (sham-immunized) at day 0 and 14. The mice were then challenged with either type A (FSC033) or type B (FSC108) strains of F. tularensis by the indicated route with the indicated dose and their survival monitored. (A) Mice (n=8-14 per group) were challenged by either i.n. or i.d. route 3 weeks later with ~50 cfu of type A or type B F. tularensis; (B) Mice (n=5-8 per group) were challenged by aerosol 2 weeks later with low dose type A F. tularensis(initial lung retained dose ~20 cfu); (C) Mice (n=4-7 per group) were challenged i.n. 3 weeks later with 100 - 1000 cfu of type A F. tularensis; (D) Groups of Balb/c mice (n = 5 - 6 per group) were immunized either i.d with 2 × 105 cfu F. tularensis LVS, by gavage with 108 cfu F. tularensisLVS or PBS (sham-immunized) at day 0 and 14. The LVS- (i.d. or oral) or sham-immunize mice were challenged by i.n. 3 weeks later with ~65 cfu or ~10 cfu of type A F. tularensis, respectively, and their survival monitored. (E). Groups of C57BL/6 mice (n = 5) were immunized by gavage with 108 cfu F. tularensis LVS or PBS (sham-immunized) at day 0 and 14. The mice were challenged by either i.n. or aerosol 2 weeks later with type A F. tularensis or LVS as indicated and their survival monitored.
To further characterize the oral LVS immunization model, we next examined (1) whether a single oral LVS immunization is optimal for protecting mice against respiratory challenge with type A F. tularensis, and (2) the duration of such protection. To this end, groups of 5 - 11 Balb/c mice were gavaged once, twice, or thrice two days apart with 5 × 108 cfu LVS and challenged i.n. with 65 cfu type A F. tularensis 4 wks after the first immunization. A two-day rather than a two-week interval immunization protocol was implemented so that all immunizations could be completed within one week rather than 6 weeks, allowing the immunized mice to all be challenged at the same time. Also, we found that two immunizations with a two-week interval showed no protective advantage over a single immunization (data not shown). As expected, all sham-immunized mice died of the infection by dpi 5 whereas significant protection was seen in all groups of LVS-immunized mice (Fig. 3A). However, there was no statistical difference between the numbers of immunization (p>0.05), suggesting that a single oral LVS immunization induces maximal protective immunity against respiratory challenge with highly virulent type A F. tularensis.
Fig. 3.
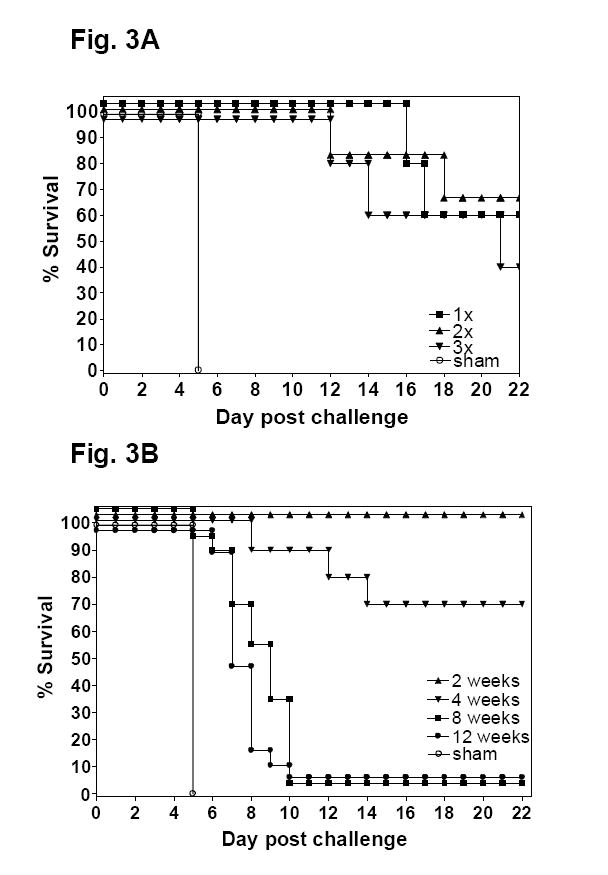
(A) Effect of the number of oral LVS immunization on the survival rate of Balb/c mice following intranasal challenge with type A F. tularensis. Groups of Balb/c mice (n = 5-11 per group) were immunized by single or multiple oral gavages with 5 × 108 cfu F. tularensis LVS every other day. The mice were challenged by i.n. 4 weeks later with 65 cfu type A F. tularensis and their survival monitored. (B). Duration of protective immunity against i.n. challenge with type A F. tularensis in Balb/c mice immunized by oral gavage with LVS. Groups of Balb/c mice (n = 6-20 per group) were immunized by oral gavage with 108 cfu F. tularensis LVS at day 0. The mice were challenged by i.n. 2, 4, 8 or 12 weeks later with ~65 cfu type A F. tularensis and their survival monitored.
To examine the duration of LVS-induced protection against respiratory type A F. tularensis infection, groups of Balb/c mice were orally immunized with 108 cfu of LVS or sham-immunized, and challenged i.n. with 62 - 103 cfu type A F. tularensis at 2, 4, 8 or 12 weeks post-immunization. As expected, all sham-immunized mice died of the infection by dpi 5 whereas the majority of LVS-immunized mice challenged at 2 or 4 wks after immunization survived the challenge (Fig. 3B). When challenged at 8 or 12 weeks post immunization, the immunized mice showed substantial survival advantages over controls with a median time to death of 9 days and 7 days, respectively. However, the majority of immunized mice eventually died of the infection (Fig. 3B). Thus, like i.d. and aerosol LVS immunization against respiratory type A F. tularensis challenge in mice [13, 15], the protective immunity induced by oral LVS immunization appears to be relatively short-lived.
3.3. Induction of antigen-specific systemic and mucosal immune responses to orally administered LVS
We next determined the types of systemic and mucosal specific immune responses elicited by oral LVS immunization in Balb/c mice. To determine the Francisella-specific antibody response, sera, fecal pellets and BAL fluids were collected from LVS- or sham-immunized mice at day 28 after oral administration of 108 cfu LVS and analyzed for levels and subtypes of antibodies directed against a sonicate whole-cell extract or purified LPS from F. tularensis LVS by ELISA. Oral LVS immunization induced low levels of Francisella-specific IgA in fecal extracts and BAL fluid (Fig. 4A). Similarly, oral immunization elicited substantial levels of SWCE-specific IgG and IgG2a, but only low levels of IgG1, in both serum and BAL fluids (Fig. 4A). The immunized mice also developed similar patterns of antibody responses to LPS purified from LVS (data not shown), suggesting that the specificity of the antibody response was primarily to LPS. These data indicated that oral LVS immunization induces both systemic and mucosal antigen-specific antibody responses with a preferable IgG2a isotype. In this regard, although it is well-recognized that antibodies play a minor role in protection against virulent type A F. tularensis infection [12, 24], the presence of systemic and mucosal Francisella-specific antibody responses, nevertheless, demonstrated the capacity of the oral immunization strategy to induce a local immune response in the lung.
Fig. 4.
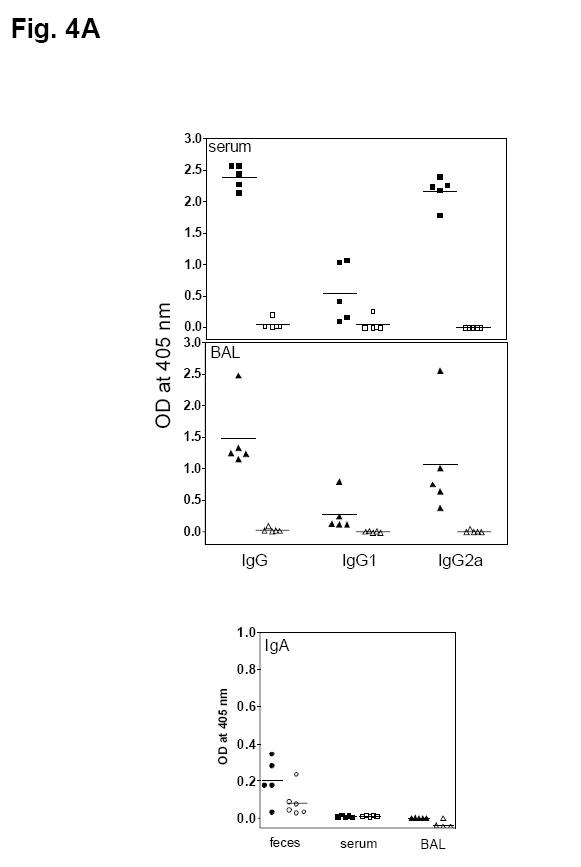
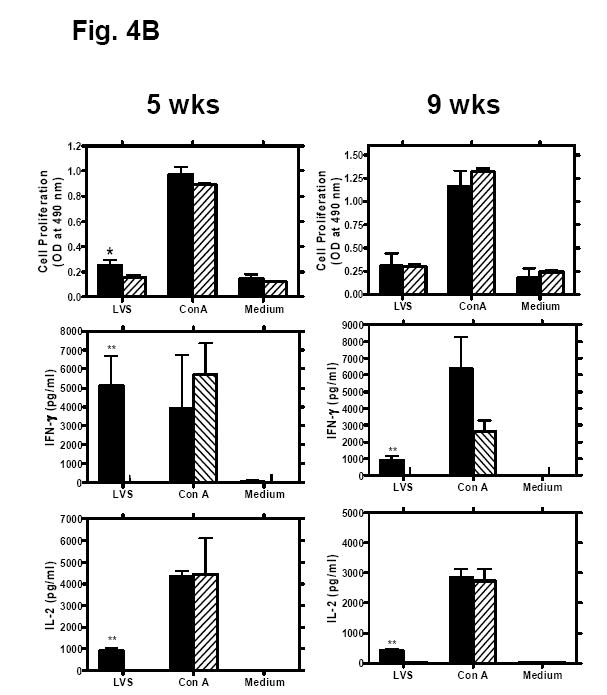
Induction of F. tularensis-specific immune responses in mice orally immunized with LVS. (A) Francisella-specific antibody responses. Groups of Balb/c mice were gavaged with either 108 cfu F. tularensis LVS (closed symbols) or PBS (open symbols) at day 0. Sera, fecal pellets, and bronchoalveolar lavage fluid were collected and processed 28 days post-immunization. The presence of Francisella-specific IgG, IgG1 and IgG2a in sera and IgA in fecal extracts, serum and lung lavage fluid was assayed by ELISA. The solid lines represent the mean of 5 mice. (B) Lymphocyte proliferation and IFN-γ and IL-2 production by splenocytes from mice orally immunized with LVS. Groups of Balb/c mice (n = 5) were orally immunized with 108 cfu F. tularensis LVS (solid bars) or PBS (hatched bars). Five or nine weeks later, spleen cells from individual mice were cultured with medium alone, Con A (5 μg/ml), or formalin-inactivated LVS (2 × 106 cells/ml). Culture supernatant fluids were collected 48 h later and assayed by Luminex® 100IS system for cytokine content. The cell proliferation was assessed 96 h later using the CellTiter 96 AQueous One Solution cell proliferation assay kit. Data are presented as means ± SD. *P < 0.05, **P < 0.01 significant difference between LVS- and shamimmunized mice.
To determine if oral LVS immunization generated an antigen-specific cellular immune response, we determined the antigen-specific lymphocyte proliferative response and the production of IFN-γ and IL-2 by the splenocytes in response to Francisella stimulation. Splenocytes harvested from LVS- or sham-immunized mice five weeks post-vaccination were cultured for 48 - 96h with formalin-inactivated LVS, Con A or medium control. Compared to sham-immunized mice, the splenocytes from LVS-immunized mice showed moderate proliferation and significant production of IL-2 (~1000 fold increase) and IFN-γ (~2000 fold increase) in response to formalin-inactivated LVS stimulation (P < 0.001)(Fig. 4B). In contrast, there was no difference in the magnitude of lymphocyte proliferation or the cytokine levels in response to Con A stimulation between sham- and LVS-immunized groups of animals (Fig. 4B). The LVS-specific T cell responses (antigen-specific proliferation and cytokine production) have also been examined at 9 wks after the immunization. Although the immunized mice showed waning of protection against i.n. challenge with type A F. tularensis at this time point, there was no significant reduction in antigen-specific IFN-γ and IL-2 production as compared to 4 weeks after the immunization (Fig. 4B). However, the T cell proliferation from these mice was reduced. Given the current lack of the knowledge about the protective antigens and the correlates of protective immunity in F. tularensis infection, the reason for the short-lived protective immunity observed in this study will be difficult to determine.
3.4. Effect of oral LVS immunization on the course of tularemia
To determine whether oral LVS immunization promotes the pulmonary clearance of F. tularensis and limits systemic dissemination of the pathogen, LVS- or sham-immunized mice were challenged i.n. with ~50 cfu type A F. tularensis and sacrificed at days 1, 3, 8 and 15 post-challenge for quantitative bacteriology (lungs and spleens) and histopathology (livers). The bacterial burdens in the lungs of LVS- and sham-immunized mice were similar at day 1 and no bacteria were recovered from the spleens of any mice at this time point (Fig. 5). At day 3 post-challenge, the number of viable bacteria increased significantly (>3 logs) in the lungs of sham-immunized mice, but increased less than one log in the lungs of LVS-immunized mice (Fig. 5). The spleens of both LVS- and sham-immunized mice were infected by this time, but the bacterial burden was significantly lower in LVS-immunized mice than sham-immunized mice (Fig. 5). Whereas all sham-immunized mice died of infection 5 days after challenge, LVS-immunized mice harbored low numbers of F. tularensis in both lungs and spleens throughout the duration of the experiment (Fig. 5). The livers from sham- and LVS-immunized mice killed at day 1 showed essentially similar histopathological changes consisting of small foci of infiltrating neutrophils and mononuclear cells with occasional degenerating or apoptosis-like hepatocytes. The number and size of inflammatory foci increased substantially in the livers of sham-immunized mice by day 3 and contained small to large numbers of neutrophils or mixed mononuclear cells and neutrophils (Fig. 6A). In contrast, the livers from LVS-immunized mice showed relatively mild changes and contained only small foci of monocytic inflammatory aggregates (Fig. 6B). All sham-immunized mice died of the infection by day 5 whereas the livers from LVS-immunized mice killed on day 15 showed the presence of small foci of mononuclear infiltration with occasionally active inflammatory responses in the parenchymal and periportal areas (Fig. 6C). These results suggest that (1) the protective immunity elicited by oral LVS immunization is likely due to the induction of both pulmonary and systemic protective immunity to reduce the rapid growth of F. tularensis rather than containment of the pathogen at the site of entry; and (2) oral LVS immunization is able to rapidly control, but not completely sterilize, a subsequent respiratory challenge with type A F. tularensis.
Fig. 5.
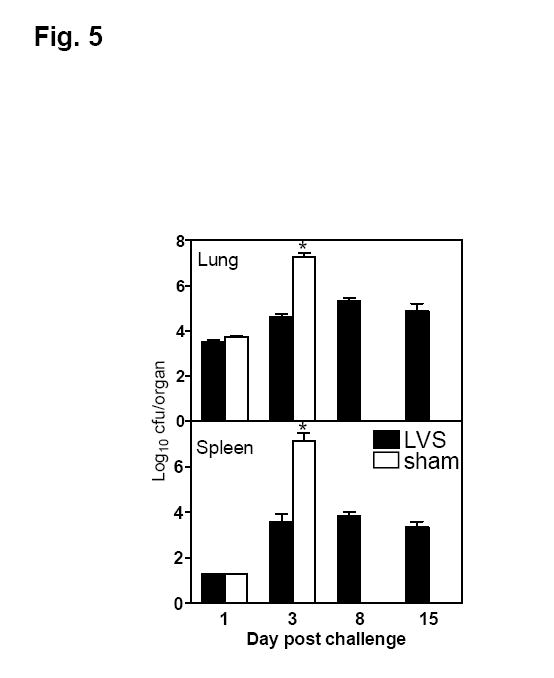
Course of infection with type A F. tularensis in Balb/c mice orally immunized with F. tularensis LVS or PBS. Mice were immunized by gavage with 108 cfu F. tularensis LVS or sham-immunized with PBS at day 0 and 14, and challenged i.n. 3 weeks later with 50 cfu type A F. tularensis. Bacterial burdens in the lungs and spleens of LVS- or sham-immunized mice on various days after challenge were determined. Results are expressed as mean ± standard deviation from 5 mice per group per time point. Results were analyzed by analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. *P < 0.05, significant difference between LVS- and sham-immunized mice.
Fig. 6.
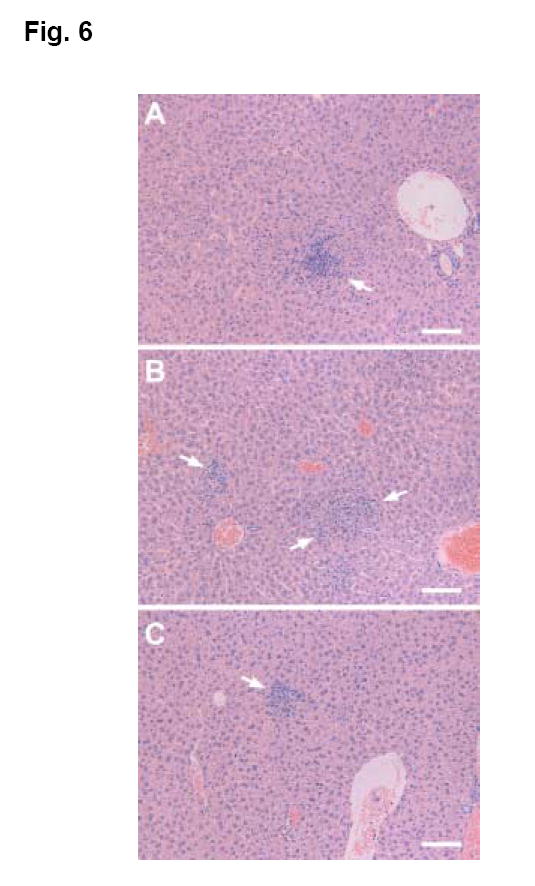
Hepatic lesions in LVS- or sham-immunized Balb/c mice after i.n. challenge with 50 cfu type A F. tularensis. The mice were orally immunized on day 0 and 14 with approximately 5 × 108 cfu LVS (LVS-immunized) or PBS (sham-immunized), and challenged by i.n. 4 wks later with type A F. tularensis. (A). The liver from a LVS-immunized mouse killed at 3 days after challenge showing the presence of a small focal infiltration of mixed neutrophils and mononuclear cells (arrow). (B). The liver from a sham-immunized mouse killed at 3 days after challenge showing the presence of multiple medium-sized inflammatory foci (arrows). (C). The presence of small inflammatory infiltrates (arrow) in the liver from a LVS-immunized mouse survived from i.n. challenge with type A F. tularensis and killed at day 15. H&E. Bar=100 μm.
4. Discussion
A safe and effective tularemia vaccine that provides solid protection against all forms of F. tularensis infection in humans would be valuable for individuals who inhabit tularemia endemic regions or who may be occupationally exposed to this pathogen. Limited epidemiological evidence and experimental studies in human volunteers and animal models indicate that systemic vaccination with LVS provides solid protection against subsequent massive systemic challenge with highly virulent type A F. tularensis, and, to a lesser extent, against the airborne pathogen [4, 5, 8, 11, 13-15, 24-26]. This could be a concern because typhoidal tularemia, the deadliest form of the disease with a mortality rate of >30% if untreated, is thought to be initiated by inhalation of the pathogen and the respiratory route is considered a likely portal in the event of a bioterrorist attack [6]. On the other hand, it has been shown previously in humans, guinea pigs, and monkeys [10, 11], and more recently in mice [14, 15] that aerosol or intranasal administration of LVS enhances protection against subsequent respiratory challenge with virulent type A F. tularensis, suggesting that the induction of local mucosal immune responses may be important for the control of tularemia initiated via the respiratory route. However, LVS retains residual virulence when administered as an aerosol and can cause overt tularemia in experimental animals and humans [8, 11, 15]. Therefore, the present study evaluated the potential of oral LVS immunization for its ability to elicit a protective immune response against systemic and respiratory tularemia using murine models of the disease.
There have been relatively few studies in the literature on oral infection or immunization with F. tularensis. It has been previously shown that oral administration of 1010 cfu LVS to human volunteers or monkeys induced antigen-specific antibody responses at least as quickly as i.d. immunization [22, 23]. This immunization regimen appeared to be safe with no overt clinical complications in either monkeys or humans [22, 23], and induced protective immunity against aerosol challenge with type A F. tularensis at least as effectively as, if not better than, i.d. immunization. The current study showed the same phenomenon in Balb/c mice. In particular, oral LVS immunization was highly effective in protecting Balb/c mice against lethal challenges, including respiratory challenges with type A and type B F. tularensis especially when challenge occurred within two months after immunization (Fig. 2). The protective efficacy was related to the size of the immunizing dose but not the number of doses administered. Immunization resulted in the induction of F. tularensis-specific systemic and mucosal antibody responses, moderate lymphocyte proliferation and increased production of IFN-γ and IL-2 by splenocytes in response to Francisella antigen stimulation. Taken together, these data suggest that oral immunization could be an attractive alternative to vaccinate humans against tularemia. However, like i.d. and aerosol immunization [13, 15], the protective immunity against respiratory tularemia induced by oral immunization was rather short-lived in Balb/c mice, and completely ineffective in C57BL/6 mice. The reasons why LVS-immunized C57BL/6 mice remained susceptible to i.n. challenge with virulent Type A F. tularensis remain unknown. A similar phenomenon has also been observed when other routes of LVS immunization were used [13, 14]. We have previously speculated [13] that LVS does not elicit the same immune response in C57BL/6 mice compared to BALB/c mice. Alternatively, LVS might elicit an identical immune response in both mouse strains, but it might only function to control tularemia in BALB/c mice. Since we currently do not known what antigens and immune responses (correlates of protection) are essential for the protection against virulent type A F. tularensis infection and lack established assays and reagents for examining Francisella-specific cellular immunity, it is therefore difficult to dissect the mechanism of protection by comparing immune responses between the two mouse strains.
Likewise, although the current study has demonstrated the effectiveness of oral LVS immunization, the protective mechanisms remain to be determined. In this study, we have shown that oral LVS immunization induces specific IgG and IgA responses in the serum and BAL fluid and specific lymphocyte responses in the spleen (Fig. 4). This suggests that the immunization induced antigen-specific T cell and antibody-secreting B cell responses at both systemic and respiratory lymphoid tissues. Although our results imply that the induction of mucosal immunity is essential for effective protection against inhalation challenge with highly virulent type A F. tularensis strain FSC033, the precise mechanisms responsible for this immunity remain to be characterized. It will also be interesting, in the future studies, to determine whether or not the short-lived protective immunity in Balb/c mice can be boosted although the selection of appropriate antigens and vaccine formulations for such boosting is likely to be challenging. Further understanding of these processes will be critical for devising improved vaccines against tularemia initiated via the pulmonary route.
We and others have suggested that it is the disseminated systemic infection and ensuing host tissue damage rather than the localized pulmonary infection that kills the host infected by the respiratory route with type A and B F. tularensis[4, 21]. In this regard, in the present study oral vaccination was successful, not by simply preventing F. tularensis dissemination from the lungs to extrapulmonary organs, since the spleens of both LVS- and sham-immunized mice became infected at the same time. Rather, the immunized mice effectively prevented virulent type A F. tularensis from proliferating to lethal levels both locally and systemically, thereby limiting the infection-associated tissue damage and inflammation. However, the present study did not allow us to determine the extent to which the reduced disseminated infection was due to a reduced reservoir in the lungs versus direct killing within extrapulmonary organs. Further dissection of such correlations will be useful for the future development of tularemia vaccines since this will address the necessity of inducing long term memory T cells in the lung.
In summary, oral LVS immunization protects Balb/c mice against lethal systemic and respiratory challenges with virulent type A and type B strains of F. tularensis. These findings warrant additional exploration of oral vaccination as an alternative strategy for administering live attenuated human tularemia vaccines. In this regard, oral immunization has been successfully used for several clinical vaccines [17, 18] and oral administration of LVS to human volunteers has been shown to be safer than respiratory administration [8, 11, 23]. The mouse model of oral LVS immunization described here will be useful for dissecting the protective mechanism against respiratory infection with virulent F. tularensis and for evaluating newly developed tularemia vaccines and other immunization strategies.
Acknowledgments
We thank Dr. John Cherwonogrodsky (Defence Research and Development Canada, Suffield, Alberta) and Dr. Malcolm Perry (National Research Council, Institute for Biological Sciences, Ottawa, Canada) for kindly providing the initial stock of FSC 033 F. tularensis and F. tularensis LVS lipopolysaccharide, respectively. We also thank Hua Shen and Maria Bùsa for technical assistance and Tom Devecseri for his expert assistance in the preparation of photomicrographs. This work was partially supported by grants R21AI59064 and R01AI 48474 from the National Institutes of Health and by the National Research Council Canada.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11(3):440–51. [PubMed] [Google Scholar]
- 2.Tarnvik A, Berglund L. Tularaemia. EurRespirJ. 2003;21(2):361–73. doi: 10.1183/09031936.03.00088903. [DOI] [PubMed] [Google Scholar]
- 3.Sjostedt A, Brenner DJ. Francisella Bergey’s manual of systematic bacteriology. Berlin: Springer-Verlag; 2002. [Google Scholar]
- 4.Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch Int Med. 1961;107:702–14. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- 5.Saslaw S, Eigelsbach HT, Wilson HE, Prior JA, Carhart S. Tularemia vaccine study. I. Intracutaneous challenge. 1961;107:689–701. doi: 10.1001/archinte.1961.03620050055006. [DOI] [PubMed] [Google Scholar]
- 6.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Tularemia as a biological weapon: medical and public health management. Jama. 2001;285(21):2763–73. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 7.Eigelsbach HT, Downs CM. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961;87:415–25. [PubMed] [Google Scholar]
- 8.Hornick RB, Eigelsbach HT. Aerogenic immunization of man with live Tularemia vaccine. Bacteriol Rev. 1966 Sep;30(3):532–8. doi: 10.1128/br.30.3.532-538.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke DS. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977;135(1):55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- 10.Hodge FA, Leif WR, Silverman MS. Susceptibility to infection with Pasteurella tularensis and the immune response of mice exposed to continuous low dose rate of gamma radiation: NRDL-TR-68-85. Res Dev Tech Rep. 1968:1–28. [PubMed] [Google Scholar]
- 11.Eigelsbach HT, Tulis JJ, Overholt EL, Griffith WR. Aerogenic immunization of the monkey and guinea pig with live tularemia vaccine. Proc Soc Exp Biol Med . 1961 Dec;108:732–4. doi: 10.3181/00379727-108-27049. [DOI] [PubMed] [Google Scholar]
- 12.Conlan J, Shen H, Webb A, Perry M. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine. 2002;20(2930):3465. doi: 10.1016/s0264-410x(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Shen H, Webb A, KuoLee R, Conlan JW. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine. 2003;21:3690–700. doi: 10.1016/s0264-410x(03)00386-4. [DOI] [PubMed] [Google Scholar]
- 14.Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun . 2005 May;73(5):2644–54. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conlan JW, Shen H, Kuolee R, Zhao X, Chen W. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alphabeta T cell- and interferon gamma- dependent mechanism. Vaccine . 2005 Mar 31;23(19):2477–85. doi: 10.1016/j.vaccine.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Conlan JW, KuoLee R, Shen H, Webb A. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb Pathog. 2002;32(3):127–34. doi: 10.1006/mpat.2001.0489. [DOI] [PubMed] [Google Scholar]
- 17.Brayden DJ. Oral vaccination in man using antigens in particles: current status. European Journal of Pharmaceutical Sciences. 2001;14(3):183–9. doi: 10.1016/s0928-0987(01)00175-0. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich G, Griot-Wenk M, Metcalfe IC, Lang AB, Viret J-F. Experience with registered mucosal vaccines. Vaccine. 2003;21(78):678–83. doi: 10.1016/s0264-410x(02)00579-0. [DOI] [PubMed] [Google Scholar]
- 19.Johansson A, Ibrahim A, Goransson I, Eriksson U, Gurycova D, Clarridge JE, III , et al. Evaluation of PCR-based methods for discrimination of Francisella species and subspecies and development of a specific PCR that distinguishes the two major subspecies of Francisella tularensis. J Clin Microbiol. 2000;38(11):4180–5. doi: 10.1128/jcm.38.11.4180-4185.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. Cell-mediated immune responses in humans after immunization with one or two doses of oral live attenuated typhoid vaccine CVD 909. Vaccine. 2007 Feb 9;25(8):1416–25. doi: 10.1016/j.vaccine.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conlan JW, Chen W, Shen H, Webb A, KuoLee R. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. MicrobPathog. 2003;34(5):239–48. doi: 10.1016/s0882-4010(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 22.Tulis JJ, Eigelsbach HT, Hornick RB. Oral vaccination against tularemia in the monkeys. Proc Soc Exp Biol Med. 1969;132(3):893–7. doi: 10.3181/00379727-132-34331. [DOI] [PubMed] [Google Scholar]
- 23.Hornick RB, Dawkins AT, Eigelsbach HT, Tulis JJ. Oral tularemia vaccine in man. Antimicrob Agents Chemother. 1966 1967:11–4. doi: 10.1128/AAC.6.1.11. [DOI] [PubMed] [Google Scholar]
- 24.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19(31):4465–72. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 25.Eigelsbach HT, Tulis JJ, McGavran MH, White JD. Live tularemia vaccine. I. Host-parasite relationship in monkeys vaccinated intracutaneously or aerogenically. J Bacteriol. 1964;84:1020–27. doi: 10.1128/jb.84.5.1020-1027.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green M, Choules G, Rogers D, Titball RW. Efficacy of the live attenuated Francisella tularensis vaccine (LVS) in a murine model of disease. Vaccine . 2005 Apr 8;23(20):2680–6. doi: 10.1016/j.vaccine.2004.03.071. [DOI] [PubMed] [Google Scholar]


