Abstract
The radiologist makes a valuable contribution to the staging of laryngeal cancer and this has a direct influence on treatment planning. This review focuses on the main anatomical concepts, patterns of tumour spread and how to detect this with optimal cross sectional imaging. Issues surrounding the relationship of tumour to the ventricular complex, submucosal laryngeal spaces, anteroposterior extension, laryngeal cartilage involvement and metastatic spread are discussed and illustrated. The impact of these imaging findings on the array of therapeutic options is described.
Keywords: Laryngeal cancer, staging, treatment planning, therapeutic options
Introduction
The incidence of laryngeal cancer ranges from 2.5 to 17.2 per 100,000 per year[1] and it represents approximately 3% of new malignancy diagnosed annually worldwide. Ninety percent of laryngeal malignancy is due to squamous cell carcinoma with lymphoma being the second most frequent diagnosis. Most patients with laryngeal carcinoma develop hoarseness however other clinical presentations include a neck mass, dysphagia, stridor and haemoptysis[2]. Impaired laryngeal function from carcinoma and its treatment results in marked disturbance of communication, breathing and swallowing.
Prior to any imaging request, a patient with suspected laryngeal carcinoma should be investigated with endoscopy which allows the tumour to be directly visualised. However, whilst the mucosal extent of the tumour and cord mobility is best assessed with endoscopy, cross sectional imaging is required to determine submucosal extent and invasion of adjacent structures. The combined information allows the tumour to be classified according to the relevant T staging (Tables 1–3)[3]. Imaging also provides important information concerning nodal metastasis, systemic metastasis, the presence of synchronous tumours and recurrent disease. Critical to the radiological evaluation of laryngeal carcinoma is an understanding of the imaging anatomy, patterns of infiltration and how the spread of tumour impacts on treatment options.
Table 1.
T staging of glottic carcinoma
| T1 | Tumour limited to vocal cord(s) with normal mobility |
|---|---|
| T1a | Tumour limited to one vocal cord |
| T1b | Tumour involves both vocal cords |
| T2 | Tumour extends to supra- and/or subglottic larynx or region outside the supraglottis (with or without impaired vocal cord mobility) |
| T3 | Tumour limited to the larynx with vocal cord fixation and/or invades paraglottic space, and/or minor thyroid cartilage erosion (e.g. inner cortex) |
| T4a | Tumour involves tissues beyond the larynx and/or laryngeal cartilage (e.g. trachea, deep extrinsic tongue muscles, strap muscles, thyroid or oesophagus) |
| T4b | Tumour invades prevertebral space, encases carotid artery or invades mediastinal structures |
Table 2.
T staging of supraglottic carcinoma
| T1 | Tumour limited to one subsite with normal vocal cord mobility |
|---|---|
| T2 | Tumour involves mucosa of more than one supraglottic subsite or glottis or extralaryngeal mucosa (e.g. piriform sinus) with normal cord mobility |
| T3 | Tumour limited to larynx with vocal cord fixation and/or invasion of postcricoid, pre-epiglottic region, paraglottic space and/or minor thyroid cartilage erosion (e.g. inner cortex) |
| T4a | Tumour involves tissues beyond the larynx and/or laryngeal cartilage (e.g. trachea, deep extrinsic tongue muscles, strap muscles, thyroid or oesophagus) |
| T4b | Tumour invades prevertebral space, encases carotid artery or invades mediastinal structures |
Table 3.
T staging of subglottic carcinoma
| T1 | Tumour limited to the subglottis |
|---|---|
| T2 | Tumour extends to the vocal cords with normal or impaired cord mobility |
| T3 | Tumour limited to larynx with vocal cord fixation |
| T4a | Tumour involves tissues beyond the larynx and/or laryngeal cartilage (e.g. trachea, deep extrinsic tongue muscles, strap muscles, thyroid or oesophagus) |
| T4b | Tumour invades prevertebral space, encases carotid artery or invades mediastinal structures |
Data from American Joint Committee on Cancer[3].
Therapeutic options for laryngeal carcinoma
Therapy for laryngeal carcinoma includes surgery, radiotherapy and chemotherapy alone or in combination. Therapeutic decisions are based on evaluation of disease extent and performance status, and are aimed at improving survival and functional outcome.
Low volume T1/T2 supraglottic and glottic tumours may be treated with definitive radiotherapy alone. Advanced tumours usually undergo a combination of surgery and radiotherapy, possibly reserving the surgery for radiotherapy failures. “Larynx preservation” regimes using definitive radiotherapy following initial volume reduction with chemotherapy (and prompt surgical salvage for treatment failures) have proved equivalent to conventional surgery and radiotherapy in some studies of advanced tumours[4].
There are several conservative voice preserving surgical options for laryngeal carcinoma. For supraglottic carcinoma this includes: laser resection with preservation of laryngeal integrity for small epiglottic tumours and speech conserving partial laryngectomy for some larger tumours. The latter includes horizontal supraglottic laryngectomy which is a resection superior to the plane of the laryngeal ventricle. Any transglottic tumour, paraglottic tumour or extensive laryngeal cartilage invasion would contraindicate such surgery. Very small glottic carcinomas (and larger tumours in some centres) may be treated with endoscopic laser resection as an alternative to radiotherapy. Some T1/T2 glottic carcinomas that do not involve more than a 1/3 of the contralateral vocal cord may be amenable to a vertical hemilaryngectomy (removal of the ipsilateral vocal cord, up to 1/3 of the contralateral vocal cord and the adjacent thyroid cartilage). Transglottic tumours may be treated with a supracricoid laryngectomy with cricohyoidpexy (cricohyoidoepiglottopexy if the suprahyoid epiglottis is preserved). This is the most extensive partial laryngeal surgery which will allow removal of the glottic as well as supraglottic structures. Fixation of the arytenoids cartilage, massive infiltration of the pre-epiglottic space and subglottic extension would contraindicate this surgery.
If partial laryngeal surgery is precluded, then a total laryngectomy is required. It is the only surgical option if there is subglottic carcinoma or extension or if the piriform sinus apex or postcricoid region are infiltrated. Invasion of cartilage, cord fixation or direct extralaryngeal spread would prompt combined surgery and radiotherapy.
Choice of imaging modality
Cross sectional imaging of the larynx may be performed with computed tomography (CT) or magnetic resonance imaging (MRI). Multislice CT allows the radiologist to evaluate almost all the relevant imaging issues. Voltage of 120–140 kVp, tube current of at least 180 mAs, display matrix of 512 × 512 pixels and a collimation of approximately 1 mm should be used. A single bolus of contrast medium is effective, however a biphasic contrast enhancement protocol may be employed. A longitudinal field of view extends from the skull base to the sternoclavicular joints with the patient breathing quietly and not swallowing. It may be helpful to perform an additional examination focused on the tumour with e-phonation (for better assessment of the laryngeal ventricle, anterior commissure and aryepiglottic folds) or modified valsalva (for better assessment of the priform sinus and post cricoid regions) manoeuvres. In addition to soft tissue windows, the bone windows (reconstructed with a bony algorithm) should be routinely evaluated if the tumour contacts ossified laryngeal cartilage. It should be ensured that axial images are reformatted in the plane of the larynx. Recent studies have explored the use of post processing such as ‘virtual endoscopy’ which maybe useful in evaluating features such as subglottic extension[5].
MRI is best performed with a high field MRI scanner. The MR examination requires a neck coil to obtain adequate resolution. Section thickness should be 4–5 mm for the neck coverage and 2–3 mm (0.1–0.2 mm interslice gap) for the focused study of the larynx. The display matrix size is 170 × 256 pixels minimum. A combination of T2-w sequences with fat saturation, T1-w sequences and T1-w fat saturated sequences with gadolinium, should be used in axial and coronal planes. The sagittal plane is also useful to assess potential tongue base involvement. Patients are asked to refrain from coughing and swallowing during the acquisition and MRI may produce poor results in the breathless or restless patient who is compromised by the tumour.
Since approaches with both CT and MRI are acceptable, the choice in practice depends on machine availability and local expertise as well as the ability of the patient to tolerate a prolonged MRI examination. MR imaging is most frequently used if there is uncertainty in assessing cartilage involvement, when this is critical to therapeutic decisions. It may also better define the margin between the tumour and thyroarytenoid muscle and involvement of the tongue base.
Ultrasound with FNA may be routinely used in some centres for the assessment of nodal metastasis. Although it may be used to visualise the anterior laryngeal structures[6], it is not sufficient for adequate primary tumour staging. Endolaryngeal high frequency ultrasound has been evaluated as a diagnostic tool[7].
Positron emission tomography (PET) and CT-PET may also be useful for the detection of recurrent tumour and in distinguishing this from post radiotherapy change[8]. A negative PET scan excludes recurrence with a high certainty. Positive PET scan findings should be assessed with biopsy and follow up PET imaging if this is negative. It should be noted that physiological uptake of [18F]fluorodeoxyglucose ([18F]FDG) is observed due to vocal cord activity, so a ‘silent protocol’ should be employed.
Key imaging considerations
Tumour volume
Tumour volume is a critical factor in predicting tumour control following radiotherapy. Supraglottic tumours measuring less than 6 ml and T3 glottic tumours measuring less than 3.5 ml have been demonstrated to have improved local control following radiotherapy[9,10]. There is also some data which suggests that tumour volume is strongly associated with local control following surgery[11]. Although tumour volume is closely associated with T stage and other findings such as cartilage invasion, it does appear to act as an independent predictor of survival.
Relationship of the tumour to the ventricular complex
A key component of the imaging assessment is to define the three anatomical sites of supraglottis, glottis and subglottis. The glottis includes the true vocal cord, anterior and posterior commissures and is defined as stretching from the ventricle to a plane 1 cm below the free margin of the vocal cord. Below this, the glottis merges with the subglottis which extends inferiorly to the caudal aspect of the cricoid cartilage. Above this, the supraglottis spans the laryngeal segment from the tip of the epiglottis to the ventricle. Its components are the epiglottis, false cord, ventricle, aryepiglottic folds and arytenoids cartilages. Transglottic is a term used to describe tumours encroaching on both glottis and supraglottis with or without subglottic components and when the site of origin is unclear (these usually develop from glottic tumours).
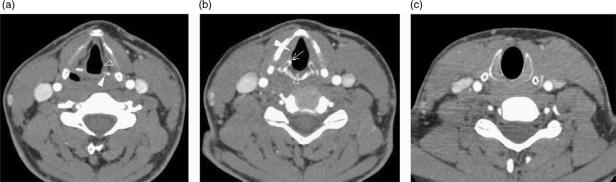
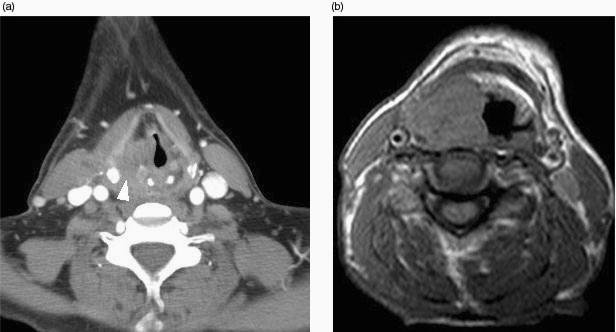
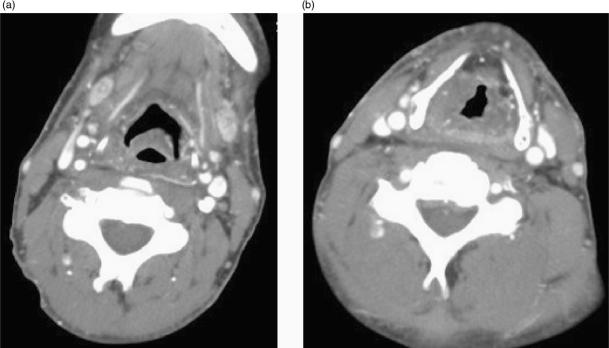
In order to define the tumour as supraglottic, glottic or subglottic, the radiologist must determine the level of the ventricular complex (comprising the true cord, false cord and the intervening ventricle). These landmarks are essentially mucosal, and the endoscopist will frequently be able to make this assessment. However, bulky tumours may hinder distal visualisation (particularly in the subglottic region) and tumour may not be seen endoscopically beneath an intact mucosa. The superior aspect of the ventricular complex is defined by the superior margin of the arytenoids cartilages (Fig. 1a). The false cords are seen at this level running parallel and superior to the true cords. The inferior aspect of the ventricular complex is defined by the true cords. This is located radiologically by identifying a transition of paraglottic fat to soft tissue in the wall of the larynx. This soft tissue represents the thyroarytenoid muscle (Fig. 1b) which stretches from the arytenoids cartilages posteriorly to the thyroid cartilage anteriorly. In addition, the true cord lies at the upper margin of the cricoid cartilage (at the level of the anteriorly pointing vocal processes of the arytenoid cartilages). The posterior commissure is seen between the arytenoids cartilages where there should be only a thin mucosal layer and the anterior commissure (Fig. 1b) is seen at the anterior junction of the two vocal cords where soft tissue should be only 1–2 mm thick[12]. The subglottic region has a characteristic appearance where the cricoid cartilage is seen as a complete ring providing the foundation of the laryngeal skeleton (Fig. 1c). Any soft tissue at the subglottic level is abnormal. The presence of an enlarged delphian node anterior to the trachea also indicates subglottic involvement.
Figure 1.
Axial CT scans through (a) false cords, (b) true cords, (c) subglottis to illustrate normal anatomy. (a) The tip of the arytenoids cartilages (white arrowhead) indicates the level of the false cords (open arrowhead) and also the superior aspect of the ventricular complex. (b) The anteriorly pointing vocal processes of the arytenoids (arrow) and the thyroarytenoid muscle (white arrowhead) are effacing the paraglottic fat within the wall of the larynx. This defines the level of the true cords and also the inferior aspect of the ventricular complex. The anterior and posterior (open arrowhead) commissures should only be represented by a thin mucosal layer. (c) There should be no soft tissue seen internal to the ring of the cricoid cartilage at subglottic level.
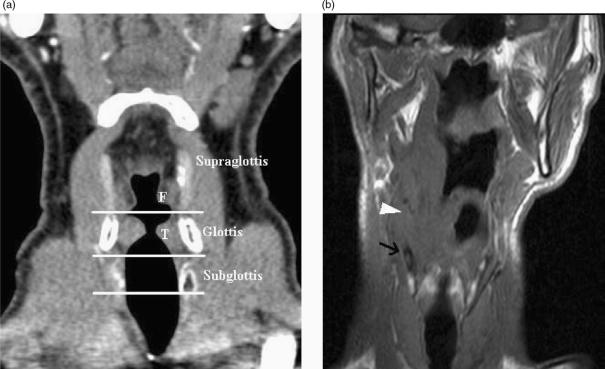
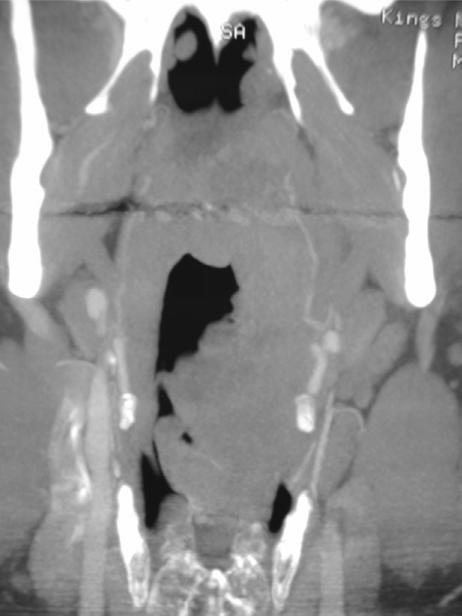
Imaging identification of transglottic extension is a further indicator that primary radiotherapy may fail. Transglottic extension by supraglottic carcinoma will contraindicate a supraglottic laryngectomy, transglottic extension by glottic carcinoma with contraindicate vertical hemilaryngectomy, whilst extension to the subglottis excludes all voice conserving partial laryngeal surgery. Coronal reformats or scanning is helpful in the assessment of such infiltration (Fig. 2), particularly if no ‘normal’ axial section is seen between the tumour and the soft tissue of the thyroarytenoid muscle.
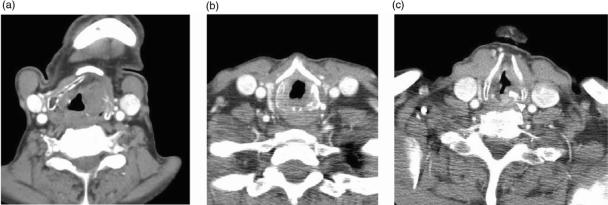
Figure 2.
(a) Coronally reformatted CT demonstrates the supraglottis, glottis and subglottis as defined by the level of the ventricular complex (false cords (F), true cords (T) and intervening ventricle). (b) T1-w coronal image demonstrates a supraglottic tumour extending inferiorly to ventricular level (arrowhead). Low T1-w signal (arrow) in the thyroid cartilage is non specific for reactive change or tumour invasion.
Involvement of the submucosal spaces
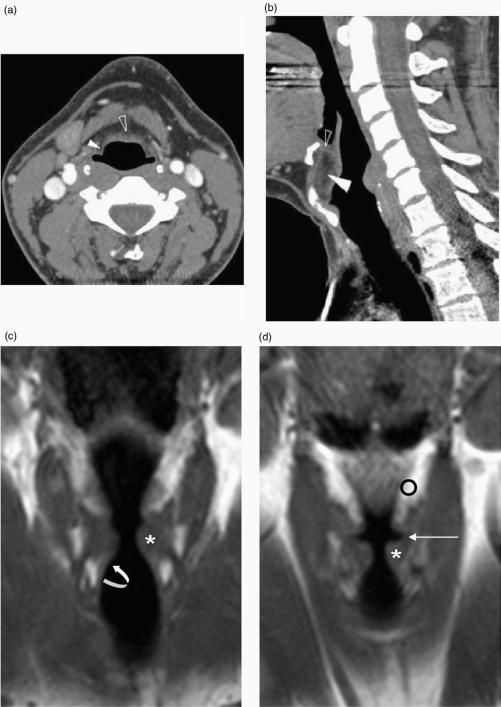
Submucosal involvement of the pre-epiglottic and paraglottic spaces is difficult to evaluate clinically and endoscopically. The fatty C-shaped pre epiglottic space is bounded anteriorly by the thyroid cartilage and thyrohyoid membrane and posteriorly by the epiglottis and quadrangular membrane (Figs. 1a and 3a,b). The fat and connective tissue within the paraglottic space is confined by the thyroid cartilage and cricothyroid membrane anterolaterally (Fig. 1a), inferomedially by the conus elasticus, superomedially by the ventricular complex or vestibule (Fig. 3c,d) and posteriorly by the piriform sinus (Fig. 1a). The pre-epiglottic space is continuous laterally with the paired paraglottic spaces (Fig. 3a) and whilst the differentiation between these spaces is important for T classification, the border zone is not detectable on imaging.
Figure 3.
(a) Axial, (b) sagittal reformatted CT images with (c) and (d) T1-w coronal MR images illustrating the submucosal laryngeal spaces. (a) The pre-epiglottic space (open arrowhead) and paraglottic space (white arrowhead) are seen in continuity. (b) More superiorly the pre-epiglottic space (white arrowhead) is bound anteriorly by the thyrohyoid membrane (see also (a)) whereas the thyroid cartilage is present anteriorly at a more inferior level (see also Fig. 1a). The epiglottis is present posteriorly. Soft tissue seen within the superior aspect of the pre-epiglottic space (open arrowhead) corresponds to the hyoepiglottic ligament. (c) The medial relations of the paraglottic fat (o) are seen to be the vestibule, the ventricle (long arrow) and the conus elasticus more inferiorly (curved arrow). Laterally it is related to thyroid cartilage and cricothyroid membrane. The true vocal cord is indicated (*).
An understanding of the pre-epiglottic and paraglottic spaces allows the radiologist to predict and identify patterns of tumour spread which have a critical impact on therapeutic decisions.
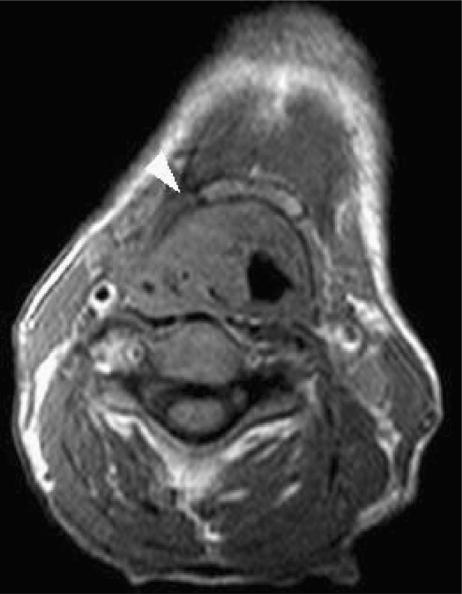
Epiglottic carcinoma may spread directly into the pre-epiglottic space through dehiscences in the epiglottic cartilage. When tumour has accessed the pre-epiglottic space, an avascular zone at its inferior border limits inferior extension, however there is a potential pathway for both subglottic extension and involvement of the adjacent thyroid cartilage. Lateral extension encroaches on the paraglottic space. The ‘pushing’ nature of tumour within the pre-epiglottic space rarely results in invasion of the hyoid bone, but both the hyoid bone and the tongue base are at risk (Fig. 4).
Figure 4.
Axial T1-w image demonstrates bulky invasion of the right pre-epiglottic space by a supraglottic carcinoma which resulted in invasion of the hyoid bone.
Paraglottic space involvement may occur secondary to glottic and supraglottic carcinoma. Carcinoma of the mid or posterior glottis may spread laterally into the paraglottic space and this often correlates with clinical evidence of vocal cord fixation. Paraglottic spread is also a key pathway for spread of false cord and aryepiglottic fold tumours. Paraglottic space tumour may extend anteriorly to the pre-epiglottic space, posteriorly to submucosally access the piriform sinus apex via the thyroarytenoid gap (Fig. 5a). The conus elasticus restricts inferior spread of paraglottic tumour so subglottic involvement is often a late finding. Inferolateral extension may occur through the cricothyroid membrane (or cricothyroid space where there is deficiency of membrane) to invade the soft tissues of the neck. Very bulky tumour will abut the carotid sheath (Fig. 5). If the carotid artery is surrounded by greater than 270° of tumour then it is unlikely to be salvaged at surgery[13].
Figure 5.
(a) Axial CT image demonstrates extension of paraglottic tumour to widen the thyroarytenoid gap and invade the piriform sinus submucosally. (b) Axial T1-w image illustrates how a bulky paraglottic tumour may extend laterally into the soft tissues of the neck through the thyrohyoid and cricohyoid membranes or through cartilage invasion. This tumour lies close to but does not encroach on the carotid sheath.
As well as its relevance in predicting and identifying tumour spread, the involvement of pre-epiglottic and paraglottic spaces has direct impact on therapeutic strategies. CT and MRI are 88–93% accurate in the assessment of tumour extension within these spaces[14]. Pre-epiglottic fat encroachment upstages supraglottic tumour to T3 and increases the possibility of cervical nodal involvement. Substantial involvement reduces the possibility of local control and preservation of laryngeal function by radiotherapy alone. Bulky pre-epiglottic disease with potential involvement of the hyoid bone will contraindicate supraglottic and supracricoid laryngectomies and may necessitate the removal of a cuff of tongue base. Supraglottic and glottic carcinomas with paraglottic invasion with or without minor cartilage invasion are staged T3 by the UICC sixth edition[3] and they represent a high risk group for recurrence post radiotherapy[15]. A glottic tumour invading deeply to the paraglottic fat usually precludes local laser resection or cordectomy. A supraglottic carcinoma infiltrating the paraglottic space inferiorly to ventricular level will preclude a supraglottic laryngectomy. It should prompt the radiologist to assess for piriform sinus, pre-epiglottic and extralaryngeal extension, which will further influence treatment decisions.
Anterior and posterior extension
In addition to defining the craniocaudal extension within the submucosal spaces relative to the ventricular complex landmarks, it is important to evaluate the anterior and posterior extension of tumour. This is particularly important at the level of the glottis.
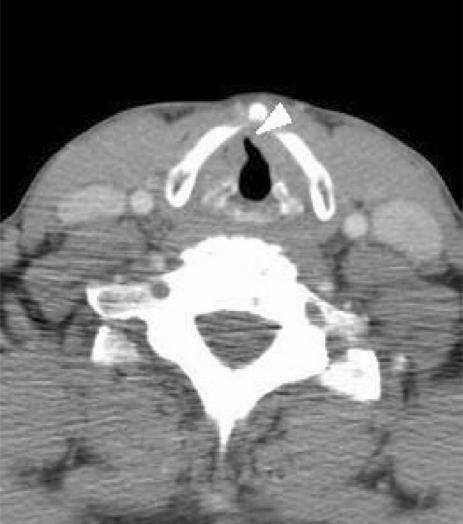
Extension of laryngeal carcinoma (usually from anterior glottic carcinoma) to the anterior commissure (Fig. 6) is significant in that it is frequently associated with thyroid cartilage invasion, particularly if there is involvement of the base of epiglottis. The cartilage is prone to invasion at this site because the internal perichondrium is deficient and the external perichondrium is thinner. Tumour may also extend from the anterior commissure to an extralaryngeal location caudal to the thyroid cartilage (via the cricothyroid membrane) and inferiorly to the subglottis. These findings would preclude partial laryngectomy and upstage the tumour. Extension of glottic carcinoma across the midline to involve greater than 1/3 of the contralateral vocal cord would also contraindicate a vertical hemilaryngectomy. CT is 75% accurate in predicting anterior commissure involvement[16].
Figure 6.
Axial CT scan shows thickening of the anterior commissure by glottic tumour (arrowhead).
Posterior extension of glottic tumour to the interarytenoid region is important since it will preclude supracricoid laryngectomies and may be a precursor to postcricoid pharyngeal extension. Such extension of laryngeal carcinoma to the pharynx or upper oesophageal segment will necessitate an additional pharyngeal resection with its associated morbidity. The radiologist should be aware of other potential pathways to the pharynx. Tumour may extend directly to the piriform sinus from the paraglottic fat and extend beneath the cricoid cartilage to the upper oesophagus from the subglottis.
Laryngeal cartilage invasion
Predicting the presence of laryngeal cartilage invasion is a key aspect of the imaging assessment. The presence of significant laryngeal cartilage invasion has been considered a negative indicator for conservative therapeutic strategies such as primary modality radiotherapy or partial laryngectomy, however recent studies have shown that tumours with limited cartilage involvement may be cured with radiotherapy[17]. The laryngeal cartilage may be evaluated with CT or MRI. CT signs of erosion (Fig. 7a,b) and extralaryngeal tumour of the thyroid, cricoid and arytenoid cartilages together with sclerosis (Fig. 7c) of the cricoid and arytenoid cartilages result in a sensitivity of 64–72% and specificity of 86–94% for cartilage involvement[18–20]. Sclerosis is the most sensitive criterion but this often corresponds to reactive inflammation, particularly with respect to the thyroid cartilage. MRI criteria for cartilage involvement by tumour are high signal on fat suppressed T2-w images and/or enhancement on post gadolinium fat suppressed T1-w images in the cartilage adjacent to the tumour, or the presence of extralaryngeal tumour. The most recent studies describing radiological–pathological correlation demonstrated slightly improved sensitivity of 89–95% and reduced specificity of 74–84% relative to CT[18–21]. The accuracy of both techniques is approximately 80% and this compares favourably with combined clinical and endoscopic examination which has a staging accuracy of 58%[19]. Additional imaging modalities such as dual isotope SPECT[22] imaging and ultrasound[6] have been evaluated but there is limited available data.
Figure 7.
Erosion of the thyroid cartilage (a) and cricoid cartilage (b) indicates cartilage involvement by tumour. Sclerosis (arrowhead) is less specific, however may be used as an indicator of cartilage involvement for the arytenoid (c) or cricoid cartilages.
Tumour is well delineated from ossified cartilage marrow fat (high T1-w signal and fatty CT attenuation) and cortical bone (low T2-w signal and calcific CT density) using either modality. This is fortunate since laryngeal cartilage is most susceptible to tumour invasion when ossified[23]. Demonstrating tumour invasion of non-ossified cartilage is problematic with CT due to similarity of the CT density. MRI is more sensitive in this situation since tumour is of increased T2-w signal relative to non-ossified cartilage. Unfortunately MRI has a tendency to overestimate cartilage involvement by tumour since it may be indistinguishable from that due to peritumoural inflammation. These changes are most commonly seen in the thyroid cartilage so specificity is lowest at this location. New criteria of moderately high T2-w signal and moderate enhancement as compared to the marked signal changes and gadolinium enhancement with inflammation, have been proposed to overcome this problem. Alternatively the lower specificity of MRI may be addressed by performing a corroborating CT scan in the presence of an MRI scan positive for cartilage invasion.
Nodal or systemic metastases and synchronous tumour
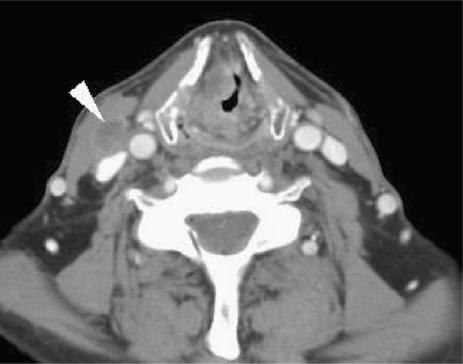
As with the other carcinomas of the head and neck, cervical lymph node metastasis is the single most important indicator of prognosis. Supraglottic carcinoma is the most likely laryngeal site to present with nodal metastases. The primary echelon of nodal drainage is to levels 2–4. Levels 1 and 5 metastases are rare and only present if proximal nodal disease is confirmed. The glottis is devoid of lymphatics, particularly anteriorly, and hence nodal involvement is present in less than 5% for T1 tumours. The subglottic drainage is to the prelaryngeal (Delphian node), and subsequently to paratracheal and superior mediastinal nodes. Imaging criteria for nodal disease including size, shape and necrosis, are well described (Fig. 8).
Figure 8.
Axial CT scan reveals a right sided necrotic lymph node secondary to a supraglottic carcinoma. The lymph node had not been palpated by the referring clinician.
The lung is the most common site for systemic metastases, with mediastinum, bone and liver sites being well recognised. Approximately 15% of patients with supraglottic carcinoma and 3% of patients with glottic carcinoma[24] will develop distant metastases within two years of diagnosis. This is often preceded by loco-regional failure. Carcinoma of the larynx is also characterised by a high incidence of second primary tumours, particularly in the setting of supraglottic carcinoma. The likelihood of metastases is low in patients with early stage disease and routine work up is generally with a chest radiograph. Although United Kingdom surgical standards dictate chest CT[25] as part of the routine staging, the data to support such an approach is limited. A CT chest may be appropriate in patients with more advanced T staging or in those with paratracheal nodal disease[26]. CT-PET may play a greater role in the future with regard to screening for distant metastases, particularly in patients with recurrent disease[27].
Post treatment imaging
Following radiotherapy, the laryngopharynx is expected to become oedematous with thickening of the epiglottis, aryepiglottic folds, arytenoids and anterior commissure (Fig. 9). The normal fat planes develop a stippled appearance. These changes may become marked and persistent beyond 6 months if there is impairment of vascular or lymphatic flow. Chondroradionecrosis may result in further soft tissue swelling, with air trapping, fragmentation, collapse and sloughing. Differentiating local recurrence from chondroradionecrosis is a difficult clinical problem with both occurring within one year of radiotherapy. Tumour recurrence is present in approximately half of patients with severe oedema but biopsies have a poor negative predictive value and may aggravate the necrosis. Despite this, endoscopy remains the preferred method to diagnose mucosal recurrences with serial follow up and PET scans contributing to the detection of deep recurrence[8]. Treatment failure should be suspected if a 4-month CT scan demonstrates a less than 50% reduction in tumour size[28].
Figure 9.
(a) Superiorly and b) inferiorly. Four months following radiotherapy for a supraglottic carcinoma, there are typical post radiotherapy appearances of oedematous thickening within supraglottic structures, thickening of fascial planes and streaky density within the fat spaces. The submandibular glands are atrophic and markedly enhancing.
Laryngeal conservation partial laryngectomies may grossly distort the normal anatomy. Distinguishing tumour from postoperative change is hindered by redundant mucosa, normal and distorted airway, obliteration of the paralaryngeal fat planes, development of postoperative granuloma and sclerotic foci within residual cartilages. Despite this, obvious cartilage destruction and soft tissue masses larger than 1 cm (particularly at ‘pexy sites’) should suggest recurrent tumour. Total laryngectomy appearances consist of a ‘neopharynx’ and a stoma placed at the third or fourth tracheal ring. Marginal recurrences are identified as nodularity, soft tissue masses and necrosis.
Other tumours of the larynx
Other tumours of the larynx comprise less than 10% of laryngeal neoplasms. They are generally submucosal in location (Fig. 10) and the role of imaging is to delineate the extent of infiltration and to guide the endoscopist to appropriate transmucosal deep biopsy site. In most cases (e.g. salivary gland tumours, neurogenic tumours, haematopoietic tumours), the imaging findings are non-specific. However the correct histology may be suggested in the setting of lipoma (fat CT density), haemangioma (high T2-w signal and calcified phleboliths), chondrogenic tumours (high T2-w signal and ‘popcorn’ calcification), melanoma metastases (high T1-w signal), amyloidoma (low T1-w and T2-w signal with poor enhancement) and lymphoma (vividly enhancing bulky supraglottic mass)[29].
Figure 10.
Coronally reformatted image from an advanced CT study demonstrates left sided supraglottic tumour. This corresponded to a venous malformation (cavernous haemangioma).
Conclusion
In conclusion, cross sectional imaging provides information on tumour volume, extension to and across the laryngeal ventricle, infiltration of pharynx, paraglottic, pre-epiglottic and extralaryngeal spaces, all of which impact on the potential for voice conserving partial laryngectomies and response to radiotherapy. Together with an assessment of nodal and systemic metastases, this enables the radiologist to play an integral role in the multidisciplinary selection of treatment options.
References
- 1.Esteve J, Kricker A, Ferlay J, Parkin NM, editors. Lyon: World Health Organization; 1993. In: Facts and figures of cancer in the European Community. International Agency for Research on Cancer Commission of the European Communities. [Google Scholar]
- 2.London: DoH; 2000. Department of Health Guidance Document HSC 2000/13 – Referral guidelines for suspected cancer. [Google Scholar]
- 3.American Joint Committee on Cancer . Cancer staging handbook. 6th. New York: Springer; 2002. edn. [Google Scholar]
- 4.Urba SG, Wolf GT, Bradford CA, et al. Neoadjuvant therapy for organ preservation in head and neck cancer. Laryngoscope. 2000;110:2074–80. doi: 10.1097/00005537-200012000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Magnano M, Bongioannini G, Cirillo S, et al. Virtual endoscopy of laryngeal carcinoma: is it useful? Otolaryngol Head Neck Surg. 2005;132:776–82. doi: 10.1016/j.otohns.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Erkan M, Tolu I, Aslan T, Guney E. Ultrasonography in laryngeal cancers. J Laryngol Otol. 1993;107:65–8. doi: 10.1017/s0022215100122182. [DOI] [PubMed] [Google Scholar]
- 7.Arens C, Eistert B, Glanz H, Waas W. Endolaryngeal high-frequency ultrasound. Eur Arch Otorhinolaryngol. 1998;255:250–5. doi: 10.1007/s004050050052. [DOI] [PubMed] [Google Scholar]
- 8.Gordin A, Daitzchman M, Doweck I. Fluorodeoxyglucose-positron emission tomography/computed tomography imaging in patients with carcinoma of the larynx: diagnostic accuracy and impact on clinical management. Laryngoscope. 2006;116:273–8. doi: 10.1097/01.mlg.0000197930.93582.32. [DOI] [PubMed] [Google Scholar]
- 9.Freeman DE, Mancuso AA, Parsons JT, et al. Irradiation alone for supraglottic larynx carcinoma: can CT findings predict treatment results? Int J Radiat Oncol Biol Phys. 1990;19:485–90. doi: 10.1016/0360-3016(90)90562-x. [DOI] [PubMed] [Google Scholar]
- 10.Lee WR, Mancuso AA, Saleh EM, et al. Can pre-treatment computed tomography findings predict local control in T3 squamous cell carcinoma of the glottic larynx treated with radiotherapy alone? Int J Radiat Oncol Biol Phys. 1993;25:683–7. doi: 10.1016/0360-3016(93)90016-o. [DOI] [PubMed] [Google Scholar]
- 11.Mukherji SK, O’Brien SM, Gerstle RJ, et al. Tumor volume: an independent predictor of outcome for laryngeal cancer. J Comput Assist Tomogr. 1999;23:50–4. doi: 10.1097/00004728-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kallmes DF, Phillips CD. The normal anterior commissure of the glottis. AJR Am J Roentgenol. 1997;168:1317–19. doi: 10.2214/ajr.168.5.9129433. [DOI] [PubMed] [Google Scholar]
- 13.Yousem DM, Hatabu H, Hurst RW, et al. Carotid invasion by head and neck masses: prediction with MR imaging. Radiology. 1995;195:715–20. doi: 10.1148/radiology.195.3.7754000. [DOI] [PubMed] [Google Scholar]
- 14.Loevner LA, Yousem DM, Montone KT, et al. Can radiologists accurately predict pre-epiglottic space invasion with MR imaging? AJR Am J Roentgenol. 1997;169:1681–7. doi: 10.2214/ajr.169.6.9393190. [DOI] [PubMed] [Google Scholar]
- 15.Marakami R, Furusawa M, Baba Y, et al. Dynamic helical CT of T1 and T2 glottic carcinomas: predictive value for local control with radiation therapy. AJNR Am J Neuroradiol. 2000;21:1320–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Barbosa MM, Araujo VJ, Jr, Boasquevisque E, et al. Anterior vocal commissure invasion in laryngeal carcinoma diagnosis. Laryngoscope. 2005;115:724–30. doi: 10.1097/01.mlg.0000161329.75600.9d. [DOI] [PubMed] [Google Scholar]
- 17.Theony HC, Delaere PR, Hermans R. Correlation of local outcome after partial laryngectomy with cartilage abnormalities on CT. AJNR Am J Neuroradiol. 2005;26:674–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Zbaren P, Becker M, Lang H. Pretherapeutic staging of hypopharyngeal carcinoma. Clinical findings. Computed tomography, and magnetic resonance imaging compared with histopathological evaluation. Arch Otolaryngol Head Neck Surg. 1997;123:908–13. doi: 10.1001/archotol.1997.01900090016003. [DOI] [PubMed] [Google Scholar]
- 19.Zbaren P, Becker M, Lang H. Pretherapeutic staging of laryngeal carcinoma. Clinical findings, computed tomography, and magnetic resonance imaging compared with histopathology. Cancer. 1996;77:1263–73. doi: 10.1002/(sici)1097-0142(19960401)77:7<1263::aid-cncr6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 20.Zbaren P, Becker M, Lang H. Staging of laryngeal cancer: endoscopy, computed tomography and magnetic resonance imaging versus histopathology. Eur Arch Otorhinolaryngol Suppl. 1997;1:S117–22. doi: 10.1007/BF02439740. [DOI] [PubMed] [Google Scholar]
- 21.Becker M, Zbaren P, Laeng H, Stoupis C, Porcellini B, Vock P. Neoplastic invasion of the laryngeal cartilage: comparison of MR imaging with CT with histopathologic correlation. Radiology. 1995;194:661–9. doi: 10.1148/radiology.194.3.7862960. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama Y, Yamamoto Y, Yokoe K, et al. Superimposed dual-isotope SPECT using 99m Tc-hydroxymethylene diphosphonate and 201 Tl-chloride t assess cartilage invasion in laryngopharyngeal cancer. Ann Nucl Med. 2004;18:527–32. doi: 10.1007/BF02984571. [DOI] [PubMed] [Google Scholar]
- 23.Carter RL, Tanner NS. Local invasion by laryngeal carcinoma- the importance of focal (metaplastic) ossification with laryngeal cartilage. Clin Otolaryngol Allied Sci. 1979;4:283–90. doi: 10.1111/j.1365-2273.1979.tb01901.x. [DOI] [PubMed] [Google Scholar]
- 24.Merino OR, Lindberg RD, Fletcher GH. An analysis of distant metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1977;40:145–51. doi: 10.1002/1097-0142(197707)40:1<145::aid-cncr2820400124>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Wilson JA, editor. 2nd ed. London: Head and Neck Surgeons; 2000. Effective head and neck cancer management. Consensus document. British Association of Otorhinolaryngologists. [Google Scholar]
- 26.Ljumanovic R, Langendijk JA, Hoekstra OS, Leemans CR, Castalijns JA. Distant metastases in head and neck carcinoma: identification of prognostic groups with MR imaging. Eur J Radiol. 2006;60:58–66. doi: 10.1016/j.ejrad.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Mukherji SK, Bradford CR. Controversies: is there a role for positron-emission tomographic CT in the initial staging of head and neck carcinoma? AJNR Am J Neuroradiol. 2006;27:243–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherji SK, Mancuso AA, Kotzur IM, et al. Radiologic appearance of the irradiate larynx. Part 11. Primary site response. Radiology. 1994;193:149–54. doi: 10.1148/radiology.193.1.8090883. [DOI] [PubMed] [Google Scholar]
- 29.Becker M, Moulin G, Kurt A-M, et al. Atypical squamous cell carcinoma of the larynx and hypopharynx: radiologic features and pathologic correlation. Eur Radiol. 1998;8:1541–51. doi: 10.1007/s003300050584. [DOI] [PubMed] [Google Scholar]